Abstract
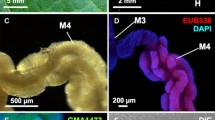
Many insects possess symbiotic bacteria in their bodies, and microbial symbionts play pivotal metabolic roles for their hosts. Members of the heteropteran superfamilies Coreoidea and Lygaeoidea stinkbugs harbor symbionts of the genus Caballeronia in their intestinal tracts. Compared with symbiotic associations in Coreoidea, those in Lygaeoidea insects are still less understood. Here, we investigated a symbiotic relationship involving the mulberry seed bug Paradieuches dissimilis (Lygaeoidea: Rhyparochromidae) using histological observations, cultivation of the symbiont, 16S rRNA gene amplicon sequencing, and infection testing of cultured symbionts. Histological observations and cultivation revealed that P. dissimilis harbors Caballeronia symbionts in the crypts of its posterior midgut. 16S rRNA gene amplicon sequencing of field-collected P. dissimilis confirmed that the genus Caballeronia is dominant in the midgut of natural populations of P. dissimilis. In addition, PCR diagnostics showed that the eggs were free of symbiotic bacteria, and hatchlings horizontally acquired the symbionts from ambient soil. Infection and rearing experiments revealed that symbiont-free aposymbiotic individuals had abnormal body color, small body size, and, strikingly, a low survival rate, wherein no individuals reached adulthood, indicating an obligate cooperative mutualism between the mulberry seed bug and Caballeronia symbionts.






Similar content being viewed by others
References
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. Interscience, New York
Kikuchi Y (2009) Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ 24:195–204. https://doi.org/10.1264/jsme2.ME09140S
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 7:17–34. https://doi.org/10.1146/annurev-ento-010814-020822
Brune A (2014) Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol 12:168–180. https://doi.org/10.1038/nrmicro3182
Sabree ZL, Kambhampati S, Moran NA (2009) Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc Natl Acad Sci U S A 106:19521–19526. https://doi.org/10.1073/pnas.0907504106
Potrikus CJ, Breznak JA (1981) Gut bacteria recycle uric acid nitrogen in termites: a strategy for nutrient conservation. Proc Natl Acad Sci 78:4601–4605. https://doi.org/10.1073/pnas.78.7.460
Itoh H, Tago K, Hayatsu M, Kikuchi Y (2018) Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat Prod Rep 35:434–454. https://doi.org/10.1039/C7NP00051K
Ishigami K, Jang S, Itoh H, Kikuchi Y (2021) Insecticide resistance governed by gut symbiosis in a rice pest, Cletus punctiger, under laboratory conditions. Biol Lett 17:1–6. https://doi.org/10.1098/rsbl.2020.0780
Sato Y, Jang S, Takeshita K, Itoh H, Koike H, Tago K, Hayatsu M, Hori T, Kikuchi Y (2021) Insecticide resistance by a host-symbiont reciprocal detoxification. Nat Commun 12:1–8. https://doi.org/10.1038/s41467-021-26649-2
Oliver KM, Russell JA, Morant NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci U S A 100:1803–1807. https://doi.org/10.1073/pnas.0335320100
Anbutsu H, Moriyama M, Nikoh N, Hosokawa T, Futahashi R, Tanahashi M, Meng XY, Kuriwada T, Mori N, Oshima K, Hattori M, Fujie M, Satoh N, Maeda T, Shigenobu S, Koga R, Fukatsu T (2017) Small genome symbiont underlies cuticle hardness in beetles. Proc Natl Acad Sci U S A 114:E8382–E8391. https://doi.org/10.1073/pnas.1712857114
Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon J-C, Fukatsu T (2010) Symbiotic bacterium modifies aphid body color. Science 330:1102–1104. https://doi.org/10.1126/science.1195463
Glasgow H (1914) The gastric caeca and the caecal bacteria of the Heteroptera. Biol Bull 26:101–170. https://doi.org/10.2307/1536004
