Abstract
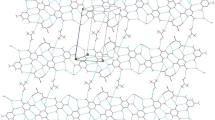
A theoretical study has been conducted on a set of supramolecular complexes based on 1,3,5-triazine-2,4,6-triamine, or melamine (M), with cyanuric acid (CA), trithiocyanuric acid (TCA), and two mono-substituted derivatives of CA with chlorine (CACl) and bromine (CABr). The study was carried out on 12 complexes, M/(CA) n , M/(TCA) n , M/(CACl) n , M/(CABr) n , with n = 1, 2 and 3, by the density functional theory employing the ω-B97XD functional with the 6-311++G(d,p) basis set. Information about the intermolecular perturbation over M and the interactions that drive the self-assembly of these species has been obtained from the quantum theory of atoms in molecules and a natural bond orbital analysis. The harmonic oscillator model of aromaticity, the para-delocalization index, the fluctuation aromatic index, and two electron charge density descriptors were used to evaluate the aromaticity of M in each complex. Results show that the hydrogen and halogen (XBs) bond interactions, which direct the self-assembly process in these complexes, are anti-cooperative. Binding energies decrease in the following order: M/(CA) n > M/(TCA) n > M/(CABr) n > M/(CACl) n (for all values of n). Brominated CA arises as a potential compound to self-assembly with M via XBs.







Similar content being viewed by others
References
Lehn J-M (1988) Supramolecular chemistry-scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew Chemie Int Ed Engl 27:89–112. doi:10.1002/anie.198800891
Atwood JL, Steed JW (2004) Encyclopedia of supramolecular chemistry, vol 1. CRC Press, New York, Besel
Lehn J-M (1973) Design of organic complexing agents strategies towards properties. Struct Bond 16:1–69
Lehn JM (1978) Cryptates: inclusion complexes of macropolycyclic receptor molecules. Pure Appl Chem 50:871–892. doi:10.1351/pac197850090871
Lehn J-M (2002) Toward complex matter: supramolecular chemistry and self-organization. Proc Natl Acad Sci USA 99:4763–4768. doi:10.1073/pnas.072065599
Wyler R, de Mendoza J, Rebek J (1993) A synthetic cavity assembles through self-complementary hydrogen bonds. Angew Chem Int Ed Engl 32:1699–1701. doi:10.1002/anie.199316991
Seto CT, Whitesides GM (1993) Molecular self-assembly through hydrogen bonding: supramolecular aggregates based on the cyanuric acid-melamine lattice. J Am Chem Soc 115:905–916
Wang K, Duan D, Zhou M, Li S, Cui T, Liu B, Liu J, Zou B, Zou G (2011) Structural properties and halogen bonds of cyanuric chloride under high pressure. J Phys Chem B 115:4639–4644. doi:10.1021/jp200966n
Shang J, Wang Y, Chen M, Dai J, Zhou X, Kuttner J, Hilt G, Shao X, Gottfried JM, Wu K (2015) Assembling molecular Sierpiński triangle fractals. Nat Chem. doi:10.1038/nchem.2211
Dey R, Bhattacharya B, Mondal P, Mondal R, Ghoshal D (2014) Fabrication of two supramolecular self-assemblies of Mn(II)-dicarboxylates with trans-4,4′-azobispyridine: analysis of H-bonding interactions with Hirshfeld surfaces and DFT calculations. J Mol Struct 1067:64–73. doi:10.1016/j.molstruc.2014.02.059
Yurenko YP, Novotný J, Mitoraj MP, Sklenár V, Michalak A, Merek R (2014) Nucleic acid quadruplexes based on 8-Halo-9-deazaxanthines: energetics and noncovalent interactions in quadruplex stems. J Chem Theory Comput 10:5353–5365
Lindsey JS (1991) Self-assembly in sinthetic routes to molecular devices. Biological principles and chemical perspectives: a review. New J Chem 15:153–180
Stupp SI, Palmer LC (2014) Supramolecular chemistry and self-assembly in organic materials design. Chem Mater 26:507–518
Ariga K, Hill JP, Lee MV, Vinu A, Charvet R, Acharya S (2008) Challenges and breakthroughs in recent research on self-assembly. Sci Technol Adv Mater 9:014109. doi:10.1088/1468-6996/9/1/014109
Paraschiv V, Crego-Calama M, Fokkens RH, Padberg CJ, Timmerman P, Reinhoudt DN (2001) Nanostructures via noncovalent synthesis: 144 hydrogen bonds bring together 27 components. J Org Chem 66:8297–8301. doi:10.1021/jo0105742
Yagai S, Nakajima T, Karatsu T, Saitow K, Kitamura A (2004) Phototriggered self-assembly of hydrogen-bonded rosette. J Am Chem Soc 126:11500–11508. doi:10.1021/ja047783z
