Abstract
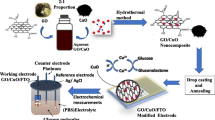
A carbon film functionalized with reduced graphene oxide (rGO) and Cu nanoparticles (NPs)-tipped carbon nanofibers (CNFs) was demonstrated to be a versatile electrode for the efficient electropolymerization of the following monomers: acryl amide, methylene blue, methyl orange, and β-cyclodextrin. These electropolymers were used as the recognition elements for the measurements of glucose, creatinine, cholesterol, and spermine solutions, respectively. The carbon film was synthesized from a phenolic precursor via suspension polymerization and subjected to multiple thermal processes, viz. carbonization, H2-reduction, and chemical vapor deposition, in an especially designed reactor. Physicochemical tests confirmed the controlled growth of the graphitic CNFs with the electrocatalytic Cu NPs located at their tips. The CNF growth was found to be critical for providing the Faradic and electrocatalytic characteristics to the electrode, whereas rGO enhanced the material electroconductivity (462 S/m). Tested in the phosphate buffer and clinical samples using differential pulse voltammetry and chronoamperometry techniques, the prepared electrode showed a fast and reproducible response, with the limits of detection of 0.18, 0.006, 0.036 and 0.0003 mM, respectively. The synthesized Cu–CNF–rGO functionalized carbon film in this study is indicated to be a potential universal substrate for electrochemical sensors of a wide range of biomarkers.
Graphical abstract








Similar content being viewed by others
References
Wilson R, Turner APF (1992) Glucose oxidase: an ideal enzyme. Biosens Bioelectron 7:165–185
Mano N, Edembe L (2013) Bilirubin oxidases in bioelectrochemistry: features and recent findings. Biosens Bioelectron 50:478–485
Malik R, Tomar VK, Mishra YK, Lin L (2020) Functional gas sensing nanomaterials: a panoramic view. Appl Phys Rev 7:021301
Lange U, Roznyatovskaya NV, Mirsky VM (2008) Conducting polymers in chemical sensors and arrays. Anal Chim Acta 614:1–26
Bai S, Hu Q, Zeng Q, Wang M, Wang L (2018) Variations in surface morphologies, properties, and electrochemical responses to nitro-analyte by controlled electropolymerization of thiophene derivatives. ACS Appl Mater Interfaces 10:11319–11327
Curulli A, Palleschi G (1997) Electropolymerization of pyrrole-2-carboxylic acid and 4,4′-dihydroxybenzophenone on platinum electrodes applications to assemble novel glucose sensors. Electroanalysis 9:1107–1112
Köhler C, Bleck L, Frei M, Zengerle R, Kerzenmacher S (2015) Poisoning of highly porous platinum electrodes by amino acids and tissue fluid constituents. ChemElectroChem 2:1785–1793
Giner J, Marinčić L, Soeldner JS, Colton CK (1981) Electrochemical glucose oxidation on a platinized platinum electrode in Krebs-Ringer solution: IV effect of amino acids. J Electrochem Soc 128:2106–2114
Lucarelli F, Marrazza G, Turner APF, Mascini M (2004) Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens Bioelectron 19:515–530
McCreery RL (2008) Advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687
Zhu H, Li L, Zhou W, Shao Z, Chen X (2016) Advances in non-enzymatic glucose sensors based on metal oxides. J Mater Chem B 4:7333–7349
Dang Y, Wang X, Cui R, Chen S, Zhou Y (2019) A novel electrochemical sensor for the selective determination of hydroquinone and catechol using synergic effect of electropolymerized nicotinic acid film and Cd-doped ZnWO4 nanoneedle. J Electroanal Chem 834:196–205
Tiwari JN, Vij V, Kemp KC, Kim KS (2016) Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 10:46–80