Kikuchi Y, Hosokawa T, Fukatsu T (2011) An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J 5:446–460. https://doi.org/10.1038/ismej.2010.150
Kikuchi Y, Prado SS, Jenkins TM (2019) Symbiotic microorganisms associated with Pentatomoidea. McPherson J. E. Invasive stink bugs and related species (Pentatomoidea). Boca Raton. CRC Press. https://doi.org/10.1201/9781315371221
Hosokawa T, Imanishi M, Koga R, Fukatsu T (2019) Diversity and evolution of bacterial symbionts in the gut symbiotic organ of jewel stinkbugs (Hemiptera: Scutelleridae). Appl Entomol Zool 54:359–367. https://doi.org/10.1007/s13355-019-00630-4
Itoh H, Matsuura Y, Hosokawa T, Fukatsu T, Kikuchi Y (2017) Obligate gut symbiotic association in the sloe bug Dolycoris baccarum (Hemiptera: Pentatomidae). Appl Entomol Zool 52:51–59. https://doi.org/10.1007/s13355-016-0453-0
Hosokawa T, Kikuchi Y, Nikon N, Meng XY, Hironaka M, Fukatsu T (2010) Phylogenetic position and peculiar genetic traits of a midgut bacterial symbiont of the stinkbug parastrachia japonensis. Appl Environ Microbiol 76:4130–4135. https://doi.org/10.1128/AEM.00616-10
Kaiwa N, Hosokawa T, Kikuchi Y, Nikoh N, Meng XY, Kimura N, Ito M, Fukatsu T (2010) Primary gut symbiont and secondary, sodalis-allied symbiont of the scutellerid stinkbug cantao ocellatus. Appl Environ Microbiol 76:3486–3494. https://doi.org/10.1128/AEM.00421-10
Kikuchi Y, Hosokawa T, Nikoh N, Fukatsu T (2012) Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae). Appl Entomol Zool 47:1–8. https://doi.org/10.1007/s13355-011-0081-7
Matsuura Y, Kikuchi Y, Meng XY, Koga R, Fukatsu T (2012) Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl Environ Microbiol 78:4149–4156. https://doi.org/10.1128/AEM.00673-12
Karamipour N, Mehrabadi M, Fathipour Y (2016) Gammaproteobacteria as essential primary symbionts in the striped shield bug, Graphosoma Lineatum (Hemiptera: Pentatomidae). Sci Rep 6:21–25. https://doi.org/10.1038/srep33168
Tada A, Kikuchi Y, Hosokawa T, Musolin DL, Fujisaki K, Fukatsu T (2011) Obligate association with gut bacterial symbiont in Japanese populations of the southern green stinkbug Nezara viridula (Heteroptera: Pentatomidae). Appl Entomol Zool 46:483–488. https://doi.org/10.1007/s13355-011-0066-6
Bistolas KSI, Sakamoto RI, Fernandes JAM, Goffredi SK (2014) Symbiont polyphyly, co-evolution, and necessity in pentatomid stinkbugs from Costa Rica. Front Microbiol 5:1–15. https://doi.org/10.3389/fmicb.2014.00349
Küchler SM, Dettner K, Kehl S (2010) Molecular characterization and localization of the obligate endosymbiotic bacterium in the birch catkin bug Kleidocerys resedae (Heteroptera: Lygaeidae, Ischnorhynchinae). FEMS Microbiol Ecol 73:408–418. https://doi.org/10.1111/j.1574-6941.2010.00890.x
Matsuura Y, Kikuchi Y, Hosokawa T, Koga R, Meng XY, Kamagata Y, Nikoh N, Fukatsu T (2012) Evolution of symbiotic organs and endosymbionts in lygaeid stinkbugs. ISME J 6:397–409. https://doi.org/10.1038/ismej.2011.103
Hosokawa T, Fukatsu T (2020) Relevance of microbial symbiosis to insect behavior. Curr Opin Insect Sci 39:91–100. https://doi.org/10.1016/j.cois.2020.03.004
Kaiwa N, Hosokawa T, Nikoh N, Tanahashi M, Moriyama M, Meng XY, Maeda T, Yamaguchi K, Shigenobu S, Ito M, Fukatsu T (2014) Symbiont-supplemented maternal investment underpinning host’s ecological adaptation. Curr Biol 24:2465–2470. https://doi.org/10.1016/j.cub.2014.08.065
Hosokawa T, Ishii Y, Nikoh N, Fujie M, Satoh N, Fukatsu T (2016) Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat Microbiol 1:1–7. https://doi.org/10.1038/nmicrobiol.2015.11
Ohbayashi T, Itoh H, Lachat J, Kikuchi Y, Mergaert P (2019) Burkholderia gut symbionts associated with European and Japanese populations of the dock bug Coreus marginatus (Coreoidea: Coreidae). Microbes Environ 34:219–222. https://doi.org/10.1264/jsme2.ME19011
