Abstract
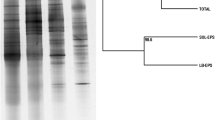
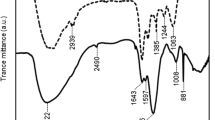
Extracellular polymeric substances (EPS) play an important role in the formation and activity of biofilms in wastewater treatment (WWT). The EPS of the denitrifying biomarker Comamonas denitrificans strain 110, produced in different culture media and growth modes, were characterized. The EPS mainly contained protein (3–37%), nucleic acids (9–50%), and carbohydrates (3–21%). The extracellular DNA was found to be important for initial biofilm formation since biofilm, but not planktonic growth, was inhibited in the presence of DNase. The polysaccharide fraction appeared to consist of at least two distinct polymers, one branched fraction (A) made up of glucose and mannose with a molecular weight around 100 kDa. The other fraction (B) was larger and consisted of ribose, mannose, glucose, rhamnose, arabinose, galactose, and N-acetylglucosamine. Fraction B polysaccharides were mainly found in capsular EPS which was the dominant type in biofilms and agar-grown colonies. Fraction A was abundant in the released EPS, the dominant type in planktonic cultures. Biofilm and agar-grown EPS displayed similar overall properties while planktonic EPS showed clear compositional disparity. This study presents results on the physiology of a key WWT organism, which may be useful in the future development of improved biofilm techniques for WWT purposes.




Similar content being viewed by others
References
Andersson S, Kuttuva Rajarao G, Land CJ, Dalhammar G (2008) Biofilm formation and interactions of bacterial strains found in wastewater treatment systems. FEMS Microbiol Lett 283:83–90
Beatson SA, Minamino T, Pallen MJ (2006) Variation in bacterial flagellins: from sequence to structure. Trends Microbiol 14:151–155
Beech I, Hanjagsit L, Kalaji M, Neal AL, Zinkevich V (1999) Chemical and structural characterization of exopolymers produced by Pseudomonas sp. NCIMB 2021 in continuous culture. Microbiology 145:1491–1497
Biermann CJ (1988) Analysis of carbohydrates by GLC and MS. CRC-Taylor & Francis, Florida
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Böckelmann U, Janke A, Kuhn R, Neu TR, Wecke J, Lawrence JR, Szewzyk U (2006) Bacterial extracellular DNA forming a defined network-like structure. FEMS Microbiol Lett 262:31–38
Böckelmann U, Lünsdorf H, Szewzyk U (2007) Ultrastructural and electron energy-loss spectroscopic analysis of an extracellular filamentous matrix of an environmental bacterial isolate. Environ Microbiol 9:2137–2144
Comte S, Guibaud G, Baudu M (2007) Effect of extraction method on EPS from activated sludge: An HPSEC investigation. J Hazard Mater 140:129–137
Dove A (2006) News feature: drugs down the drain. Nat Med 12:376–377
Flemming H-C, Wingender J (2002) Extracellular polymeric substances (EPS): structural, ecological and technical aspects. In: Bitton G (ed) Encyclopedia of environmental microbiology. Wiley, New York, pp 1223–1231
Flemming H-C, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells”. J Bacteriol 189:7945–7947
Frings CS, Fendley TW, Dunn RT, Queen CA (1972) Improved determination of total serum lipids by the sulfo-phospho-vanillin reaction. Clin Chem 18:673–674
Gao B, Zhu X, Xu C, Yue Q, Li W, Wei J (2008) Influence of extracellular polymeric substances on microbial activity and cell hydrophobicity in biofilms. J Chem Technol Biotechnol 83:227–232
Guerry P (2007) Campylobacter flagella: not just for motility. Trends Microbiol 15:456–461
Gumaelius L, Magnusson G, Pettersson B, Dalhammar G (2001) Comamonas denitrificans sp. nov., an efficient denitrifying bacterium isolated from activated sludge. Int J Syst Evol Microbiol 51:999–1006
Gumaelius L, Smith EH, Dalhammar G (1996) Potential biomarker for denitrification of wastewaters: effects of process variables and cadmium toxicity. Wat Res 30:3025–3031
