Genetic diversity of gliadin-coding alleles in bread wheat (Triticum aestivum L.) from Northern Kazakhstan
- Published
- Accepted
- Received
- Academic Editor
- Yuriy Orlov
- Subject Areas
- Genetics, Plant Science
- Keywords
- Allele frequency, Protein electrophoresis, Genetic polymorphism, Gli loci, Gliadins, Bread wheat
- Copyright
- © 2019 Utebayev et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Genetic diversity of gliadin-coding alleles in bread wheat (Triticum aestivum L.) from Northern Kazakhstan. PeerJ 7:e7082 https://doi.org/10.7717/peerj.7082
Abstract
Background
Spring bread wheat (Triticum aestivum L.) represents the main cereal crop in Northern Kazakhstan. The quality of wheat grain and flour strongly depends on the structure of gluten, comprised of gliadin and glutenin proteins. Electrophoresis spectra of gliadins are not altered by environmental conditions or plant growth, are easily reproducible and very useful for wheat germplasm identification in addition to DNA markers. Genetic polymorphism of two Gli loci encoding gliadins can be used for selection of preferable genotypes of wheat with high grain quality.
Methods
Polyacrylamide gel electrophoresis was used to analyse genetic diversity of gliadins in a germplasm collection of spring bread wheat from Northern Kazakhstan.
Results
The highest frequencies of gliadin alleles were found as follows, in Gli1: -A1f (39.3%), -B1e (71.9%), and -D1a (41.0%); and in Gli-2: -A2q (17.8%), -B2t (13.5%), and -D2q (20.4%). The combination of these alleles in a single genotype may be associated with higher quality of grain as well as better adaptation to the dry environment of Northern Kazakhstan; preferable for wheat breeding in locations with similar conditions.
Introduction
Wheat flour remains one of main ingredients in quite a diverse range of foods for human consumption and provides the major proteins gliadins and glutenins. In particular, glutenin can make up at least 40% of the total protein in grain and flour (Qi et al., 2006; Metakovsky et al., 2018). The genetic control of gliadin includes two major genes, Gli-1 and Gli-2, mapped to the short arms of chromosome groups 1 and 6, respectively, with corresponding homeologous genes, Gli-A1, -B1, -D1 and Gli-A2, -B2, -D2 (Metakovsky, Branlard & Graybosch, 2006; Metakovsky et al., 2018). Multiple alleles are typically found for both Gli loci. Each Gli allele encodes the transcription of clusters of subunits, with several components of gliadin proteins showing linked inheritance. Gliadin groups can differ in the number of components, their electrophoretic mobility and molecular weight, and levels of expression (Sozinov & Poperelya, 1980; Obukhova & Shumny, 2016). By its nature, gliadin is a complex protein with several components that can be separated using polyacrylamide gel electrophoresis in aluminium-lactate buffer (pH = 3.1) (Bushuk & Zillman, 1978). The original protocol of gliadin electrophoresis has been since modified (Tkachuk & Metlish, 1980; Khan, Hamada & Patek, 1985; Metakovsky & Novoselskaya, 1991), and was used as the basis for the International standard procedure ISO (ISO, 1993). Gli alleles and their components have been widely studied and identified in International wheat germplasm collections, resulting in published Catalogues. The genetic polymorphism in the composition of Gli alleles in a given genotype was summarised as the ‘Gliadin genetic formula’ (GGF) in the Catalogues for bread wheat (Metakovsky, 1991; Metakovsky et al., 2018) and for durum wheat (Melnikova, Kudryavtseva & Kudryavtsev, 2012).
As reported in many publications, wheat cultivars produced in each separate country often have similar GGF despite the absence of any selection pressure based on gliadins (Xynias, Kozub & Sozinov, 2006; Aguiriano et al., 2008; Salavati et al., 2008; Melnikova et al., 2010; Novoselskaya-Dragovich et al., 2011; Hailegiorgis, Lee & Yun, 2017). A linkage between Gli alleles and other genes or a group of genes encoding favourable traits can be preferable and beneficial for wheat breeding (Chebotar et al., 2012). Therefore, a high frequency of Gli alleles can be used as simple and convenient method based on protein marker analysis for wheat germplasm identification and application in further breeding programs in the same environment.
Currently, molecular markers based on DNA analysis are widely used for genotyping and genetic identification in various crops (Shavrukov, 2016; Jatayev et al., 2017; Scheben, Batley & Edwards, 2017; Burridge et al., 2018). The application of molecular markers was successful in the study of wheat genes controlling such traits as 1,000-grain weight, protein and gluten content (Zhang et al., 2018), grain hardness (Nirmal et al., 2016), flour production from grain milling (Nirmal et al., 2017), and bread quality (Henry, Furtado & Rangan, 2018). Genome editing using CRISPR/Cas9 technology represents a novel method in plants (Khlestkina & Shumny, 2016; Liang et al., 2018; Borisjuk et al., 2019), for production of wheat with low gluten content (Sánchez-León et al., 2018), as required by people allergic to some components of gliadin in traditional wheat cultivars (Palosuo et al., 2001; Pastorello et al., 2007).
