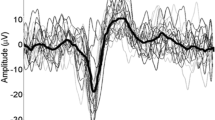
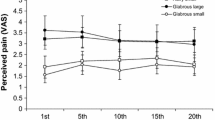
Abstract
The endogenous analgesia (EA) system is psychophysically evaluated using various paradigms, including conditioned pain modulation (CPM) and offset analgesia (OA) testing, respectively, the spatial and temporal filtering processes of noxious information. Though both paradigms assess the function of the EA system, it is still unknown whether they reflect the same aspects of EA and consequently whether they provide additive or equivalent data. Twenty-nine healthy volunteers (15 males) underwent 5 trials of different stimulation conditions in random order including: (1) the classic OA three-temperature stimulus train (‘OA’); (2) a three-temperature stimulus train as control for the OA (‘OAcon’); (3) a constant temperature stimulus (‘constant’); (4) the classic parallel CPM (‘CPM’); and (5) a combination of OA and CPM (‘OA + CPM’). We found that in males, the pain reduction during the OA + CPM condition was greater than during the OA (P = 0.003) and CPM (P = 0.07) conditions. Furthermore, a correlation was found between OA and CPM (r = 0.62, P = 0.01) at the time of maximum OA effect. The additive effect found suggests that the two paradigms represent at least partially different aspects of EA. The moderate association between the CPM and OA magnitudes indicates, on the other hand, some commonality of their underlying mechanisms.






Similar content being viewed by others
References
Arendt-Nielsen L, Sluka KA, Nie HL (2008) Experimental muscle pain impairs descending inhibition. Pain 140:465–471
Baad-Hansen L, Poulsen HF, Jensen HM, Svensson P (2005) Lack of sex differences in modulation of experimental intraoral pain by diffuse noxious inhibitory controls (DNIC). Pain 116:359–365
Beck AT, Beck RW (1972) Screening depressed patients in family practice. A rapid technique. Postgrad Med 52:81–85
Bouhassira D, Bing Z, Le Bars D (1990) Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. J Neurophysiol 64:1712–1723
Bouhassira D, Villanueva L, Le Bars D (1992) Effects of systemic morphine on diffuse noxious inhibitory controls: role of the periaqueductal grey. Eur J Pharmacol 216:149–156
Derbyshire SW, Osborn J (2009) Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. Neuroimage 47:1002–1006
Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL III (2009) Sex, gender and pain: a review of a recent clinical and experimental findings. J Pain 10:447–485
Ge HY, Madeleine P, Arendt-Nielsen L (2004) Sex differences in temporal characteristics of descending inhibitory control: an evaluation using repeated bilateral experimental induction of muscle pain. Pain 110:72–78
Gebhart GF (2004) Descending modulation of pain. Neurosci Biobehav Rev 27:729–737
Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR (2009) Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. J Pain 10:180–190
Granot M, Goldstein Ferber S (2005) The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity. Clin J Pain 21:439–445
Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, Yarnitsky D (2008) Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain 136:142–149
Grill JD, Coghill RC (2002) Transient analgesia evoked by noxious stimulus offset. J Neurophysiol 87:2205–2208
King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL III (2009) Deficiency in endogenous modulation of prolonged heat pain in patients with irritable bowel syndrome and temporomandibular disorder. Pain 143:172–178
Kong J, Tu PC, Zyloney C, Su TP (2010) Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res 211:215–219
Kosek E, Ordeberg G (2000) Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 88:69–78
Le Bars D (2002) The whole body receptive field of multireceptive neurons. Prog Brain Res 40:29–44
Le Bars D, Dickenson AH, Besson J (1979a) Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurons in the rat. Pain 6:283–304
Le Bars D, Dickenson AH, Besson J (1979b) Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on nonconvergent neurons, supraspinal involvement and theoretical implications. Pain 6:305–327
Lovick T, Bandler R (2005) The organization of the midbrain periaqueductal grey and the integration of pain behaviors. In: Hunt SP, Koltzenburg M (eds) The neurobiology of pain. Oxford, New York, pp 267–287