Takeshita K, Matsuura Y, Itoh H, Navarro R, Hori T, Sone T, Kamagata Y, Mergaert P, Kikuchi Y (2015) Burkholderia of plant-beneficial group are symbiotically associated with bordered plant bugs (Heteroptera: Pyrrhocoroidea: Largidae). Microbes Environ 30:321–329. https://doi.org/10.1264/jsme2.ME15153
Itoh H, Aita M, Nagayama A, Meng X, Kamagata Y, Navarro R, Hori T, Ohgiya S, Kikuchi Y (2014) Evidence of environmental and vertical transmission of Burkholderia symbionts in the oriental chinch bug, Cavelerius saccharivorus (Heteroptera: Blissidae). Appl Environ Microbiol 80:5974–5983. https://doi.org/10.1128/AEM.01087-14
Kikuchi Y, Meng XY, Fukatsu T (2005) Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl Environ Microbiol 71:4035–4043. https://doi.org/10.1128/AEM.71.7.4035-4043.2005
Takeshita K, Kikuchi Y (2017) Riptortus pedestris and Burkholderia symbiont: an ideal model system for insect–microbe symbiotic associations. Res Microbiol 168:175–187. https://doi.org/10.1016/j.resmic.2016.11.005
Kuechler SM, Matsyuura Y, Dettner K, Kikuchi Y (2016) Phylogenetically diverse Burkholderia associated with midgut crypts of spurge bugs, Dicranocephalus spp. (Heteroptera: Stenocephalidae). Microbes Environ 31:145–153. https://doi.org/10.1264/jsme2.ME16042
Xu Y, Buss EA, Boucias DG (2016) Culturing and characterization of gut symbiont Burkholderia spp. from the Southern chinch bug, Blissus insularis (Hemiptera: Blissidae). Appl Environ Microbiol 82:3319–3330. https://doi.org/10.1128/AEM.00367-16
Dobritsa AP, Samadpour M (2016) Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int J Syst Evol Microbiol 66:2836–2846. https://doi.org/10.1099/ijsem.0.001065
Sawana A, Adeolu M, Gupta RS (2014) Molecular signatures and phylogenomic analysis of the genus burkholderia: proposal for division of this genus into the emended genus burkholderia containing pathogenic organisms and a new genus paraburkholderia gen. nov. harboring environmental species. Front Genet 5. https://doi.org/10.3389/fgene.2014.00429
Beukes CW, Palmer M, Manyaka P, Chan WY, Avontuur JR, van Zyl E, Huntemann M, Clum A, Pillay M, Palaniappan K, Varghese N, Mikhailova N, Stamatis D, Reddy TBK, Daum C, Shapiro N, Markowitz V, Ivanova N, Kyrpides N, Woyke T, Blom J, Whitman WB, Venter SN, Steenkamp ET (2017) Genome data provides high support for generic boundaries in Burkholderia sensu lato. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.01154
Lopes-Santos L, Castro DBA, Ferreira-Tonin M, Corrêa DBA, Weir BS, Park D, Ottoboni LMM, Neto JR, Destéfano SAL (2017) Reassessment of the taxonomic position of Burkholderia andropogonis and description of Robbsia andropogonis gen. nov., comb. nov. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol 110:727–736. https://doi.org/10.1007/s10482-017-0842-6
Estrada-de los Santos P, Palmer M, Chávez-Ramírez B, Beukes C, Steenkamp ET, Briscoe L, Khan N, Maluk M, Lafos M, Humm E, Arrabit M, Crook M, Gross E, Simon MF, dos Reis Junior FB, Whitman WB, Shapiro N, Poole PS, Hirsch AM, Venter SN, James EK (2018) Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): implications for the evolution of diazotrophy and nodulation in the Burkholderiaceae. Genes (Basel) 9. https://doi.org/10.3390/genes9080389
Lin QH, Lv YY, Gao ZH, Qiu LH (2020) Pararobbsia silviterrae gen. nov., sp. nov., isolated from forest soil and reclassification of Burkholderia alpina as Pararobbsia alpina comb. nov. Int J Syst Evol Microbiol 70:1412–1420. https://doi.org/10.1099/ijsem.0.003932
