Abstract
We have demonstrated that cellulosic CuI nanoparticles could perform as an efficient heterogeneous catalyst for the synthesis of useful organoboron compounds. Desired β-borylation products were all obtained in good to excellent yields under mild conditions. This catalyst could be recovered easily and still work effectively in six runs. Notably, asymmetric synthesis of organoboron compounds was accomplished by applying a chiral phosphine ligand. This newly developed protocol provides an efficient and sustainable pathway for the formation of C-B bonds and largely extends the applied range of cellulose.
Graphical Abstract
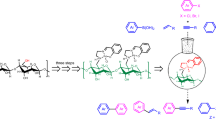
Asymmetric and efficient synthesis of organoboron compounds was accomplished by cellulosic CuI nanoparticles combining with a chiral phosphine ligand. This newly reported strategy provides a green and sustainable method for the construction of C–B bonds and largely extends the applied range of cellulose.













Similar content being viewed by others
References
Anastas P, Eghbali N (2010) Chem Soc Rev 39:301–312
Gawande MB, Bonifácio VDB, Luque R, Branco PS, Varma RS (2013) Chem Soc Rev 42:5522–5551
Astruc D (2020) Chem Rev 120(2):461–463
Astruc D, Lu F, Ruiz J (2005) Angew Chem Int Chem 44(48):7852–7872
Noujima A, Mitsudome T, Mizugaki T, Jitsukawa K, Kaneda K (2011) Angew Chem Int Chem 50:2986–2989
Mitsudome T, Mikami Y, Matoba M, Mizugaki T, Jitsukawa K, Kaneda K (2012) Angew Chem Int Chem 51:136–139
Mitsudome T, Takahashi Y, Ichikawa S, Mizugaki T, Jitsukawa K, Kaneda K (2013) Angew Chem Int Chem 52:1481–1485
Xiao B, Niu Z, Wang YG, Jia W, Shang J, Zhang L, Wang D, Fu Y, Zeng J, He W, Wu K, Li J, Yang J, Liu L, Li Y (2015) J Am Chem Soc 137:3791–3794
Das BC, Thapa P, Karki R, Schinke C, Das S, Kambhampati S, Banerjee SK, Veldhuizen PV, Verma A, Weiss LM, Evans T (2013) Future Med Chem 5(6):653–676
Byun Y, Yan J, Madhoun ASA, Johnsamuel J, Yang W, Barth RF, Eriksson S, Tjarks W (2005) J Med Chem 48:1188–1198
Hall DG (2005) Boronic acids: preparation and applications in organic synthesis and medicine. Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim
Fyfe JWB, Watson AJB (2017) Chem Rev 3(1):31–55
O’Farrell AM, Vliet AV, Farha KA, Cherrington JM, Campbell DA, Li X (2007) Clin Ther 29(8):1692–1705
Paramore A, Frantz S (2003) Nat Rev Drug Discov 2:611–612
Dorsey BD, Iqbal M, Chatterjee S, Menta E, Bernardini R, Bernareggi A, Cassarà PG, D’Arasmo G, Ferretti E, Munari SD, Oliva A, Pezzoni G, Allievi C, Strepponi I, Ruggeri B, Ator MA, Williams M, Mallamo JP (2008) J Med Chem 51(4):1068–1072
Cvek B (2012) Drugs Future 37(8):561–565
Burkhardt ER, Matos K (2006) Chem Rev 106(7):2617–2650
Miyaura N, Suzuki A (1995) Chem Rev 95(5):2457–2483
Lennox AJJ, Lloyd-Jones GC (2014) Chem Soc Rev 43:412–443
Bonet A, Odachowski M, Leonori D, Essafi S, Aggarwal VK (2014) Nat Chem 6:584–589
Muncipinto G, Moquist PN, Schreiber SL, Schaus SE (2011) Angew Chem Int Ed 123(35):8322–8325
Miura T, Nishida Y, Morimoto M, Murakami M (2013) J Am Chem Soc 135(31):11497–11500
Ripin DHB, Cai W, Brenek SJ (2000) Tetrahedron Lett 41(31):5817–5819
Wang H, Jing C, Noble A, Aggarwal VK (2020) Angew Chem Int Ed 59(39):16859–16872