Martucci KT, Eisenach JC, Tong C, Coghill RC (2012a) Opioid-independent mechanisms supporting offset analgesia and temporal sharpening of nociceptive information. Pain 153:1232–1243
Martucci KT, Yelle MD, Coghill RC (2012b) Differential effects of experimental central sensitization on the time-course and magnitude of offset analgesia. Pain 153:463–472
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, Sarton EY (2011a) Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain 152:656–663
Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A (2011b) Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 115:1063–1071
Nir RR, Granovsky Y, Yarnitsky D, Sprecher E, Granot M (2011) A psychophysical study of endogenous analgesia: the role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur J Pain 15:491–497
Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia—imaging a shared neuronal network. Science 295:1737–1740
Piché M, Arsenault M, Rainville P (2009) Cerebral and cerebrospinal processes underlying counter irritation analgesia. J Neurosci 29:14236–14246
Pud D, Eisenberg E, Sprecher E, Rogowski Z, Yarnitsky D (2004) The tridimensional personality theory and pain: harm avoidance and reward dependence traits correlate with pain perception in healthy volunteers. Eur J Pain 8:31–38
Pud D, Sprecher E, Yarnitsky D (2005) Homotopic and heterotopic effects of endogenous analgesia in healthy volunteers. Neurosci Lett 380:209–213
Pud D, Granovsky Y, Yarnitsky D (2009) The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144:16–19
Quiton RL, Greenspan JD (2007) Sex differences in endogenous pain modulation by distracting and painful conditioning stimulation. Pain 132:S134–S149
Ram KC, Eisenberg E, Haddad M, Pud D (2008) Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain—new perspective of opioid induced hyperalgesia. Pain 139:431–438
Ruscheweyh R, Marziniak M, Stumpenhorst F, Reinholz J, Knecht S (2009) Pain sensitivity can be assessed by self-rating: development and validation of the pain sensitivity questionnaire. Pain 146:65–74
Schouenborg J, Dickenson A (1985) Effects of a distant noxious stimulation on A and C fiber-evoked flexion reflexes and neuronal activity in the dorsal horn of the rat. Brain Res 328:23–32
Seminowics DA, Davis KD (2006) Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 120:297–306
Sillery E, Bittar RG, Robson MD, Behrens TE, Stein J, Aziz TZ, Johansen-Berg H (2005) Connectivity of the human periventricular-periaqueductal gray region. J Neurosurg 103:1030–1034
Spielberger CD, Gorsuch RL, Lushene R (1970) Manual for the state-trait anxiety inventory. Consulting Psychologist Press, Palo Alto
Staud R, Robinson ME, Vierck CJ Jr, Price DD (2003) Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain 101:167–174
Sullivan MJL, Bishop S, Pivik J (1995) The pain catastrophizing scale: development and validation. Psychol Assess 7:524–532
Van Wijk G, Veldhijzen DS (2010) Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain 11:408–419
Villanueva L (2009) Diffuse noxious inhibitory control (DNIC) as a tool for exploring dysfunction of endogenous pain modulatory systems. Pain 143:161–162
Weissman-Fogel I, Sprecher E, Pud D (2008) Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res 186:79–85
Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A (2004) Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 53:1595–1601
Willer JC, Le Bars D, De Broucker T (1990) Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. Eur J Pharmacol 182:347–355
Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O (2010) Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 14:339
Yelle MD, Rogers JM, Coghill RC (2008) Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain 134:174–186
Yelle MD, Oshiro Y, Kraft RA, Coghill RC (2009) Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci 29:10264–10271
Conflict of interest
No funding sources were provided. The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honigman, L., Yarnitsky, D., Sprecher, E. et al. Psychophysical testing of spatial and temporal dimensions of endogenous analgesia: conditioned pain modulation and offset analgesia. Exp Brain Res 228, 493–501 (2013). https://doi.org/10.1007/s00221-013-3580-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3580-7