Ohbayashi T, Cossard R, Lextrait G, Hosokawa T, Lesieur V, Takeshita K, Tago K, Mergaert P, Kikuchi Y (2022) Intercontinental diversity of Caballeronia gut symbionts in the conifer pest bug Leptoglossus occidentalis. Microbes Environ 37:1–9. https://doi.org/10.1264/jsme2.ME22042
Peeters C, Meier-Kolthoff JP, Verheyde B, De Brandt E, Cooper VS, Vandamme P (2016) Phylogenomic study of Burkholderia glathei-like organisms, proposal of 13 novel Burkholderia species and emended descriptions of burkholderia sordidicola, Burkholderia zhejiangensis, and Burkholderia grimmiae. Front Microbiol 7:1–19. https://doi.org/10.3389/fmicb.2016.00877
Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission : a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73:4308–4316. https://doi.org/10.1128/AEM.00067-07
Kikuchi Y, Hosokawa T, Fukatsu T (2011) Specific developmental window for establishment of an insect-microbe gut symbiosis 77:4075–4081. https://doi.org/10.1128/AEM.00358-11
Lee JB, Park KE, Lee SA, Jang SH, Eo HJ, Jang HA, Kim CH, Ohbayashi T, Matsuura Y, Kikuchi Y, Futahashi R, Fukatsu T, Lee BL (2017) Gut symbiotic bacteria stimulate insect growth and egg production by modulating hexamerin and vitellogenin gene expression. Dev Comp Immunol 69:12–22. https://doi.org/10.1016/j.dci.2016.11.019
Kim JK, Lee JB, Huh YR, Jang HA, Kim CH, Yoo JW, Lee BL (2015) Burkholderia gut symbionts enhance the innate immunity of host Riptortus pedestris. Dev Comp Immunol 53:265–269. https://doi.org/10.1016/j.dci.2015.07.006
Vinokurov NN (2019) Paradieuches dissimilis (Distant, 1883)—new genus and new species of seed bug (Heteroptera: Lygaeidae) in the fauna of Russia from the South of the Far East Russ. Entomol J 28:1–4. https://doi.org/10.15298/rusentj.28.1.01
Kwon T-S, Jung S, Park Y-S (2021) Inverse relationship of Hemiptera richness with temperature in South Korea. Korean J Ecol Environ 54:102–107. https://doi.org/10.11614/KSL.2021.54.2.102
Tomohide Y, Mikio T, Izumi Y, Mitsuru K, Tetsuo K (1993) Terrestrial heteropterans: a field guide to Japanese bugs. Zenkoku noson Kyoiku Kyokai, Tokyo
Vanderzant ES, Pool MC, Richardson CD (1962) The role of ascorbic acid in the nutrition of three cotton insects. J Insect Physiol 8:287–297. https://doi.org/10.1016/0022-1910(62)90032-X
Chippendale GM (1975) Ascorbic acid: an essential nutrient for a plant feeding insect, Diatraea grandiosella. J Nutr 105:499–507. https://doi.org/10.1093/jn/105.4.499
Tago K, Itoh H, Kikuchi Y, Hori T, Sato Y, Nagayama A, Okubo T, Navarro R, Aoyagi T, Hayashi K, Hayatsu M (2014) A fine-scale phylogenetic analysis of free-living burkholderia species in sugarcane field soil. Microbes Environ 29:434–437. https://doi.org/10.1264/jsme2.ME14122
Fukatsu T, Nikoh N (1998) Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (insecta Homoptera). Appl Environ Microbiol 64:3599–3606. https://doi.org/10.1128/aem.64.10.3599-3606.1998
Vergunst AC, Meijer AH, Renshaw SA, O’Callaghan D (2010) Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect Immun 78:1495–1508. https://doi.org/10.1128/IAI.00743-09
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Edgar RC (2016) UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. bioRxiv 081257. https://doi.org/10.1101/081257
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Letunic I, Bork P (2007) Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23:127–128. https://doi.org/10.1093/bioinformatics/btl529
Ohbayashi T, Takeshita K, Kitagawa W, Nikohc N, Koga R, Meng XY, Tago K, Hori T, Hayatsu M, Asano K, Kamagata Y, Lee BL, Fukatsu T, Kikuchi Y (2015) Insect’s intestinal organ for symbiont sorting. Proc Natl Acad Sci U S A 112:E5179–E5188. https://doi.org/10.1073/pnas.1511454112
