Abstract
Objectives
To compare neoadjuvant chemotherapy (NAC) plus concurrent chemoradiotherapy (CCRT) to CCRT alone in children and adolescents (age ≤ 18 years) with locoregionally advanced nasopharyngeal carcinoma (CA-LANPC, stage III-IVA).
Materials and methods
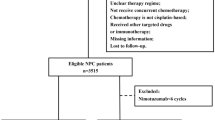
195 CA-LANPC patients who were treated through CCRT with or without NAC between 2008 and 2018 were enrolled in this study. A matched cohort composed of CCRT patients and NAC-CCRT patients was generated by propensity score matching (PSM) at a 1:2 ratio. Survival outcomes and toxicities were compared between the CCRT group and NAC-CCRT group.
Results
Of the 195 patients, 158 (81%) received NAC plus CCRT, and 37 (19%) received CCRT alone. The NAC-CCRT group had higher EBV DNA levels (≥ 4000 copy/mL), more advanced TNM stage (stage IV disease), and lower incidence of a high radiation dose (> 6600 cGy) than the CCRT group. To avoid bias in treatment selection within retrospectively analysis, 34 patients from the CCRT group were matched with 68 patients from the NAC-CCRT group. In the matched cohort, the 5-year DMFS rate was 94.0% in the NAC-CCRT group versus 82.4% in the CCRT group, with marginal statistical significance (HR = 0.31; 95%CI 0.09–1.10; P = 0.055). During treatment, the accumulate incidence of severe acute toxicities (65.8% vs 45.9%; P = 0.037) in the NAC-CCRT group was higher than the CCRT group. However, the CCRT group had significantly higher accumulate incidence of severe late toxicities (30.3% vs 16.8%; P = 0.041) than the NAC-CCRT group.
Conclusions
Addition of NAC to CCRT tended to improve long-term DMFS in CA-LANPC patients with acceptable toxicity. However, relative randomized clinical trial is still needed in the future.




Similar content being viewed by others
Availability of data and materials
Data statement: Key raw data were uploaded onto the Research Data Deposit public platform (RDD), with the approval RDD number of RDDA2022396736.
References
Amin MB, Greene FL, Edge SB et al (2017) The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99
Austin PC (2009) The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making 29(6):661–677
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Buehrlen M, Zwaan CM, Granzen B et al (2012) Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults: preliminary results from the prospective, multicenter study NPC-2003-GPOH/DCOG. Cancer 118(19):4892–4900
Casanova M, Bisogno G, Gandola L et al (2012) A prospective protocol for nasopharyngeal carcinoma in children and adolescents: the Italian Rare Tumors in Pediatric Age (TREP) project. Cancer 118(10):2718–2725
Chen BB, Lu SY, Peng H et al (2020) comparison of long-term outcomes and sequelae between children and adult nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 106(4):848–856
Chen YP, Ismaila N, Chua MLK et al (2021) Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol 39(7):840–859
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
ICRU Report (1999) Prescribing, recording, and reporting photon beam therapy, vol 62. International Commission on Radiation Units and Measurements, Maryland
ICRU Report (2010) Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT), vol 83. International Commission on Radiation Units and Measurements, Maryland
Jin YN, Yao JJ, Zhang F et al (2017) Is pretreatment Epstein-Barr virus DNA still associated with 6-year survival outcomes in locoregionally advanced nasopharyngeal carcinoma? J Cancer 8(6):976–982
Jin YN, Yao JJ, You YF et al (2021) Optimal cumulative cisplatin dose during concurrent chemoradiotherapy among children and adolescents with locoregionally advanced nasopharyngeal carcinoma: a real-world data study. Radiother Oncol 161:83–91
Jin YN, Cao HJ, Gong XH et al (2022) Does three cycles of neoadjuvant chemotherapy prior to concurrent chemoradiotherapy provide benefits for all childhood patients with locoregionally advanced nasopharyngeal carcinoma? J Cancer Res Clin Oncol 148(10):2569–2579
Li Y, Tang LQ, Liu LT et al (2018) induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma in children and adolescents: a matched cohort analysis. Cancer Res Treat 50(4):1304–1315
Li WF, Chen NY, Zhang N et al (2019) Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer 145(1):295–305
Liang YJ, Wen DX, Luo MJ et al (2021) Induction or adjuvant chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in paediatric nasopharyngeal carcinoma in the IMRT era: a recursive partitioning risk stratification analysis based on EBV DNA. Eur J Cancer 159:133–143
Lu S, Wei J, Sun F et al (2019) Late sequelae of childhood and adolescent nasopharyngeal carcinoma survivors after radiation therapy. Int J Radiat Oncol Biol Phys 103(1):45–51
Pfister DG, Spencer S, Adelstein D et al (2020) Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18:873–898
Qiu WZ, Peng XS, Xia HQ, Huang PY, Guo X, Cao KJ (2017) A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol 143(8):1563–1572
Qiu W, Lv X, Guo X, Yuan Y (2020) Clinical implications of plasma Epstein-Barr Virus DNA in children and adolescent nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Front Oncol 10:356
Rodriguez-Galindo C, Krailo MD et al (2019) Treatment of childhood nasopharyngeal carcinoma with induction chemotherapy and concurrent chemoradiotherapy: results of the children’s oncology group ARAR0331 study. J Clin Oncol 37(35):3369–3376
Sultan I, Casanova M, Ferrari A, Rihani R, Rodriguez-Galindo C (2010) Differential features of nasopharyngeal carcinoma in children and adults: a SEER study. Pediatr Blood Cancer 55(2):279–284
Tang LL, Chen YP, Chen CB et al (2021) The Chinese society of clinical oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond) 41(11):1195–1227
Wang L, Wu Z, Xie D et al (2019) Reduction of target volume and the corresponding dose for the tumor regression field after induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer Res Treat 51(2):685–695
Yang H, Chen X, Lin S et al (2018) Treatment outcomes after reduction of the target volume of intensity-modulated radiotherapy following induction chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a prospective, multi-center, randomized clinical trial. Radiother Oncol 126(1):37–42
Yang Q, Cao SM, Guo L et al (2019) Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer 119:87–96
Zhang MX, Li J, Shen GP et al (2015) Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 51(17):2587–2595
Zhang Y, Chen L, Hu GQ et al (2019) Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 381(12):1124–1135
Funding
This work was supported by grants from the Natural Science Foundation of Guangdong, China (2021A1515220128; 2020A1515111075), Excellent Young Researchers Program of the 5th Affiliated Hospital of SYSU (WYYXQN-2021015), National Natural Science Foundation of China (82002557), Guangdong Medical Science and Technology Research Fund (C2018063), Science and Technology Program of Zhuhai, China (2220004000192, 202002030070), and Investigator-Initiated Clinical Trial foundation of the 5th Affiliated Hospital of SYSU (YNZZ2021-04).
Author information
Authors and Affiliations
Contributions
Study concepts: LPX and JJY. Study design: YNJ, ZHR, WWC. Data acquisition: YNJ and WWC. Quality control of data and algorithms: YNJ, LY, WY and XFP. Data analysis and interpretation: WJZ and TM. Statistical analysis: WJZ. Manuscript preparation: YNJ, ZHR, WWC. Manuscript editing: TM. Manuscript review: All authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, YN., Ruan, ZH., Cao, WW. et al. Concurrent chemoradiotherapy with or without neoadjuvant chemotherapy in pediatric patients with stage III-IVa nasopharyngeal carcinoma: a real-world propensity score-matched cohort study. J Cancer Res Clin Oncol 149, 11929–11940 (2023). https://doi.org/10.1007/s00432-023-05041-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05041-1