Abstract
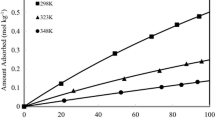
Diffusivity of methane in CHA-type zeolite (Si/Al = 160) was investigated by uptake rate method using a volumetric adsorption setup. The methane diffusivities were measured over a range of temperature (298-393 K) and pressure (0-0.9 bar) to study the effect of temperature and pressure on the diffusion coefficients. The results showed that the diffusivities decrease with pressure while they increase with increase in temperature. Furthermore, activation energy of diffusion decreases with pressure. The limiting values of activation energy and diffusivity of methane (at 298 K) at p = 0 (zero loading) were estimated to be 18.0 kJ/mol and ~3.2 × 10−13 m2/s, respectively. The results were compared with the diffusivities of CH4 in 12-, 10- and 8-MR zeolites.







Similar content being viewed by others
Abbreviations
- C0 :
-
initial loading of the zeolite particles, (mol/kg)
- D:
-
diffusivity, (m2/s)
- D∞ :
-
diffusivity at infinite temperature, (m2/s)
- E:
-
activation energy of diffusion, (kJ mol-1)
- m* :
-
mass of adsorptive in storage vessel prior to adsorption, (mol)
- mf :
-
mass of adsorptive in gas phase, (mol)
- mt :
-
mass adsorbed on the adsorbent at time t, (mol/kg)
- m∞ :
-
mass adsorbed at the equilibrium (t→∞), (mol/kg)
- M:
-
molar mass of adsorptive gas, (kg/kmol)
- p:
-
pressure of adsorptive gas, (bar)
- ri :
-
radius of adsorbents particle, (m)
- R:
-
universal gas constant, (83.14 cm3 bar mol-1 K-1)
- t:
-
time, (s)
- T:
-
absolute temperature, (K)
- VAC :
-
volume of adsorption chamber, (cm3)
- VSV :
-
volume of storage vessel, (cm3)
- VsHe :
-
volume of a (porous) sorbent measured by helium expansion experiments, (cm3)
- wi :
-
weight fraction of the particles of radius ri, (-)
- Z:
-
gas compressibility factor, (-)
- σ2 :
-
variance of a measurable quantity, (unit2)
References
Hudson MR, Queen WL, Mason JA, Fickel DW, Lobo RF, Brown CM (2012) Unconventional, highly selective CO2 adsorption in zeolite SSZ-13. J Am Chem Soc 134(4):1970–1973
Shang J, Li G, Singh R, Xiao P, Danaci D, Liu JZ, Webley PA (2014) Adsorption of CO2, N2, and CH4 in Cs-exchanged chabazite: a combination of van der Waals density functional theory calculations and experiment study. J Chem Phys 140(8):084705
Maghsoudi H, Soltanieh M, Bozorgzadeh H, Mohamadalizadeh A (2013) Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 19(5):1045–1053
Li S, Martinek JG, Falconer JL, Noble RD, Gardner TQ (2005) High-pressure CO2/CH4 separation using SAPO-34 membranes. Ind Eng Chem Res 44(9):3220–3228
Venna SR, Carreon MA (2011) Amino-functionalized SAPO-34 membranes for CO2/CH4 and CO2/N2 separation. Langmuir 27(6):2888–2894
Kosinov N, Auffret C, Gücüyener C, Szyja BM, Gascon J, Kapteijn F, Hensen EJ (2014) High flux high-silica SSZ-13 membrane for CO2 separation. J Mater Chem A 2(32):13083–13092
Maghsoudi H, Soltanieh M (2014) Simultaneous separation of H2S and CO2 from CH4 by a high silica CHA-type zeolite membrane. J Membr Sci 470:159–165
Wu T, Diaz MC, Zheng Y, Zhou R, Funke HH, Falconer JL, Noble RD (2015) Influence of propane on CO2/CH4 and N2/CH4 separations in CHA zeolite membranes. J Membr Sci 473:201–209
Krishna R, Van Baten J, Garcia-Perez E, Calero S (2006) Diffusion of CH4 and CO2 in MFI, CHA and DDR zeolites. Chem Phys Lett 429(1-3):219–224
Beerdsen E, Dubbeldam D, Smit B (2006) Loading dependence of the diffusion coefficient of methane in nanoporous materials. J Phys Chem B 110(45):22754–22772
Jee SE, Sholl DS (2009) Carbon dioxide and methane transport in DDR zeolite: insights from molecular simulations into carbon dioxide separations in small pore zeolites. J Am Chem Soc 131(22):7896–7904
Demontis P, Fois ES, Suffritti GB, Quartieri S (1990) Molecular dynamics studies on zeolites. 4. Diffusion of methane in silicalite. J Phys Chem 94(10):4329–4334
Garcia-Sanchez A, Dubbeldam D, Calero S (2010) Modeling adsorption and self-diffusion of methane in LTA zeolites: the influence of framework flexibility. J Phys Chem C 114(35):15068–15074
Zimmermann NE, Jakobtorweihen S, Beerdsen E, Smit B, Keil FJ (2007) In-depth study of the influence of host− framework flexibility on the diffusion of small gas molecules in one-dimensional zeolitic pore systems. J Phys Chem C 111(46):17370–17381
Vidoni A, Ruthven D (2012) Diffusion of methane in DD3R zeolite. Microporous Mesoporous Mater 159:57–65
Yucel H, Ruthven DM (1980) Diffusion in 4A zeolite. Study of the effect of crystal size. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 76:60–70
Mohr R, Vorkapic D, Rao M, Sircar S (1999) Pure and binary gas adsorption equilibria and kinetics of methane and nitrogen on 4A zeolite by isotope exchange technique. Adsorption 5(2):145–158
Yucel H, Ruthven DM (1980) Diffusion in 5A zeolite. Study of the effect of crystal size. J Chem Soc Faraday Trans 1 Phys Chemi Condens Phases 76:71–83
Caro J, Hǒcevar S, Kärger J, Riekert L (1986) Intracrystalline self-diffusion of H2O and CH4 in ZSM-5 zeolites. Zeolites 6(3):213–216
Silva JA, Schumann K, Rodrigues AE (2012) Sorption and kinetics of CO2 and CH4 in binderless beads of 13X zeolite. Microporous Mesoporous Mater 158:219–228
Lauerer A, Binder T, Haase J, Kärger J, Ruthven D (2015) Diffusion of propene in DDR crystals studied by interference microscopy. Chem Eng Sci 138:110–117
Pourmahdi Z, Maghsoudi H (2017) Adsorption isotherms of carbon dioxide and methane on CHA-type zeolite synthesized in fluoride medium. Adsorption 23(6):799–807
Keller JU, Staudt R (2005) Gas adsorption equilibria: experimental methods and adsorptive isotherms. Springer Science & Business Media
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maghsoudi, H., Nozari, V. & Zamzami, S.R. Diffusion of methane in high-silica CHA zeolite. Heat Mass Transfer 55, 1619–1625 (2019). https://doi.org/10.1007/s00231-018-02547-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-018-02547-0