Abstract
Purpose
The aim of this study is to investigate the changes in the volume of individual thalamic nuclei in patients with temporal lobe epilepsy (TLE) and hippocampal sclerosis (HS).
Methods
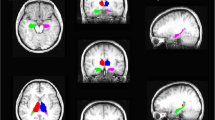
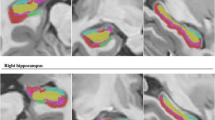
We enrolled 80 TLE patients with HS and 40 healthy controls. All of the subjects underwent 3D T1-weighted imaging. The hippocampus subfields and thalamic nuclei were segmented using the FreeSurfer program. We investigated volume changes in thalamic nuclei according to the HS side involved, and types or antiepileptic drug (AED) response compared with healthy controls.
Results
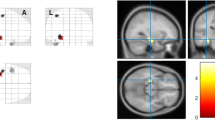
Compared with healthy controls, patients with HS showed atrophy of thalamic nuclei involving right and left parafascicular nuclei. In the right HS, the atrophy of the right thalamic nucleus was more prominent than that of the left thalamic nucleus, whereas the reduction in the volume of left thalamic nuclei was more prominent in patients with left HS. The reduction in thalamic nuclear volumes was more prominent in HS type 1 (atrophy of both CA1 and CA4 regions) than in other HS types. The suprageniculate nuclear volumes were significantly increased in patients with drug-controlled epilepsy.
Conclusions
Our study demonstrates thalamic nuclear atrophy in TLE patients with HS. In addition, the atrophy of individual thalamic nuclei varied according to the HS side, the HS types, and the AED response. These findings suggest that the role of thalamic nuclei varied in TLE with HS subtypes and displayed varying degrees of vulnerability in the pathology networks associated with the hippocampus.



Similar content being viewed by others
References
Blumcke I (2009) Neuropathology of focal epilepsies: a critical review. Epilepsy Behav 15(1):34–39. https://doi.org/10.1016/j.yebeh.2009.02.033
Kuzniecky RI, Knowlton RC (2002) Neuroimaging of epilepsy. Semin Neurol 22(3):279–288. https://doi.org/10.1055/s-2002-36647
Sadler RM (2006) The syndrome of mesial temporal lobe epilepsy with hippocampal sclerosis: clinical features and differential diagnosis. Adv Neurol 97:27–37
Blumcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Bernasconi N, Bien CG, Cendes F, Coras R, Cross JH, Jacques TS, Kahane P, Mathern GW, Miyata H, Moshe SL, Oz B, Ozkara C, Perucca E, Sisodiya S, Wiebe S, Spreafico R (2013) International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54(7):1315–1329. https://doi.org/10.1111/epi.12220
Jardim AP, Neves RS, Caboclo LO, Lancellotti CL, Marinho MM, Centeno RS, Cavalheiro EA, Scorza CA, Yacubian EM (2012) Temporal lobe epilepsy with mesial temporal sclerosis: hippocampal neuronal loss as a predictor of surgical outcome. Arq Neuropsiquiatr 70(5):319–324
Kwan BYM, Salehi F, Kope R, Lee DH, Sharma M, Hammond R, Burneo JG, Steven D, Peters T, Khan AR (2017) Evaluation of ex-vivo 9.4T MRI in post-surgical specimens from temporal lobe epilepsy patients. J Neuroradiol 44(6):377–380. https://doi.org/10.1016/j.neurad.2017.05.007
Steve TA, Jirsch JD, Gross DW (2014) Quantification of subfield pathology in hippocampal sclerosis: a systematic review and meta-analysis. Epilepsy Res 108(8):1279–1285. https://doi.org/10.1016/j.eplepsyres.2014.07.003
Jones EG (2007) The thalamus, 2nd edn. Cambridge University Press, Cambridge
Maller JJ, Welton T, Middione M, Callaghan FM, Rosenfeld JV, Grieve SM (2019) Revealing the hippocampal connectome through super-resolution 1150-direction diffusion MRI. Sci Rep 9(1):2418–2413. https://doi.org/10.1038/s41598-018-37905-9
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6(7):750–757. https://doi.org/10.1038/nn1075
Aracri P, de Curtis M, Forcaia G, Uva L (2018) Enhanced thalamo-hippocampal synchronization during focal limbic seizures. Epilepsia 59(9):1774–1784. https://doi.org/10.1111/epi.14521
Park KM, Kim SE, Shin KJ, Ha SY, Park J, Kim TH, Mun CW, Lee BI, Kim SE (2017) Effective connectivity in temporal lobe epilepsy with hippocampal sclerosis. Acta Neurol Scand 135(6):670–676. https://doi.org/10.1111/ane.12669
Zheng L, Bin G, Zeng H, Zou D, Gao J, Zhang J, Huang B (2018) Meta-analysis of voxel-based morphometry studies of gray matter abnormalities in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Brain Imaging Behav 12(5):1497–1503. https://doi.org/10.1007/s11682-017-9797-5
Li J, Zhang Z, Shang H (2012) A meta-analysis of voxel-based morphometry studies on unilateral refractory temporal lobe epilepsy. Epilepsy Res 98(2–3):97–103. https://doi.org/10.1016/j.eplepsyres.2011.10.002
Jo HJ, Kenny-Jung DL, Balzekas I, Benarroch EE, Jones DT, Brinkmann BH, Matt Stead S, Van Gompel JJ, Welker KM, Worrell GA (2019) Nuclei-specific thalamic connectivity predicts seizure frequency in drug-resistant medial temporal lobe epilepsy. Neuroimage Clin 21:101671. https://doi.org/10.1016/j.nicl.2019.101671
