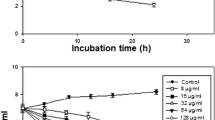
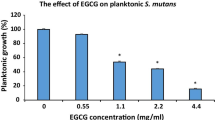
Abstract
The high rate of microbes and their biological activity in the patient’s mouth is a concern in the domains of dental caries and periodontal disease. The study aimed to shed light on the relationship between graphene oxide’s nanoparticles (nGOs) antimicrobial properties and the growth of dental pathogenic bacteria. The forty swab samples were frequently collected from the patient’s cavity mouth between November 2019 and January 2020, from patients who visited dentist clinics in Baghdad by taking swabs from mouth cavities with various dental caries with two age groups (5–17) and (18–60) from male and female to streaking them on Brain–Heart Infusion (BHI) agar, then identified by re-streaking on Mitis Salivarius Bacitracin (MSB) agar. All isolates were confirmed as Streptococcus mutans after API 20 Strep method. As well as the Colony Forming Units (CFU) were then determined after diluting the bacterial cell suspensions to obtain cell samples containing 1.5 × 108 CFU/ ml. The collagen-binding adhesin (cnm) and glucosyltransferases (gtf) of S. mutans genes were identified using polymerase chain reaction (PCR) method before and after exposure to the nGOs, which were prepared in different pulse laser energy (500, 600, and 700 mJ) with presence and absence of the magnetic field, and the data have been analyzing. After counting the CFU, the nGOs shows high effectiveness inhibiting the growth of S. mutans. This research provides definitive answers about the relationship between nGOs, antibacterial caries, and periodontal disease.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within this article collected continuously from dental caries of patients who attended dentist clinics in Baghdad city from November 2019 to January 2020.
References
Ge Z, Yang L, Xiao F, Wu Y, Yu T, Chen J, Zhang Y. Graphene family nanomaterials: properties and potential applications in dentistry. Intern J Biomater. 2018;2018:1539678.
Flaih ON, Najeeb LM, Mohammed RK. Investigating the portability bacteria proteus mirabilis isolated from wounds and burns infections to form swarming and study the virulence factor genetically. J Univ Anbar Pure Sci. 2015;9(3):44–7.
Wu R, Zhao Q, Lu S, Fu Y, Yu D, Zhao W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J Appl Oral Sci. 2018;27:e20180042.
Wade WG. The oral microbiome in health and disease. Pharmacol Res Off J Itali Pharmacol Soc. 2013;69(1):137–43.
He J, Zhu X, Qi Z, Wang C, Mao X, Zhu C, Tang Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl Mater Interf. 2015;7(9):5605–11. https://doi.org/10.1021/acsami.5b01069.
Li Y, Tanner A. Effect of antimicrobial interventions on the oral microbiota associated with early childhood caries. Pediat Dent. 2015;37(3):226–44.
Rago I, Bregnocchi A, Zanni E, D’Aloia AG, De Angelis F, Bossu M, Sarto MS (2015) Antimicrobial activity of graphene nanoplatelets against Streptococcus mutans. 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO. pp 9–12.
Flaih ON, Najeb LM, Mohammad RK. Determine the biofilm formed by using ELISA technology for gram-negative bacteria isolated from wounds and burns infections, and the study of the production of the biofilm molecularly. Ibn AL-Haitham J Pure Appl Sci. 2017;30(1):325–38.
Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Japan Dent Sci Rev. 2018;54(1):22–9.
Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192(12):3024–32. https://doi.org/10.1128/JB.01649-09.
Rodbari RJ, Wendelbo R, Jamshidi LCLA, Nascimento L. Study of physical and chemical characterization of nanocomposite polystyrene/graphene oxide high acidity can be applied in thin films. J Chil Chem Soc. 2016;61(3):3120–4.
Nakano K, Nomura R, Taniguchi N, Lapirattanakul J, Ooshima T. Molecular characterization of Streptococcus mutans strains containing the cnm gene encoding a collagen-binding adhesin. Archiv Oral Biol. 2010;55(1):34–9.
Lapirattanakul J, Nakano K, Nomura Ooshima T. Multilocus sequence typing analysis of Streptococcus mutans strains with the cnm gene encoding collagen-binding adhesin. J Med Microbiol. 2011;60(11):1677–84. https://doi.org/10.1099/jmm.0.033415-0?crawler=true.
Abranches J, Miller JH, Martinez AR, Lemos JA. The collagen-binding protein cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect Immun. 2011;79(6):2277–84. https://doi.org/10.1128/iai.00767-10.
