Abstract
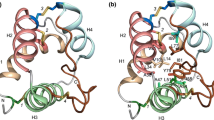
In Lotus japonicus, as in most plants, long-chain fatty acids are important components of cuticular wax, one of the principal functions of which is to act as a barrier to water loss in response to drought stress. It is thought that lipid transfer proteins (LTPs) are involved in the process of cuticle formation. We previously described LjLTP10 as an LTP involved in cuticle formation during acclimation response to drought stress in L. japonicus. The structural model of LjLTP10 had two residues (K33 and R45) in the hydrophobic cavity, although the role of these residues was unclear. In the present work, we investigated the molecular mechanism involved in the transport of lipid precursors in L. japonicus and clarified the importance of the residues K33 and R45. First, in silico site-directed mutagenesis studies were carried out on the LjLTP10 structure. Structural analysis showed that LjLTP10 mutants possess similar structures but their hydrophobic cavities are somewhat different. Unfavorable energies for the interactions of the mutant proteins with different ligands were found by molecular docking and molecular dynamics simulations. We also examined the contributions of energetic parameters to the free energy of the protein–ligand complex using the MM-GBSA method. Results showed that the different complexes present similar, favorable van der Waals interactions, whereas electrostatic interactions were not favored in the mutant structures. Our study indicates that the residues K33 and R45 play a crucial role in maintaining the binding pocket structure required for lipid transport.






Similar content being viewed by others
References
Kader J-C (1996) Lipid-transfer proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 47:627–654
Jenks MA, Rashotte AM, Tuttle HA, Feldman KA (1996) Mutants in Arabidopsis thaliana altered in epicuticular wax and leaf morphology. Plant Physiol 110:377–385
Bringe K, Schumacher CFA, Schmitz-Eiberger MA, Steiner U, Oerke EC (2006) Ontogenetic variation in chemical and physical characteristics of adaxial apple leaf surfaces. Phytochemistry 67:161–170
Cameron KD, Teece MA, Smart LB (2006) Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol 140:176–183
Tapia G, Acuña H, Inostroza L (2012) Plant drought tolerance: some genetics and agronomics relevant aspects for breeding in forage species. In: Neves DF, Sanz JD (eds) Droughts: new research. Nova, Hauppauge, pp 157–188
Diaz P, Borsani O, Márquez AJ, Monza J (2005) Osmotically induced proline accumulation in Lotus corniculatus leaves is affected by light and nitrogen source. Plant Growth Regul 46:223–232
Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004) Plant cuticular lipid export requires an ABC transporter. Science 306:702–704
Luo B, Xue XY, Hu WL, Wang LJ, Chen XY (2007) An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol 48:1790–1802
Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Hofer R, Schreiber L, Chory J, Aharoni A (2007) The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 145:1345–1360
Tapia G, Morales-Quintana L, Parra C, Berbel A, Alcorta MA (2013) Study of nsLTPs in Lotus japonicus genome reveal a specific epidermal cell member (LjLTP10) regulated by drought stress in aerial organs with a putative role in cutin formation. Plant Mol Biol 82:485–501
Boutrot F, Chantret N, Gautier MF (2008) Genome-wide analysis of the rice and Arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genomics 9:86
Shin DH, Lee JY, Hwang KY, Kim KK, Su SW (1995) High-resolution crystal structure of the non-specific lipid-transfer protein from maize seedlings. Structure 3:189–199
Desormeaux A, Blochet J, Pezolet M, Marion D (1992) Amino acid sequence of a non-specific wheat phospholipid transfer protein and its conformation as revealed by infrared and Raman spectroscopy. Role of disulfide bridges and phospholipids in the stabilization of a helix structure. Biochim Biophys Acta 1121:137–152
Marion D, Bakan B, Elmorjani K (2007) Plant lipid binding proteins: properties and applications. Biotechnol Adv 25:195–197
Allison JR, Moll G-P, van Gunsteren WF (2010) Investigation of stability and disulfide bond shuffling of lipid transfer proteins by molecular dynamics simulation. Biochemistry 49:6916–6927
Charvolin D, Douliez J-P, Marion D, Cohen-Addad C, Pebay-Peyroula E (1999) The crystal structure of a wheat nonspecific lipid transfer protein (ns-LTP1) complexed with two molecules of phospholipid at 2.1 Å resolution. Eur J Biochem 264:562–568
Hoh F, Pons JL, Gautier MF, de Lamotte F, Dumas C (2005) Structure of a liganded type 2 non specific lipid-transfer protein from wheat and the molecular basis of lipid binding. Acta Crystallogr D 61:397–406
Salcedo G, Sánchez-Monge R, Barber D, Díaz-Perales A (2007) Plant non-specific lipid transfer proteins: an interface between plant defence and human allergy. Biochim Biophys Acta 1771:781–791
Rickers J, Tober I, Spener F (1984) Purification and binding characteristics of a basic fatty acid binding protein from Avena sativa seedlings. Biochim Biophys Acta 794:313–319
Rickers J, Spener F, Kader LC (1985) A phospholipid transfer protein that binds long-chain fatty acid. FEBS Lett 180:29–32
