Abstract
Purpose
We investigated the role of gap junctions (GJs) in embryological differentiation, and observed the morphological behavior of the inner cell mass (ICM) by time-lapse movie observation (TLM) with gap junction inhibitors (GJis).
Methods
ICR mouse embryos were exposed to two types of GJis in CZB medium: oleamide (0 to 50 μM) and 1-heptanol (0 to 10 mM). We compared the rate of blastocyst formation at embryonic day 4.5 (E4.5) with E5.5. We also observed and evaluated the times from the second cleavage to each embryonic developing stage by TLM. We investigated embryonic distribution of DNA, Nanog protein, and Connexin 43 protein with immunofluorescent staining.
Results
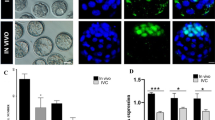
In the comparison of E4.5 with E5.5, inhibition of gap junction intercellular communication (GJIC) delayed embryonic blastocyst formation. The times from the second cleavage to blastocyst formation were significantly extended in the GJi-treated embryos (control vs with oleamide, 2224 ± 179 min vs 2354 ± 278 min, p = 0.013). Morphological differences were traced in control versus GJi-treated embryos until the hatching stage. Oleamide induced frequent severe collapses of expanded blastocysts (77.4 % versus 26.3 %, p = 0.0001) and aberrant ICM divisions connected to sticky strands (74.3 % versus 5.3 %, p = 0.0001). Immunofluorescent staining indicated Nanog-positive cells were distributed in each divided ICM.
Conclusions
GJIC plays an important role in blastocyst formation, collapses of expanded blastocysts, and the ICM construction in mouse embryos.




Similar content being viewed by others
References
Shirayoshi Y, Okada TS, Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983;35:631–8.
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410.
Zhou JZ, Jiang JX. Gap junction and hemichannel-independent actions of connexins on cell and tissue functions – An update. FEBS Lett. 2014;588:1186–92.
Houghton FD. Role of gap junctions during early embryo development. Reproduction. 2005;129:129–35.
Houghton FD, Barr KJ, Walter G, Gabriel HD, Grümmer R, Traub O, et al. Functional significance of Gap junctional coupling in preimplantation development. Biol Reprod. 2002;66:1403–12.
Bloor DJ, Wilson Y, Kibschull M, Traub O, Leese HJ, Winterhager E, et al. Expression of connexins in human preimplantation embryos in vitro. Reprod Biol Endocrinol. 2004;2:25.
Harris A., Locke D. Connexins: A Guide. Humana Press, a part of Springer Science, New York, USA; 2009. pp 207–219, 270–271, 287–301.
Oyamada M, Takebe K, Endo A, Hara S, Oyamada Y. Connexin expression and gap-junctional intercellular communication in ES cells and iPS cells. Front Pharmacol. 2013. doi:10.3389/fphar.2013.00085.
Lo CW, Gilula NB. Gap junctional communication in the preimplantation mouse embryo. Cell. 1979;18:399–409.
Becker DL, Leclerc-David C, Warner A. The relationship of gap junctions and compaction in the preimplantation mouse embryo. Dev Suppl.1992:113–118.
Becker DL, Davies CS. Role of gap junctions in the development of the preimplantation mouse embryo. Microsc Res Tech. 1995;31:364–74.
Vance MM, Wiley LM. Gap junction intercellular communication mediates the competitive cell proliferation disadvantage of irradiated mouse preimplantation embryos in aggregation chimeras. Radiat Res. 1999;152:544–51.
Mio Y, Maeda K. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos. Am J Obstet Gynecol. 2008;199:660.e1–5. doi:10.1016/j.ajog.2008.07.023.
Mio Y, Iwata K, Yumoto K, Maeda K. Human embryonic behavior observed with time-lapse cinematography. J Health Med Info. 2014;5:143. doi:10.4172/2157-7420.1000143.
Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans. 2001;29:606–12.
Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–40.