Hughes EW (1941) The crystal structure of melamine. J Am Chem Soc 63:1737–1752. doi:10.1021/ja01851a069
Dewar MJS, Paoloni L (1957) The electronic structure of melamine. Trans Faraday Soc 53:261–271
Meier RJ, Coussens B (1990) The molecular structure of hydrazine and melamine: rotational barriers and hybridisation. J Mol Struct THEOCHEM 209:303–312. doi:10.1016/0166-1280(90)80084-2
Wang Y, Pittman CU, Saebo S (1993) Investigation of the structure and properties of ammeline, melamine, and 2,4-diamino-1,3,5-triazine by ab initio calculations. J Org Chem 58:3085–3090. doi:10.1021/jo00063a030
Li Z, Chen G, Xu Y, Wang X, Wang Z (2013) Study of the structural and the spectral characteristics of [C3N3(NH2)3] n (n = 1–4) clusters. J Phys Chem A 117:12511–12518
Silly F, Shaw AQ, Castell MR, Briggs GAD, Mura M, Martsinovich N, Kantorovich L (2008) Melamine structures on the Au(111) surface. J Phys Chem C 112:11476–11480. doi:10.1021/jp8033769
Chiş V, Mile G, Ştiufiuc R, Leopold N, Oltean M (2009) Vibrational and electronic structure of PTCDI and melamine–PTCDI complexes. J Mol Struct 924–926:47–53. doi:10.1016/j.molstruc.2008.12.038
Atalay Y, Avcı D, Başoğlu A, Okur İ (2005) Molecular structure and vibrational spectra of melamine diborate by density functional theory and ab initio Hartree–Fock calculations. J Mol Struct THEOCHEM 713:21–26. doi:10.1016/j.theochem.2004.09.044
Thomas R, Kulkarni GU (2007) A hydrogen-bonded channel structure formed by a complex of uracil and melamine. Beilstein J Org Chem 3:17. doi:10.1186/1860-5397-3-17
Makowski SJ, Lacher M, Lermer C, Schnick W (2012) Supramolecular hydrogen-bonded structures between melamine and N-heterocycles. J Mol Struct 1013:19–25. doi:10.1016/j.molstruc.2012.01.010
Xu J, Wu G, Wang Z, Zhang X (2012) Generation of 2D organic microsheets from protonated melamine derivatives: suppression of the self assembly of a particular dimension by introduction of alkyl chains. Chem Sci 3:3227. doi:10.1039/c2sc20871g
Prior TJ, Armstrong JA, Benoit DM, Marshall KL (2013) The structure of the melamine–cyanuric acid co-crystal. CrystEngComm 15:5838. doi:10.1039/c3ce40709h
Wang Y, Wei B, Wang Q (1990) Crystal structure of melamine cyanuric acid complex (1:1) trihydrochloride, MCA·3HCl. J Crystallogr Spectrosc Res 20:79–84
Li S, Sun L, Chung Y, Weber SG (1999) Artificial receptor-facilitated solid-phase microextraction of arbiturates. Anal Chem 71:2146–2151. doi:10.1021/ac980587o
Bader RWF (1990) Atoms in molecules: a quantum theory. Clarendon Press, Oxford
Krygowski TM (1993) Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of. pi.-electron systems. J Chem Inf Comput Sci 33:70–78
Krygowski TM, Cyranski MK (2001) Structural aspects of aromaticity. Chem Rev 101:1385–1419
Poater J, Fradera X, Duran M, Solà M (2003) The delocalization index as an electronic aromaticity criterion: application to a series of planar polycyclic aromatic hydrocarbons. Chem Eur J 9:400–406
Matito MD, Solà M (2005) The aromatic fluctuation index (FLU): a new aromaticity index based on electron delocalization. J Chem Phys 122:014109
Howard ST, Krygowski TM (1997) Benzenoid hydrocarbon aromaticity in terms of charge density descriptors. Can J Chem 75:1174–1181
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, and Pople JA (2004) Gaussian 03, Revision D.01. Gaussian, Inc., Wallingford CT
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Kozuch S, Martin JML (2013) Halogen bonds: benchmarks and theoretical analysis. J Chem Theory Comput 9:1918–1931. doi:10.1021/ct301064t
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–559
AIMAll Version 12.06.03, Todd A. Keith, TK Gristmill Software, Overland Park KS, USA, 2012 aim.tkgristmill.com
Glendening ED, Reed AE, Carpenter JE, Weinhold F NBO Version 3.1
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comp Chem 33:580–592
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Song HJ, Xiao HM, Dong HS, Zhu WH (2006) Cooperative effects and strengths of hydrogen bonds in open-chain cis-triaziridine clusters (n = 2–8): a DFT investigation. J Phys Chem A 110:2225–2230. doi:10.1021/jp055385s