Nakano K, Hokamura K, Taniguchi N, Ooshima T. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun. 2011;2(1):485. https://doi.org/10.1128/IAI.00767-10.
Majeed AA, Al-Aubydi MA. Assessment the Modulation effect of using green synthesis ZnO NPs against multidrug resistant Klebsiella pneumoniae isolated from respiratory tract infection. Iraqi J Sci. 2019. https://doi.org/10.24996/ijs.2019.60.6.5.
Yang Y, Asiri AM, Tang Z, Du D, Lin Y. Graphene based materials for biomedical applications. Mater Today (Kidlington, England). 2013;16(10):365–73.
Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Controll Release Off J Controll Release Soc. 2014;173:75–88.
Gao L, Li Q, Li R, Yan L, Zhou Y, Chen K, Shi H. Highly sensitive detection for proteins using graphene oxide-aptamer based sensors. Nanoscale. 2015;7(25):10903–7.
Jin J, Zhang L, Shi M, Zhang Y, Wang Q. Ti-GO-Ag nanocomposite: the effect of content level on the antimicrobial activity and cytotoxicity. Intern J Nanomed. 2017;12:4209–24.
Yoo SY, Park SJ, Jeong DK, Kook J-K. Isolation and characterization of the mutans streptococci from the dental plaques in Koreans. J Microbiol. 2007;45(3):246–55.
Al-Shamari RK, Al-Khteeb SN. Molecular characterization aminoglycosids resistance Pseudomonas aeruginosa. Iraqi J Sci. 2016;57(2B):1150–7.
Mohammed RK . Molecular Identification of Geobacillus WCH 70 Isolate according to Nitrate reductase gene sequence. Al-Anbar Journal of Veterinary Sciences. 2016;9(2):75–80.
Nomura R, Nakano K, Taniguchi N, Ooshima T. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J Med Microbiol. 2009;58(4):469–75. https://doi.org/10.1099/jmm.0.007559-0?crawler=true.
Oho T, Yamashita Y, Shimazaki Y, Kushiyama M, Koga T. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol Immunol. 2000;15(4):258–62. https://doi.org/10.1034/j.1399-302x.2000.150408.x.
Di Giulio M, Zappacosta R, Di Lodovico S, Cellini L. Antimicrobial and antibiofilm efficacy of graphene oxide against chronic wound microorganisms. Antimicrob Agents Chemother. 2018;62(7):e00547-e618. https://doi.org/10.1128/AAC.00547-18.
Chen T, Yu WH, Izard J, Dewhirst FE. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. J Biolog Databases Curat. 2010;2010:baq013. https://doi.org/10.1093/database/baq013/405450?view=long.
Zarei M, Jamnejad A, Khajehali E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J Microbiol. 2014;7(1):e8720.
Li WR, Xie XB, Shi QS, Chen YB. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115–22. https://doi.org/10.1007/s00253-009-2159-5.
Chatterjee T, Chatterjee BK, Majumdar D, Chakrabarti P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochem Biophys Acta. 2015;1850(2):299–306.
Tu Y, Lv M, Xiu P, Zhou R. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol. 2013;8(8):594–601.
Hu W, Peng C, Luo W, Fan C. Graphene-based antibacterial paper. ACS Nano. 2010;4(7):4317–23. https://doi.org/10.1021/nn101097v.
Flayyih AS, Hassani HH, Wali MH. Identification of Streptococcus mutans from human dental plaque and dental caries using 16srrna gene. Iraqi J Sci. 2016;57(1C):552–7.
Ferraro M, Vieira AR. Explaining gender differences in caries: a multifactorial approach to a multifactorial disease. Intern J Dent. 2010;2010:649643.
Zanni E, De Bellis G, Uccelletti Bracciale D. Graphite nanoplatelets and Caenorhabditis elegans: insights from an in vivo model. Nano Lett. 2012;12(6):2740–4. https://doi.org/10.1021/nl204388p.
Liu S, Zeng TH, Hofmann M, Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5(9):6971–80. https://doi.org/10.1021/nn202451x.
Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim JH. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Intern J Nanomed. 2012;7:5901–14.
Acknowledgements
We would like to thank everyone who helped make this work possible in the Biotechnology Lab at the Department of Biotechnology, College of Science, and University of Baghdad by providing a location, materials, and instruments
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study concerning bacterial samples. The University of Baghdad Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm there are no research participants need to provided informed consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammed, R.K., Ibrahim, A.A. The anti-adherence activity and bactericidal effect of GO against Streptococcus mutans from Iraqi dental patients. Odontology 111, 863–869 (2023). https://doi.org/10.1007/s10266-023-00791-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-023-00791-3