Guan X, Cravatt BF, Ehring GR, Hall JE, Boger DL, Lerner RA, et al. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J Cell Biol. 1997;139:1785–92.
Nelson WL, Makielski JC. Block of sodium current by heptanol in voltage-clamped canine cardiac Purkinje cells. Circ Res. 1991;68:977–83.
Mori T, Amano T, Shimizu H. Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured in vitro. Biol Reprod. 2000;62:913–9.
Pan G, Thomson JA. Nanog works together with other key pluripotent factors such as Oct4 and Sox2 to control a set of target genes that have important functions in ES cell pluripotency. Cell Res. 2007;17:42–9.
Ke Q, Li L, Cai B, Liu C, Yang Y, Gao Y, et al. Connexin 43 is involved in the generation of human-induced pluripotent stem cells. Hum Mol Genet. 2013;22:2221–33.
Chen AA, Tan L, Suraj V, Reijo Pera R, Shen S. Biomarkers identified with time-lapse imaging discovery, validation, and practical application. Fertil Steril. 2013;99:1035–43.
Niimura S. Time-lapse videomicrographic analyses of contractions in mouse blastocysts. J Reprod Dev. 2003;49:413–23.
Acknowledgments
We would like to thank Yasuyuki Mio and his colleagues for their constructive comments, face-to-face discussion, and helpful advice on analyzing the TLM data regarding the aberrant ICM behavior, and Masahito Tachibana for practical suggestions on the IF protocol, especially Nanog protein. We appreciate Mr. Jeffrey G. Stocker's contributions to proofreading.
All staff in the Department of Obstetrics and Genecology, Akita University Graduate School of Medicine provided considerable long-term encouragement. In particular, Hideya Kodama and Katsuya Kabashima provided valuable advice. Hisataka Hasegawa contributed constructive discussion to this work. We greatly appreciate the protocols provided by “Cell Community in early mammalian development” in Grant-in-Aid for Scientific Research on Innovative Areas.
Funding
This work was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) [Grant Number 25462549]; and a Grant of National Centre for Child Health and Development (24–6).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Morphological parameters might indicate the efficiency of gap junction intercelluler communication in embryos.
Electronic supplementary material
Below is the link to the electronic supplementary material.
TLM observation of mouse embryos. 1A: TLM observation of mouse embryos co-cultured with 0.1 % DMSO. They developed smoothly and finished hatching. The recording interval was 3 min at 15 frames per second. Scale bar: 100 μm (MPG 48940 kb)
1B: TLM observation of mouse embryos co-cultured with oleamide 50 μM. Aberrant ICM behavior and frequent collapses were recorded. The recording interval was 3 min at 15 frames per second. Scale bar: 100 μm (MPG 43864 kb)
TLM observation of human freeze/thaw embryos. Two embryos were obtained from a patient. They were conventionally fertilized and frozen on day 5. Both of them were frozen at 3AA (blastocyst grading system introduced by Gardner and Schoolcraft in 1999). The aberrant ICM divisions were traced in both embryos. The embryo on the left has finished hatching. The embryo on the right contained fragment blastomeres, exhibited delayed expansion, and failed to finish hatching. The recording interval was 3 min at 15 frames per second. Scale bar: 100 μm (MPG 29904 kb)
Immunofluorescent staining and confocal imaging of divided ICMs. These are confocal images taken using the Zeiss LSM 780 confocal microscope. 3A: Mouse embryo cultured with oleamide 50 μM. The same embryo as that in Fig. 4B (MPG 16222 kb)
3B: Human freeze/thawed blastocyst. Same embryo as that in Fig. 4D green, Cx43; red, Nanog; blue, DNA (MPG 26928 kb)
Rights and permissions
About this article
Cite this article
Togashi, K., Kumagai, J., Sato, E. et al. Dysfunction in gap junction intercellular communication induces aberrant behavior of the inner cell mass and frequent collapses of expanded blastocysts in mouse embryos. J Assist Reprod Genet 32, 969–976 (2015). https://doi.org/10.1007/s10815-015-0479-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0479-1