Martinson VG, Gawryluk RMR, Gowen BE, Curtis CI, Jaenike J, Perlman SJ (2020) Multiple origins of obligate nematode and insect symbionts by a clade of bacteria closely related to plant pathogens. Proc Natl Acad Sci U S A 117:31979–31986. https://doi.org/10.1073/pnas.2000860117
Hypša V, Aksoy S (1997) Phylogenetic characterization of two transovarially transmitted endosymbionts of the bedbug Cimex lectularius (Heteroptera: Cimicidae). Insect Mol Biol 6:301–304. https://doi.org/10.1046/j.1365-2583.1997.00178.x
Campbell BC, Purcell AH (1993) Phylogenetic affiliation of BEV, a bacterial parasite of the leafhopper Euscelidius variegatus, on the basis of 16S rDNA sequences. Curr Microbiol 26:37–41. https://doi.org/10.1007/BF01577240
Kuechler SM, Dettner K, Kehl S (2011) Characterization of an obligate intracellular bacterium in the midgut epithelium of the bulrush bug Chilacis typhae (Heteroptera, Lygaeidae, Artheneinae). Appl Environ Microbiol 77:2869–2876. https://doi.org/10.1128/AEM.02983-10
Nikoh N, Tsuchida T, Maeda T, Yamaguchi K, Shigenobu S, Koga R, Fukatsu T (2018) Genomic insight into symbiosis-induced insect color change by a facultative bacterial endosymbiont, “candidatus rickettsiella viridis.” MBio 9. https://doi.org/10.1128/mBio.00890-18
Taylor M, Mediannikov O, Raoult D, Greub G (2012) Endosymbiotic bacteria associated with nematodes, ticks and amoebae. FEMS Immunol Med Microbiol 64:21–31. https://doi.org/10.1111/j.1574-695X.2011.00916.x
Rosenwald LC, Sitvarin MI, White JA (2020) Endosymbiotic Rickettsiella causes cytoplasmic incompatibility in a spider host. Proc R Soc B Biol Sci 287. https://doi.org/10.1098/rspb.2020.1107
Acevedo TS, Fricker GP, Garcia JR, Alcaide T, Berasategui A, Stoy KS, Gerardo NM (2021) The importance of environmentally acquired bacterial symbionts for the squash bug (Anasa tristis), a significant agricultural pest. Front Microbiol 12:1–18. https://doi.org/10.3389/fmicb.2021.719112
Boucias DG, Garcia-Maruniak A, Cherry R, Lu H, Maruniak JE, Lietze VU (2012) Detection and characterization of bacterial symbionts in the Heteropteran, Blissus insularis. FEMS Microbiol Ecol 82:629–641. https://doi.org/10.1111/j.1574-6941.2012.01433.x
Xu Y, Buss EA, Boucias DG (2016) Impacts of antibiotic and bacteriophage treatments on the gut-symbiont-associated Blissus insularis (Hemiptera: Blissidae). Insects 7. https://doi.org/10.3390/insects7040061
Ravenscraft A, Thairu MW, Hansen AK, Hunter MS (2020) Continent-scale sampling reveals fine-scale turnover in a beneficial bug symbiont. Front Microbiol 11:1–13. https://doi.org/10.3389/fmicb.2020.01276
Hunter MS, Umanzor EF, Kelly SE, Whitaker SM, Ravenscraft A (2022) Development of common leaf-footed bug pests depends on the presence and identity of their environmentally acquired symbionts. Appl Environ Microbiol 88. https://doi.org/10.1128/aem.01778-21
Łukasik P, van Asch M, Guo H, Ferrari J, Charles H (2013) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16:214–218. https://doi.org/10.1111/ele.12031
Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M (2002) High Wolbachia density in insecticide-resistant mosquitoes. Proc R Soc B Biol Sci 269:1413–1416. https://doi.org/10.1098/rspb.2002.2022
Hosokawa T, Koga R, Kikuchi Y, Meng X, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A 107:769–774. https://doi.org/10.1073/pnas.091147610
Nadal-Jimenez P, Siozios S, Halliday N, Cámara M, Hurst GDD (2022) Symbiopectobacterium purcellii, gen. nov., sp. nov., isolated from the leafhopper Empoasca decipiens. Int J Syst Evol Microbiol 72:1–13. https://doi.org/10.1099/ijsem.0.005440
Husnik F, McCutcheon JP (2016) Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc Natl Acad Sci U S A 113:E5416–E5424. https://doi.org/10.1073/pnas.1603910113