Sandford C, Aggarwal VK (2017) Chem Commun 53(40):5481–5494
Ito H, Yamanaka H, Tateiwa JI, Hosomi A (2000) Tetrahedron Lett 41(35):6821–6825
Kou T, Jun T, Tatsuo I, Norio M (2000) Chem Lett 29(2):126–127
Bonet A, Guláys H, Koshevoy IO, Estevan F, Sanaú M, Úbeda MA, Fernández E (2010) Chem Eur J 16(21):6382–6390
Bell NJ, Cox AJ (2004) Chem Commun (16):1854–1855
Hirano K, Yorimitsu H, Oshima K (2007) Org Lett 9(24):5031–5033
Takushi S, Takahiro A, Kenji T, Li Z, Hisao N (2009) Chem Commun (40):5987–8989
Kajiwara T, Terabayashi T, Yamashita M, Nozaki K (2008) Angew Chem Int Ed 47(35):6606–6610
Parra A, Trulli L, Tortosa M (2020) PATAI’S chemistry of functional groups. Wiley, Hoboken, pp 1–82
Mun S, Lee JE, Yun J (2006) Org Lett 8(21):4887–4889
Thorpe SB, Calderone JA, Santos WL (2012) Org Lett 14(7):1918–1921
Stavber G, Časar Z (2014) ChemCatChem 6(8):2162–2174
Kobayashi S, Xu P, Endo T, Ueno M, Kitanosono T (2012) Angew Chem Int Ed 51(51):12763–12766
Kitanosono T, Xu P, Isshiki S, Zhu L, Kobayashi S (2014) Chem Commun 50(66):9336–9339
Kitanosono T, Xu P, Kobayashi S (2014) Chem Asian J 9(1):179–188
Zhu L, Kitanosono T, Xu P, Kobayashi S (2015) Chem Commun 51(58):11685–11688
Zhu L, Kitanosono T, Xu P, Kobayashi S (2015) Beilstein J Org Chem 11:2007–2011
Niu Z, Chen J, Chen Z, Ma M, Song C, Ma Y (2015) J Org Chem 80(1):602–608
Zhou XF, Sun YY, Wu YD, Dai JJ, Xu J, Huang Y, Xu HJ (2016) Tetrahedron 72(37):5691–5698
Wu W, Han B, Yan F, Ding Y, Li B, Wang L, Zhu L (2018) Nanomaterials 8(5):326–335
Zhu L, Wang LS, Li BJ, Fu BQ, Zhang CP (2016) Chem Commun 52(38):6371–6374
Zhu L, Li B, Wang S, Wang W, Wang L, Ding Y, Qin C (2018) Polymers 10(4):385–394
Xu Y, Zhang L, Cui Y (2008) J Appl Polym Sci 110(5):2996–3000
Reddy KR, Kumar NS (2006) Synlett 14:2246–2250
Chavan PV, Pandit KS, Desai UV, Kulkarni MA, Wadgaonkar PP (2014) RSC Adv 4(79):42137–42146
Bahsis L, Ayouchia HBE, Anane H, Benhamou K, Kaddami H, Julve M, Stiriba S-E (2018) Int J Biol Macromol 119:849–856
Gao S, Li Z, Yang S, Jiang K, Li Y, Zeng H, Li L, Wang H (2009) ACS Appl Mater Interfaces 1(9):2080–2085
Lee JE, Yun J (2007) Angew Chem Int Ed 47(1):145–147
Isegawa M, Sameera WMC, Sharma AK, Kitanosono T, Kato M, Kobayashi S, Morokuma K (2017) ACS Catal 7(8):5370–5380
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 21774029), the Natural Science Foundation of Hubei Province of China (Nos. 2019CFB237, 2019CFB354), Hubei University Excellent Young and Middle-aged Science and Technology Innovation Team Project (No.T201816), the Natural Science Foundation of Xiaogan City (Nos. XGKJ201910047, XGKJ2020010053). Lei Zhu thanks the “Chutian Scholar” Program of Hubei Province. Lijie Zhou and Biao Han thanks the High Level Master Degree Thesis Cultivation Project of Hubei Engineering University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, L., Han, B., Zhang, Y. et al. Cellulosic CuI Nanoparticles as a Heterogeneous, Recyclable Catalyst for the Borylation of α,β-Unsaturated Acceptors in Aqueous Media. Catal Lett 151, 3220–3229 (2021). https://doi.org/10.1007/s10562-021-03571-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03571-2