Xie F, Yang M, Jiang M, Huang XJ, Liu WQ, Xie PH (2019) Carbon-based nanomaterials–a promising electrochemical sensor toward persistent toxic substance. TrAC Trends Anal Chem 119:115624
Settu K, Lai YC, Liao CT (2021) Carbon nanotube modified laser-induced graphene electrode for hydrogen peroxide sensing. Mater Lett 300:130106
Chen HC, Su WR, Yeh YC (2020) Functional channel of SWCNTs/Cu2O/ZnO NRs/graphene hybrid electrodes for highly sensitive nonenzymatic glucose sensors. ACS Appl Mater Interfaces 12:32905–32914
Abousalman-Rezvani Z, Eskandari P, Roghani-Mamaqani H, Salami-Kalajahi M (2020) Functionalization of carbon nanotubes by combination of controlled radical polymerization and “grafting to” method. Adv Colloid Interf Sci 278:102126
Shoja Y, Rafati AA, Ghodsi J (2016) Electropolymerization of Ni-LD metallopolymers on gold nanoparticles enriched multi-walled carbon nanotubes as nano-structure electrocatalyst for efficient voltammetric sertraline detection in human serum. Electrochim Acta 203:281–291
Shrivastava S, Bairagi PK, Verma N (2020) Spermine biomarker of cancerous cells voltammetrically detected on a poly(β-cyclodextrin) - electropolymerized carbon film dispersed with Cu-CNFs. Sens Actuators B Chem 313:128055
Pandey I, Bairagi PK, Verma N (2018) Electrochemically grown polymethylene blue nanofilm on copper-carbon nanofiber nanocomposite: An electrochemical sensor for creatinine. Sensors Actuators, B Chem 277:562–570
Bairagi PK, Verma N (2018) Electrochemically deposited dendritic poly (methyl orange) nanofilm on metal-carbon-polymer nanocomposite: a novel non-enzymatic electrochemical biosensor for cholesterol. J Electroanal Chem 814:134–143
Bairagi PK, Verma N (2019) Electro-polymerized polyacrylamide nano film grown on a Ni-reduced graphene oxide- polymer composite: a highly selective non-enzymatic electrochemical recognition element for glucose. Sensors Actuators B Chem 289:216–225
Pumera M, Ambrosi A, Bonanni A, Chng ELK, Poh HL (2010) Graphene for electrochemical sensing and biosensing. TrAC Trends Anal Chem 29:954–965
Gao J, Li H, Li M, Wang G, Long Y, Li P, Li C, Yang B (2021) Polydopamine/graphene/MnO2 composite-based electrochemical sensor for in situ determination of free tryptophan in plants. Anal Chim Acta 1145:103–113
Li S, Cheng C, Thomas A (2017) Carbon-based microbial-fuel-cell electrodes: from conductive supports to active catalysts. Adv Mater 29:1602547
Pant D, Singh A, Bogaert GV, Olsen SI, Nigam PS, Diels L, Vanbroekhoven K (2012) Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv 2:1248–1263
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Vander Wal RL, Ticich TM, Curtis VE (2001) Substrate-support interactions in metal-catalyzed carbon nanofiber growth. Carbon 39:2277–2289
Baker RTK (1989) Catalytic growth of carbon filaments. Carbon 27:315–323
Wu H, Zhou Z, Chen L, Li W, Han Q, Li C, Xu Z, Qian X (2018) PECVD-induced growing of diverse nanomaterials on carbon nanofibers under various conditions. Mater Lett 216:381–389
Hammel E, Tang X, Trampert M, Schmitt T, Mauthner K, Eder A, Potschke P (2004) Carbon nanofibers for composite applications. Carbon 42:1153–1158
Prajapati YN, Verma N (2018) Fixed bed adsorptive desulfurization of thiophene over Cu/Ni-dispersed carbon nanofiber. Fuel 216:381–389
Seyring M, Simon A, Voigt I, Ritter U, Rettenmayr M (2017) Quantitative crystallographic analysis of individual carbon nanofibers using high resolution transmission electron microscopy and electron diffraction. Carbon 116:347–355
Ramos A, Cameán I, García AB (2013) Graphitization thermal treatment of carbon nanofibers. Carbon 59:2–32