Kuechler SM, Renz P, Dettner K, Kehl S (2012) Diversity of symbiotic organs and bacterial endosymbionts of: Lygaeoid bugs of the families blissidae and lygaeidae (Hemiptera: Heteroptera: Lygaeoidea). Appl Environ Microbiol 78:2648–2659. https://doi.org/10.1128/AEM.07191-11
da Mota FF, Marinho LP, de Moreira CJC, Lima MM, Mello CB, Garcia ES, Carels N, Azambuja P (2012) Cultivation-independent methods reveal differences among bacterial gut microbiota in triatomine vectors of Chagas disease. PLoS Negl Trop Dis 6:1–13. https://doi.org/10.1371/journal.pntd.0001631
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. https://doi.org/10.1038/nrmicro1969
Kikuchi Y, Fukatsu T (2003) Diversity of Wolbachia endosymbionts in Heteropteran bugs. Appl Environ Microbiol 69:6082–6090. https://doi.org/10.1128/AEM.69.10.6082-6090.2003
Itoh H, Jang S, Takeshita K, Ohbayashi T, Ohnishi N, Meng XY, Mitani Y, Kikuchi Y (2019) Host–symbiont specificity determined by microbe–microbe competition in an insect gut. Proc Natl Acad Sci U S A 116:22673–22682. https://doi.org/10.1073/pnas.1912397116
Itoh H, Hori T, Sato Y, Nagayama A, Tago K, Hayatsu M, Kikuchi Y (2018) Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J 12:909–920. https://doi.org/10.1038/s41396-017-0021-9
Kim J, Jung M, Lee D (2022) Characterization of Burkholderia bacteria clade compositions in soil and Riptortus pedestris ( Hemiptera : Alydidae ) in South Korea. J Asia Pac Entomol 25:1–8. https://doi.org/10.1016/j.aspen.2022.101976
Jang S, Kikuchi Y (2020) Re-opening of the symbiont sorting organ with aging in Riptortus pedestris. J Asia Pac Entomol 23:1089–1095. https://doi.org/10.1016/j.aspen.2020.09.005
Mainali BP, Kim HJ, Yoon YN, Oh IS, Do BS (2014) Evaluation of different leguminous seeds as food sources for the bean bug Riptortus pedestris. J Asia Pac Entomol 17:115–117. https://doi.org/10.1016/j.aspen.2013.11.007
Kim H-B, Kweon H, Ju W-T, Jo Y-Y, Kim Y-S (2019) Nutrient compositions of Korean mulberry fruits (Morus sp.) dried with low temperature vacuum dryer using microwave. Int J Ind Entomol 42:14–20. https://doi.org/10.7852/ijie.2021.42.1.14
Ohbayashi T, Futahashi R, Terashima M, Barrière Q, Lamouche F, Takeshita K, Meng XY, Mitani Y, Sone T, Shigenobu S, Fukatsu T, Mergaert P, Kikuchi Y (2019) Comparative cytology, physiology and transcriptomics of Burkholderia insecticola in symbiosis with the bean bug Riptortus pedestris and in culture. ISME J 13:1469–1483. https://doi.org/10.1038/s41396-019-0361-8
Salem H, Kaltenpoth M (2021) Beetle–bacterial symbioses: endless forms most functional. Annu Rev Entomol 67:201–219. https://doi.org/10.1146/annurev-ento-061421-063433
Noh MY, Muthukrishnan S, Kramer KJ, Arakane Y (2016) Cuticle formation and pigmentation in beetles. Curr Opin Insect Sci 17:1–9. https://doi.org/10.1016/j.cois.2016.05.004
Acknowledgements
We thank H. Ooi and A. Sawaguchi (AIST) for insect rearing.
Funding
This study was supported by JSPS Research Fellowships for Young Scientists to SJ (21F21090) and by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) KAKENHI to YK (20H03303).
Author information
Authors and Affiliations
Contributions
KI, SJ, and YK designed and developed the study. KI, SJ, and HI performed 16S rRNA amplicon sequencing and data analysis. KI and SJ performed other experiments and statistical analyses. KI, SJ, and YK wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ishigami, K., Jang, S., Itoh, H. et al. Obligate Gut Symbiotic Association with Caballeronia in the Mulberry Seed Bug Paradieuches dissimilis (Lygaeoidea: Rhyparochromidae). Microb Ecol 86, 1307–1318 (2023). https://doi.org/10.1007/s00248-022-02117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02117-2