Abstract
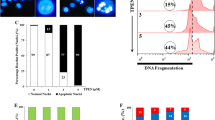
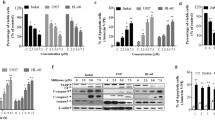
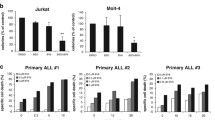
The cathepsin B inhibitor benzyloxycarbonyl-phenylalanine-alanine-chloromethyl ketone (z-FA-CMK) was recently found to induce apoptosis at low concentrations in Jurkat T cells, while at higher concentrations, the cells die of necrosis. In the present study, we showed that z-FA-CMK readily depletes intracellular glutathione (GSH) with a concomitant increase in reactive oxygen species (ROS) generation. The toxicity of z-FA-CMK in Jurkat T cells was completely abrogated by N-acetylcysteine (NAC), suggesting that the toxicity mediated by z-FA-CMK is due to oxidative stress. We found that l-buthionine sulfoximine (BSO) which depletes intracellular GSH through the inhibition of GSH biosynthesis in Jurkat T cells did not promote ROS increase or induce cell death. However, NAC was still able to block z-FA-CMK toxicity in Jurkat T cells in the presence of BSO, indicating that the protective effect of NAC does not involve GSH biosynthesis. This is further corroborated by the protective effect of the non-metabolically active d-cysteine on z-FA-CMK toxicity. Furthermore, in BSO-treated cells, z-FA-CMK-induced ROS increased which remains unchanged, suggesting that the depletion of GSH and increase in ROS generation mediated by z-FA-CMK may be two separate events. Collectively, our results demonstrated that z-FA-CMK toxicity is mediated by oxidative stress through the increase in ROS generation.









Similar content being viewed by others
References
Aggarwal A, Misro MM, Maheshwari A, Sehgal N, Nandan D (2010) N-acetylcysteine counteracts oxidative stress and prevents hCG-induced apoptosis in rat Leydig cells through down regulation of caspase-8 and JNK. Mol Reprod Dev 77(10):900–909
Ahmed NK, Martin LA, Watts LM, Palmer J, Thornburg L, Prior J et al (1992) Peptidyl fluoromethyl ketones as inhibitors of cathepsin B. Implication for treatment of rheumatoid arthritis. Biochem Pharmacol 44(6):1201–1207
Angliker H, Wikstrom P, Rauber P, Shaw E (1987) The synthesis of lysyifluoromethanes and their properties as inhibitors of trypsin, plasmin and cathepsin B. Biochem J 241:871–875
Aoshiba K, Tamaoki J, Nagai A (2001) Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 281:1392–1401
Apostolova N, Gomez-Sucerquia LJ, Moran A, Alvarez A, Blas-Garcia A, Esplugues A (2010) Enhanced oxidative stress and increased mitochondrial mass during efavirenz-induced apoptosis in human hepatic cells. Br J Pharmacol 160:2069–2084
Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW et al (2002) Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ 9(3):252–263
Cai H, Dikalov S, Griendling KK, Harrison DG (2007) Detection of reactive oxygen species and nitric oxide in vascular cells and tissues. Methods in Molecular Medicine 139:293–311
Cai J, Jones DP (1999) Mitochondrial redox signaling during apoptosis. J Bioenerg Biomembr 31:327–334
Cazanave S, Berson A, Haouzi D, Vadrot N, Fau D, Grodet A et al (2007) High hepatic glutathione stores alleviate Fas-induced apoptosis in mice. J Hepatol 46:858–868
Chao YIY, Ferrari G, Greene LA (1995) N-acetylcysteine-promoted survival of PC12 cells is glutathione-independent but transcription-dependent. J Biol Chem 270(45):26827–26832
Chen J, Rogers SC, Kavdia M (2013) Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann Biomed Eng 41(2):327–337
Demuth H (1990) Recent developments in inhibiting cysteine and serine proteases. J Enzym Inhib 3:249–278
Deneka SM (2000) Thiol-based antioxidants. Curr Top Cell Regul 36:151–180
Esser RE, Angelo RA, Murphey MD, Watts LM, Thornburg LP, Palmer J et al (1993) Cysteine proteinase inhibitors decrease articular cartilage and bone destruction in chronic inflammatory arthritis. Arthritis Rheum 37:236–247
Fabian Z, O’Brien P, Pajecka K, Fearnhead HO (2009) TPCK-induced apoptosis and labelling of the largest subunit of RNA polymerase II in Jurkat cells. Apoptosis 14:1154–1164
Forman HJ, Zhang HQ, Rinna A (2009) Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med 30:1–12
Franco R, Panayiotidis MI, Cidlowski JA (2007) Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem 282:30452–30465
Friesen C, Kiess Y, Debatin KM (2004) A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ 11:S73–S85