Znamenskiy VS, Green ME (2007) Quantum calculations on hydrogen bonds in certain water clusters show cooperative effects. J Chem Theory Comput 3:103–114. doi:10.1055/s-0029-1237430.Imprinting
Jalili S, Aghdastinat H (2008) Study of hydrogen bonding in dihydroxyacetone and glyceraldehyde using computational methods. J Mol Struct THEOCHEM 857:7–12. doi:10.1016/j.theochem.2008.01.026
Grabowski SJ, Bilewicz E (2006) Cooperativity halogen bonding effect—Ab initio calculations on H2CO···(ClF) n complexes. Chem Phys Lett 427:51–55. doi:10.1016/j.cplett.2006.06.060
Ranganathan A, Pedireddi VR, Rao CNR (1999) Hydrothermal synthesis of organic channel structures: 1:1 hydrogen-bonded adducts of melamine with cyanuric and trithiocyanuric acids. J Am Chem Soc 121:1752–1753
Carroll MT, Bader RFW (1988) An analysis of the hydrogen bond in BASE-HF complexes using the theory of atoms in molecules. Mol Phys 65:695–722
Koch U, Popelier PLA (1995) Characterization of C–H–O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754. doi:10.1021/j100024a016
Amezaga NJM, Pamies SC, Peruchena NM, Sosa GL (2010) Halogen bonding: a study based on the electronic charge density. J Phys Chem A 114:552–562. doi:10.1021/jp907550k
Han N, Zeng Y, Sun C, Li X, Sun Z, Meng L (2014) N···I halogen bonding interactions: in fluence of lewis bases on their strength and characters. J Phys Chem A 118:7058–7065
Duarte DJR, Sosa GL, Peruchena NM (2013) Nature of halogen bonding. A study based on the topological analysis of the Laplacian of the electron charge density and an energy decomposition analysis. J Mol Model 19:2035–2041. doi:10.1007/s00894-012-1624-8
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Grabowski SJ (2001) Ab initio calculations on conventional and unconventional hydrogen bonds study of the hydrogen bond strength. J Phys Chem A 105:10739–10746
Szatyłowicz H, Sadlej-Sosnowska N (2010) Characterizing the strength of individual hydrogen bonds in DNA base pairs. J Chem Inf Model 50:2151–2161. doi:10.1021/ci100288h
Matta CF, Castillo N, Boyd RJ (2006) Extended weak bonding interactions in DNA: π-stacking (base-base), base–backbone, and backbone–backbone interactions. J Phys Chem B 110:563–578
Reiher M, Sellmann D, Hess BA (2001) Stabilization of diazene in Fe(II)–sulfur model complexes relevant for nitrogenase activity. I. A new approach to the evaluation of intramolecular hydrogen bond energies. Theor Chem Acc 106:379–392
Cremer D, Kraka E (1984) Chemical bonds without bonding electron density—does the difference electron-density analysis suffice for a description of the chemical bond? Angew Chem 23:627–628
Angelina EL, Duarte DJR, Peruchena NM (2013) Is the decrease of the total electron energy density a covalence indicator in hydrogen and halogen bonds? J Mol Model 19:2097–2106. doi:10.1007/s00894-012-1674-y
Angelina EL, Peruchena NM (2011) Strength and nature of hydrogen bonding interactions in mono- and di-hydrated formamide complexes. J Phys Chem A 115:4701–4710. doi:10.1021/jp1105168
Wang W, Wong NB, Zheng W, Tiang A (2004) Theoretical study on the blueshifting halogen bond. J Phys Chem A 108:1799–1805
Krygowski TM, Szatylowicz H, Stasyuk OA, Dominikowska J, Palusiak M (2014) Aromaticity from the viewpoint of molecular geometry: application to planar systems. Chem Rev 114:6383–6422. doi:10.1021/cr400252h
Parreira RLT, Galembeck SE (2006) Computational study of pyrylium cation–water complexes: hydrogen bonds, resonance effects, and aromaticity. J Mol Struct THEOCHEM 760:59–73. doi:10.1016/j.theochem.2005.11.020
Acknowledgments
We would like to thank SECYT UTN (Secretaría de Ciencia y Tecnología – Universidad Tecnológica Nacional), for financial support. A.N.P. is a fellowship researcher of CONICET, Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas), and N.M.P. is a career researcher of CONICET, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “CHITEL 2015 - Torino - Italy”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petelski, A.N., Duarte, D.J.R., Pamies, S.C. et al. Intermolecular perturbation in the self-assembly of melamine. Theor Chem Acc 135, 65 (2016). https://doi.org/10.1007/s00214-015-1795-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1795-3