Engelborghs S, Marien P, Martin JJ, De Deyn PP (1998) Functional anatomy, vascularisation and pathology of the human thalamus. Acta Neurol Belg 98(3):252–265
Kumar VJ, van Oort E, Scheffler K, Beckmann CF, Grodd W (2017) Functional anatomy of the human thalamus at rest. Neuroimage 147:678–691. https://doi.org/10.1016/j.neuroimage.2016.12.071
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51(6):1069–1077. https://doi.org/10.1111/j.1528-1167.2009.02397.x
Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, van der Kouwe A, Alzheimer's Disease Neuroimaging I, Fischl B, Caballero-Gaudes C, Paz-Alonso PM (2018) A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 183:314–326. https://doi.org/10.1016/j.neuroimage.2018.08.012
Feger J, Bevan M, Crossman AR (1994) The projections from the parafascicular thalamic nucleus to the subthalamic nucleus and the striatum arise from separate neuronal populations: a comparison with the corticostriatal and corticosubthalamic efferents in a retrograde fluorescent double-labelling study. Neuroscience 60(1):125–132
Brown HD, Baker PM, Ragozzino ME (2010) The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci 30(43):14390–14398. https://doi.org/10.1523/JNEUROSCI.2167-10.2010
Vogt BA, Hof PR, Friedman DP, Sikes RW, Vogt LJ (2008) Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal, and intralaminar thalamic nuclei. Brain Struct Funct 212(6):465–479. https://doi.org/10.1007/s00429-008-0178-0
Langlois M, Polack PO, Bernard H, David O, Charpier S, Depaulis A, Deransart C (2010) Involvement of the thalamic parafascicular nucleus in mesial temporal lobe epilepsy. J Neurosci 30(49):16523–16535. https://doi.org/10.1523/JNEUROSCI.1109-10.2010
Zhang Z, Li JJ, Lu QC, Gong HQ, Liang PJ, Zhang PM (2016) Interaction between thalamus and hippocampus in termination of amygdala-kindled seizures in mice. Comput Math Methods Med 2016:9580724. https://doi.org/10.1155/2016/9580724
Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, Torres N, Pastor J, Selway R, Sola RG, Alarcon G (2013) Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 54(10):1823–1833. https://doi.org/10.1111/epi.12352
Son BC, Shon YM, Choi JG, Kim J, Ha SW, Kim SH, Lee SH (2016) Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg 94(3):187–197. https://doi.org/10.1159/000446611
Blumcke I, Thom M, Wiestler OD (2002) Ammon’s horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 12(2):199–211
Goubran M, Bernhardt BC, Cantor-Rivera D, Lau JC, Blinston C, Hammond RR, de Ribaupierre S, Burneo JG, Mirsattari SM, Steven DA, Parrent AG, Bernasconi A, Bernasconi N, Peters TM, Khan AR (2016) In vivo MRI signatures of hippocampal subfield pathology in intractable epilepsy. Hum Brain Mapp 37(3):1103–1119. https://doi.org/10.1002/hbm.23090
Thom M, Zhou J, Martinian L, Sisodiya S (2005) Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain 128(Pt 6):1344–1357. https://doi.org/10.1093/brain/awh475
Benedek G, Pereny J, Kovacs G, Fischer-Szatmari L, Katoh YY (1997) Visual, somatosensory, auditory and nociceptive modality properties in the feline suprageniculate nucleus. Neuroscience 78(1):179–189
Paroczy Z, Nagy A, Markus Z, Waleszczyk WJ, Wypych M, Benedek G (2006) Spatial and temporal visual properties of single neurons in the suprageniculate nucleus of the thalamus. Neuroscience 137(4):1397–1404. https://doi.org/10.1016/j.neuroscience.2005.10.068
Eordegh G, Nagy A, Berenyi A, Benedek G (2005) Processing of spatial visual information along the pathway between the suprageniculate nucleus and the anterior ectosylvian cortex. Brain Res Bull 67(4):281–289. https://doi.org/10.1016/j.brainresbull.2005.06.036
Hicks TP, Stark CA, Fletcher WA (1986) Origins of afferents to visual suprageniculate nucleus of the cat. J Comp Neurol 246(4):544–554. https://doi.org/10.1002/cne.902460410
Hu Y, Mi X, Xu X, Fang W, Zeng K, Yang M, Li C, Wang S, Li M, Wang X (2015) The brain activity in Brodmann area 17: a potential bio-marker to predict patient responses to antiepileptic drugs. PLoS One 10(10):e0139819. https://doi.org/10.1371/journal.pone.0139819
Guo C, Ferreira D, Fink K, Westman E, Granberg T (2019) Repeatability and reproducibility of FreeSurfer, FSL-SIENAX and SPM brain volumetric measurements and the effect of lesion filling in multiple sclerosis. Eur Radiol 29(3):1355–1364. https://doi.org/10.1007/s00330-018-5710-x
Su JH, Thomas FT, Kasoff WS, Tourdias T, Choi EY, Rutt BK, Saranathan M (2019) Thalamus optimized multi atlas segmentation of thalamic nuclei from structural MRI. Neuroimage 194:272–282. https://doi.org/10.1016/j.neuroimage.2019.03.021
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, HJ., Seo, S.A. & Park, K.M. Quantification of thalamic nuclei in patients diagnosed with temporal lobe epilepsy and hippocampal sclerosis. Neuroradiology 62, 185–195 (2020). https://doi.org/10.1007/s00234-019-02299-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02299-6