Gillibert, M., Dehry, Z., Terrier, M., El Benna, J., and Lederer, F. (2005). Another biological effect of tosylphenylalanylchloromethane (TPCK): it prevents p47 phox phosphorylation and translocation upon neutrophil stimulation. BiochemJ, 386: 549–556
Gmunder H, Roth HS, Eck HP, Gallas H, Mihm S, Droege W (1990) Interleukin-2 mRNA expression, lymphokine production and DNA synthesis in glutathione-depleted T cells. Cell Immunol 130:520–528
Griffith OW (1982) Mechanism of action, metabolism and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 257:13704–13712
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-N-butyl homocysteine sulfoximine). J Biol Chem 254:7558–7560
Guha P, Dey A, Sen R, Chatterjeeb M, Chattopadhyay S, Bandyopadhyay SK (2010) Intracellular GSH depletion triggered mitochondrial Bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J Pharmacol Exp Ther 336(1):206–214
Ha K-H, Byun M-S, Choi J, Jeong J, Lee K-J, Jue D-M (2009) N-tosyl-L-phenylalanine chloromethyl ketone inhibits NF-kB activation by blocking specific cysteine residues of IkB kinase B and p65/RelA. Biochemistry 48:7271–7278
Hampton MB, Orrenius S (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414(3):552–556
Heussler VT, Fernandez PC, Machado J Jr, Botteron C, Dobbelaere DAE (1999) N-acetylcysteine blocks apoptosis induced by N-a-tosyl-L-phenylalanine chloromethyl ketone in transformed T-cells. Cell Death Differ 6:342–350
Jitkaew S, Trebinska A, Grzybowska E, Carlsson G, Nordstrom A, Lehtio J et al (2009) Nα-tosyl-L-phenylalanine chloromethyl ketone (TPCK) induces caspase-dependent apoptosis in transformed human B cell lines with transcriptional downregulation of anti-apoptotic HS1-associated protein X-1 (HAX-1). J Biol Chem 284:27827–27837
Johnson VL, Ko SC, Holmstrom TH, Eriksson JE, Chow SC (2000) Effector caspases are dispensable for the early nuclear morphological changes during chemical-induced apoptosis. J Cell Sci 113(Pt 17):2941–2953
Jones DP, Maellaro E, Jiang S, Slater AFG, Orrenius S (1995) Effects of N-acetyl-L-cysteine on T-cell apoptosis are not mediated by increased cellular glutathione. Immunol Lett 45:205–209
Kirkland RA, Franklin JL (2001) Evidence for redox regulation of cytochrome C release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition. J Neurosci 21:1949–1963
Kowaltowski AJ, Vercesi AE (1998) Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26(3/4):463–471
Lawrence CP, Kadioglu A, Yang A-L, Coward WR, Chow SC (2006) The cethepsin B inhibitor, z-FA-FMK, inhibits human T cell proliferation in vitro and modulates host response to pneumococcal infection in vivo. J Immunol 177:3827–3836
Lennon SV, Martin SJ, Cotter TG (1991) Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif 24:203–204
Liow KY, Chow SC (2013) The cathepsin B inhibitor, z-FA-CMK is toxic and readily induced cell death in human T lymphocytes. Toxicol Appl Pharmacol 272:559–567
Mailloux RJ, Harper ME (2012) Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab 23(9):451–458
Mailloux RJ, McBride SL, Harper M-E (2013) Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci 38(12):592–602
Martin HL, Teismann P (2009) Glutathione—a review on its role and significance in Parkinson’s disease. The Journal of the Federation of American Societies for Experimental Biology 23:3263–3272
Meister A, Griffith OW (1979) Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: possible chemotherapeutic implications. Cancer Treatment Reports 63:1115–1121
Merad-Boudia M, Nicole A, Santiard-Baron D, Saille C, Ceballos-Picot I (1998) Mitochondrial impairment as an early event in the process of apoptosis induced by glutathione depletion in neuronal cells: relevance to Parkinson’s disease. Biochem Pharmacol 56:645–655
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Mytilineou C, Kramer BC, Yabut JA (2002) Glutathione depletion and oxidative stress. Parkinsonism and Related Disorders 8:385–387
Nakagawa S, Arai Y, Mazda O, Kishida T, Takahashi KA, Sakao K et al (2010) N-acetylcysteine prevents nitric oxide-induced chondrocyte apoptosis and cartilage degeneration in an experimental model of osteoarthritis. J Orthop Res 28(2):156–163
Nobel CS, Burgess DH, Zhivotovsky B, Burkitt MJ, Orrenius S, Slater AF (1997) Mechanism of dithiocarbamate inhibition of apoptosis: thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem Res Toxicol 10:636–643