Kachlany SC, Levery SB, Kim JS, Reuhs BL, Lion LW, Ghiorse WC (2001) Structure and carbohydrate analysis of the exopolysaccharide capsule of Pseudomonas putida G7. Environ Microbiol 3:774–784
Kives J, Orgaz B, Sanjose C (2006) Polysaccharide differences between planktonic and biofilm-associated EPS from Pseudomonas fluorescens B52. Colloids Surf B Biointerfaces 52:123–127
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu H, Fang HHP (2002) Extraction of extracellular polymeric substances (EPS) of sludges. J Biotechnol 95:249–256
Liu H-H, Yang Y-R, Shen X-C, Zhang Z-L, Shen P, Xie Z-X (2008) Role of DNA in bacterial aggregation. Curr Microbiol 57:139–144
Mandal SM, Ray B, Dey S, Pati BR (2007) Production and composition of extracellular polysaccharide synthesized by a Rhizobium isolate of Vigna mungo (L.) Hepper. Biotechnol Lett 29:1271–1275
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S.-I, Lee YC (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339:69–72
McConville MJ, Homans SW, Thomas-Oates JE, Dell A, Bacic A (1990) Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem 265:7385–7394
Qureshi N, Annous B, Ezeji T, Karcher P, Maddox I (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microbial Cell Factories 4:24
Rodgers M, Zhan XM (2003) Moving-medium biofilm reactors. Rev Environ Sci Biotechnol 2:213–224
Romaní A, Fund K, Artigas J, Schwartz T, Sabater S, Obst U (2008) Relevance of polymeric matrix enzymes during biofilm formation. Microb Ecol 56(3):427–436. doi:https://doi.org/10.1007/s00248-00007-09361-00248
Sawardeker JS, Sloneker JH, Jeanes A (1965) Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal Chem 37:1602–1604
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077
Sheng G-P, Zhang M-L, Yu H-Q (2008) Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Colloids Surf B 62:83–90
Späth R, Flemming HC, Wuertz S (1998) Sorption properties of biofilms. Water Sci Technol 37:207–210
Stewart PS (2006) Matrix mysteries hold keys to controlling biofilms. In: Biofilms Perspectives, February. Available at http://www.biofilmsonline.com/biofilmsonline/pdfs/biofilm_perspectives/Perspectives_Feb2006.pdf Accessed 16 Dec 2008
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210
Sutherland IW (1990) Biotechnology of microbial exopolysaccharides. Cambridge University Press, New York
Talaga P, Vialle S, Moreau M (2002) Development of a high-performance anion-exchange chromatography with pulsed-amperometric detection based quantification assay for pneumococcal polysaccharides and conjugates. Vaccine 20:2474–2484
Ternes T (2007) The occurrence of micopollutants in the aquatic environment: a new challenge for water management. Water Sci Technol 55:327–332
Toutain CM, Caizza NC, Zegans ME, O’Toole GA (2007) Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Res Microbiol 158:471–477
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295:1487
Wilderer PA, McSwain BS (2004) The SBR and its biofilm application potentials. Water Sci Technol 50:1–10
Wuertz S, Spaeth R, Hinderberger A, Griebe T, Flemming HC, Wilderer PA (2001) A new method for extraction of extracellular polymeric substances from biofilms and activated sludge suitable for direct quantification of sorbed metals. Water Sci Technol 43:25–31
Acknowledgments
The authors are grateful to Gustav Sundqvist, Jens Eklöf, and Associate Professor Qi Zhou at the Department of Wood Biotechnology, School of Biotechnology, KTH, for their support with chromatographic instruments and Kaj Kauko for assistance with SEM. Special thanks to Associate Professor Harry Brumer and Professor Vincent Bulone for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andersson, S., Dalhammar, G., Land, C.J. et al. Characterization of extracellular polymeric substances from denitrifying organism Comamonas denitrificans . Appl Microbiol Biotechnol 82, 535–543 (2009). https://doi.org/10.1007/s00253-008-1817-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1817-3