Worsley MA, Pham TT, Yan A, Shin SJ, Lee JRI, Bagge-Hansen M, Mickelson W, Zettl A (2014) Synthesis and characterization of highly crystalline graphene aerogels. ACS Nano 8:11013–11022
Singh S, Pophali A, Omar RA, Kumar R, Kumar P, Mondal DP, Pant D, Verma N (2021) A nickel oxide-decorated in situ grown 3-D graphitic forest engrained carbon foam electrode for microbial fuel cells. Chem Commun 57:879–882
Some S, Kim Y, Yoon Y, Yoo H, Lee S, Park Y, Lee H (2013) High-quality reduced graphene oxide by a dual-function chemical reduction and healing process. Sci Rep 3:1929
Bozal-Palabiyik B, Kurbanoglu S, Uslu B, Ozkan SA, Zuman P (2016) Diffusion, adsorption and electrode kinetics of electro-oxidations on a stationary solid electrode. Electroanalysis 28:2947–2955
Bard A, Faulkner L (2001) Electrochemical methods – fundamentals and applications, 2nd edn. Wiley, New York
Barsan MM, Pinto EM, Brett CMA (2011) Methylene blue and neutral red electropolymerisation on AuQCM and on modified AuQCM electrodes: an electrochemical and gravimetric study. Phys Chem Chem Phys 13:5462–5471
Reddaiah K, Madhusudana Reddy T, Raghu P (2012) Electrochemical investigation of L-dopa and simultaneous resolution in the presence of uric acid and ascorbic acid at a poly (methyl orange) film coated electrode: a voltammetric study. J Electroanal Chem 682:164–171
Roa Morales G, Ramírez Silva T, Galicia L (2003) Carbon paste electrodes electrochemically modified with cyclodextrins. J Solid State Electrochem 7:355–360
Lu W, Smela E, Adams P, Zuccarello G, Mattes BR (2004) Development of solid-in-hollow electrochemical linear actuators using highly conductive polyaniline. Chem Mater 16:1615–1621
Pelzer J, Scholz F, Henrion G, Nitschke L (1989) Optimization of parameters for differential pulse voltammetry at the hanging mercury drop electrode. Electroanalysis 1:437–440
Piletsky SA, Piletskaya EV, Sergeyeva TA, Panasyuk TL, A. V. El’Skaya, (1999) Molecularly imprinted self-assembled films with specificity to cholesterol. Sens Actuators B Chem 60:216–220
Reynolds ER, Yacynych AM (1994) Direct sensing platinum ultramicrobiosensors for glucose. Biosens Bioelectron 9:283–293
Martin MJ, Browner WS, Hulley SB, Kuller LH, Wentworth D (1986) Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet 328:933–936
Jones CA, McQuillan GM, Kusek JW, Eberhardt MS, Herman WH, Coresh J, Salive M, Jones CP, Agodoa LY (1998) Serum creatinine levels in the US population: third national health and nutrition examination survey. Am J Kidney Dis 32:992–999
Kano Y, Soda K, Nakamura T, Saitoh M, Kawakami M, Konishi F (2007) Increased blood spermine levels decrease the cytotoxic activity of lymphokine-activated killer cells: a novel mechanism of cancer evasion. Cancer Immunol Immunother 56:771–781
Góralski P (1994) Hydrogen bonds between cholesterol and nitrogen bases—a thermodynamic study. Thermochim Acta 235:31–38
Krebs HA (1950) Chemical composition of blood plasma and serum. Annu Rev Biochem 19:409–430
Acknowledgements
The authors acknowledge the Science and Engineering Research Board (Government of India, New Delhi) for the project grant (SERB-CRG/2019/000122) to perform the present study. The authors are also thankful to the Center for Environmental Science and Engineering, IIT Kanpur, for providing the infrastructure to conduct research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, H., Verma, N. A Cu–CNF–rGO-functionalized carbon film indicated as a versatile electrode for sensing of biomarkers using electropolymerized recognition elements. J Mater Sci 57, 6345–6360 (2022). https://doi.org/10.1007/s10853-022-07029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07029-7