Osseni RA, Debbasch C, Christen MO, Rat P, Warnet JM (1999) Tacrine-induced reactive oxygen species in a human liver cell line: the role of anethole dithiolethione as a scavenger. Toxicol in Vitro 13:683–688
Otto HH, Schirmeister T (1997) Cysteine proteases and their inhibitors. Chem Rev 97:133–171
Pace V, Castoldi L, Pregnolato M (2013) α-Amino-α′-halomethylketones: synthetic methodologies and pharmaceutical applications as serine and cysteine protease inhibitors. Mini Reviews in Medicinal Chemistry 13:988–996
Perez-G M, Cortes JR, Rivas MD, Masa F, Zamorano J (2008) Treatment of cells with n-alpha-tosyl-l-phenylalanine-chloromethyl ketone induces the proteolytic loss of STAT6 transcription factor. MolImmunol 45:3896–3901
Pong S-S, Nuss DL, Koch G (1975) Inhibition of initiation of protein synthesis in mammalian tissue culture cells by L-1-tosylamido-2-phenylethyl chloromethyl ketone. J Biol Chem 250:240–245
Powers JC, Asgian JL, Ekici OD, James KE (2002) Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev 102(12):4639–4750
Powers JC, Gupton BF, Harley AD, Nishino N, Whitley RJ (1977) Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta 485:156–166
Rajah T, Chow SC (2014) The inhibition of human T cell proliferation by the caspase inhibitor z-VAD-FMK is mediated through oxidative stress. Toxicol Appl Pharmacol 278(2):100–106
Ranga V, Kleinerman J, Ip MPC, Sorensen J, Powers JC (1981) Effects of oligopeptide chloromethylketone administered after elastase: renal toxicity and lack of prevention of experimental emphysema. Am Rev Respir Dis 124:613–618
Rasnick D (1985) Synthesis of peptide fluoromethyl ketones and the inhibition of human cathepsin B. Anal Biochem 149(2):461–465
Rauber P, Angliker H, Walker B, Shaw E (1986) The synthesis of peptidylfluoromethanes and their properties as inhibitors of serine proteinases and cysteine proteinases. Biochem J 239:633–640
Rice GC, Bump EA, Shrieve DC, Lee W, Kovacs M (1986) Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res 46:6105–6110
Rossman T, Norris C, Troll W (1974) Inhibition of macromolecular synthesis in Escherichia coli by protease inhibitors. J Biol Chem 249:3412–3417
Schoellmann G, Shaw E (1962) Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry 2(2):252–255
Schotte P, Schauvliege R, Janssens S, Beyaert R (2001) The cathepsin B inhibitor z-FA-FMK inhibits cytokine production in macrophages stimulated by lipopolysaccharide. J Biol Chem 276:21153–21157
Smith HJ (1978) Perspectives in the design of small molecule enzyme inhibitors as useful drugs. J Theor Biol 73:531–538
Steenvoorden DPT, Beijersbergen van Henegouwen GMJ (1998) Glutathione synthesis is not involved in protection by N-acetylcysteine against UVB-induced systemic immunosuppression in mice. Photochem Photobiol 68(1):97–100
Sun HL, Tsai AC, Pan SL, Ding QQ, Yamaguchi H, Lin CN et al (2009) EPOX inhibits angiogenesis by degradation of Mcl-1 through ERK inactivation. Clin Cancer Res 15(15):4904–4914
Sun SY (2010) N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther 9(2):109–110
Van Noorden CJ, Smith RE, Rasnick D (1988) Cysteine proteinase activity in arthritic rat knee joints and the effects of a selective systemic inhibitor, z-Phe-AlaCH2F. J Rheumatol 15:1525–1535
Weis M, Schlegel J, Kass GEN, Holmstrîm TH, Peters I, Eriksson JE et al (1995) Cellular events in Fas/APO-1-mediated apoptosis in JURKAT T lymphocytes. Exp Cell Res 219:699–708
Wispriyono B, Matsuoka M, Igisu H, Matsuno K (1998) Protection from cadmium cytotoxicity by N-acetylcysteine in LLC-PK1 cells. J Pharmacol Exp Ther 287:344–351
Young PR, Connors White AL, Dzido GA (1994) Kinetic analysis of the intracellular conjugation of monochlorobimane by IC-21 murine macrophage glutathione-S-transferase. Biochim Biophys Acta 1201:461–465
Zafarullah M, Li WQ, Sylvester J, Ahmad M (2003) Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60:6–20
Zhang FJ, Lau SS, Monks TJ (2011) The cytoprotective effect of N-acetyl-L-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol Sci 120(1):87–97
Funding
This work was supported by the internal funding provided by Monash University Malaysia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Liow, K.Y., Chow, S.C. The cathepsin B inhibitor z-FA-CMK induces cell death in leukemic T cells via oxidative stress. Naunyn-Schmiedeberg's Arch Pharmacol 391, 71–82 (2018). https://doi.org/10.1007/s00210-017-1436-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1436-6