Arondel V, Vergnolle C, Tchang F, Kader JC (1990) Bifunctional lipid-transfer: fatty acid-binding proteins in plants. Mol Cell Biochem 98:49–56
Tsuboi S, Osafune T, Tsugeki R, Nishimura M, Yamada M (1992) Nonspecific lipid transfer protein in castor bean cotyledon cells: subcellular localization and a possible role in lipid metabolism. Biogeosciences 111:500–508
Van Ree R (2002) Clinical importance of non-specific lipid transfer proteins as food allergens. Biochem Soc Trans 30:910–913
Maldonado AM, Doerner P, Dixonk RA, Lamb CJ, Cameron RK (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419:399–403
Molina A, Segura A, Garcia-Olmedo F (1993) Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett 316:119–122
de Oliveira A, Moreira V (2007) Role of plant lipid transfer proteins in plant cell physiology—a concise review. Peptides 28:1144–1153
Lee JY, Min K, Cha H, Hwang DHSKY, Suh SW (1998) Rice nonspecific lipid transfer protein: the 1.6 Å crystal structure in the unliganded state reveals a small hydrophobic cavity. J Mol Biol 276:437–448
Morales-Quintana L, Fuentes L, Gaete-Eastman C, Herrera R, Moya-León MA (2011) Structural characterization and substrate specificity of VpAAT1 protein related to ester biosynthesis in mountain papaya fruit. J Mol Graph Model 29:635–642
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
MacKerell AD Jr, Bashford D, Bellott M, Dunbrack RL Jr, Evanseck J, Field M, Fischer JS, Gao J, Guo H, Ha S, Joseph D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Roux B, SchlenkrichM SJ, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M (1992) Self-consistent parameterization of biomolecules for molecular modeling and condensed phase simulations. FASEB J 6:A143
Schlenkrich M, Brickmann J, MacKerell AD Jr, Karplus M (1996) In: Roux B, Merz KM (eds) A molecular perspective from computation and experiment. Birkhauser, Boston, pp 31–81
Jorgensen WL, Chandresekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Abagyan R, Totrov M, Kuznetsov D (1994) ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15:488–506
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, MacKerell AD Jr (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force field. J Comput Chem 31:671–690
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Homeyer N, Gohlke H (2012) Free energy calculations by the molecular mechanics Poisson−Boltzmann surface area method. Mol Informatics 31:114–122
Vergara-Jaque A, Comer J, Monsalve L, González-Nilo FD, Sandoval C (2013) Computationally efficient methodology for atomic-level characterization of dendrimer−drug complexes: a comparison of amine- and acetyl-terminated PAMAM. J Phys Chem B 117:6801–6813
Samuel D, Liu Y-J, Cheng C-S, Lyu P-C (2002) Solution structure of plant nonspecific lipid transfer protein-2 from rice (Oryza sativa). J Biol Chem 277:35267–35273
Han GW, Lee JY, Song HK, Chang C, Min K, Moon J, Shin DH, Kopka ML, Sawaya MR, Yuan HS, Kim TD, Choe J, Lim D, Moon HJ, Suh SW (2001) Structural basis of nonspecific lipid binding in maize lipid-transfer protein complexes revealed by high-resolution X-ray crystallography. J Mol Biol 308:263–278
Lerche MH, Kragelund BB, Bech LM, Poulsen FM (1997) Barley lipid-transfer protein complexed with palmitoyl CoA: the structure reveals a hydrophobic binding site that can expand to fit both large and small lipidlike ligands. Structure 5:291–306
Lerche MH, Poulsen FM (1998) Solution structure of barley lipid transfer protein complexed with palmitate. Two different binding modes of palmitate in the homologous maize and barley nonspecific lipid transfer proteins. Protein Sci 7:2490–2498
Cheng CS, Samuel D, Liu YJ, Shyu JC, Lai SM, Lin KF, Lyu PC (2004) Binding mechanism of nonspecific lipid transfer proteins and their role in plant defense. Biochemistry 43:13628–13636
Pasquato N, Berni R, Folli C, Folloni S, Cianci M, Pantano S, Helliwell JR, Zanotti G (2006) Crystal structure of peach Pru p 3, the prototypic member of the family of plant non-specific lipid transfer protein pan-allergens. J Mol Biol 356:684–694
Yeats TH, Rose JKC (2008) The biochemistry and biology of extracellular plant lipid transfer proteins (LTPs). Protein Sci 17:191–198
Tassin S, Broekaert WF, Marion D, Acland DP, Ptak M, Vovelle F, Sodano P (1998) Solution structure of Ace-AMP1, a potent antimicrobial protein extracted from onion seeds. Structural analogies with plant nonspecific lipid transfer proteins. Biochemistry 37:3623–3637
Acknowledgments
C. Parra-Palma acknowledges CONICYT for providing a doctoral scholarship. The Center of Bioinformatics and Molecular Simulations (CBSM) of the University of Talca provided the ICM program. We acknowledge the helpful comments and suggestions made by the two anonymous reviewers of this manuscript. FONDECYT initiation project #11090243 and CONICYT PAI/ACADEMIA #79140027 supported this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Valenzuela-Riffo, F., Tapia, G., Parra-Palma, C. et al. Understanding the roles of Lys33 and Arg45 in the binding-site stability of LjLTP10, an LTP related to drought stress in Lotus japonicus . J Mol Model 21, 270 (2015). https://doi.org/10.1007/s00894-015-2807-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2807-x