Nevertheless, molecular markers are relatively expensive in the equipment and reagents required, in typically well-established molecular laboratories. In contrast, biochemical markers based on proteins such as enzymes and storage proteins offer an alternative method involving cheaper and simpler protocols for crop breeding including wheat (Shewry & Halford, 2001; Ghanti et al., 2009; Al-Doss et al., 2010; Netsvetaev, Akinshina & Bondarenko, 2010; Hailegiorgis, Lee & Yun, 2017). Additionally, protein synthesis is encoded by genes, and modulation of gene expression in response to changes in the environment directly results in different levels of the corresponding proteins.
The aim of this study was to identify and analyse the genetic diversity of the Gli alleles in spring bread wheat (Triticum aestivum L.) collection from Northern Kazakhstan, and to address the question of which alleles of gliadins with highest frequencies are typical for modern wheat produced and cultivated in the dry environment of this region.
Materials and Methods
Wheat germplasm and geographic locations
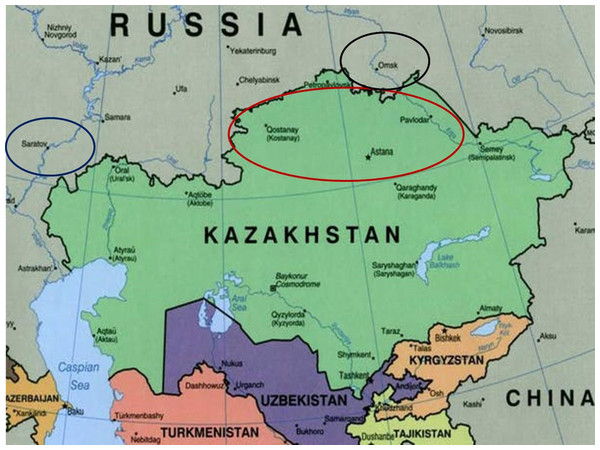
A seed collection of 70 bread wheat cultivars was provided by the A.I. Barayev Research and Production Centre of Grain Farming, Shortandy, Kazakhstan. The studied wheat accessions were bred and produced at different times by Breeding Institutions (Karabalyk Agricultural Breeding Station and Pavlodar Research Institute of Agriculture) in Northern Kazakhstan, as listed in Supplemental Information 1. Additional data for various wheat germplasms from Kazakhstan and neighbouring regions, used for comparison of the results obtained for genetic diversity of Gli alleles in wheats, were retrieved from papers published earlier (Supplemental Information 2). In the map (Fig. 1), Northern Kazakhstan and two nearby regions in Russia with wheat Breeding Research Organisations—Saratov (European part) and Omsk (Siberia) are indicated by ovals. Briefly, Northern Kazakhstan is located at latitude 51°–55°N and longitude 61°–79°E, with a territory of about 565K km2 comprised largely of steppe or low-hilled forest. The strong continental climate is characterized by a cold and long winter with high winds, but a hot and short summer season. Average winter/summer temperatures are about −18 °C and 20 °C but extreme levels of −45 °C and 41 °C, respectively can also be reached.
Figure 1: A map of Kazakhstan and nearby regions of Russia.
The red oval shows Northern Kazakhstan, while the Russian regions, Saratov (European part) and Omsk (Siberia), are shown in blue and black, respectively (Kusznir, 2015). The map was taken from the website: http://theconversation.com/russias-borders-moscows-long-alliance-with-kazakhstan-is-strong-but-not-unbreakable-36457.Electrophoresis and identification of Gli alleles
Polyacrylamide gel electrophoresis was carried out following a method published earlier (Metakovsky & Novoselskaya, 1991). Gliadins were extracted from individually milled seeds by adding 150 µL of 70% ethanol. Acrylamide polymerization was initiated by 50 µL of 3% H2O2 in 45 mL of gel solution. A vertical gel tank, model VE-20 (Helicon, Moskva, Russia) was used and the gels were 17.8 × 17.5 × 1 (mm) in size. Electrophoresis was conducted at optimal temperature below 20 °C, at 520 V for 4 h. 10% trichloroacetic acid supplied with 0.05% of Coomassie Brilliant Blue R-250 in ethanol (Sigma-Aldrich, St. Louis, MO, USA) was used for gel fixation and staining. The identification of gliadin components was conducted using the Protein Catalogue (Metakovsky, 1991). Genes that encoded gliadins were identified in accordance to the Gene Catalogue developed by McIntosh et al. (2008) for Gli-1 (-A1, -B1, and -D1) and for Gli-2 (-A2, -B2, and -D2). Alleles of the Gli locus were designated as additional Latin letters and total GGFs were used as recommended for wheat cv. Chinese Spring with the following in full GGF: Gli-A1a, Gli-B1a, Gli-D1a, Gli-A2a, Gli-B2a, Gli-D2a; and abbreviated GGF: a , a , a , a , a , a.
Computer and statistical analysis
Intra-population diversity (μ ± Sμ) and frequency of rare alleles (h ± Sh) were calculated following the method published by Zhivotovsky (1991), while genetic diversity (H) was calculated by the formula described by Nei, where pi is the frequency of alleles (Nei, 1973)
Phylogenetic tree construction and clustering analysis among the studied wheat genotypes was carried out using the computer program software Statistica 6.0. (Statsoft, USA) following instructions for Ward’s method with Manhattan distances and applied for GGFs. The ‘Data Standardization’ option was applied to transform the allele identifications in letters into numbers suitable for the computer program software (http://documentation.statsoft.com/STATISTICAHelp.aspx?path=Cluster/ClusterAnalysis/Examples/Example1JoiningTreeClustering).
Results
Gli allele diversity
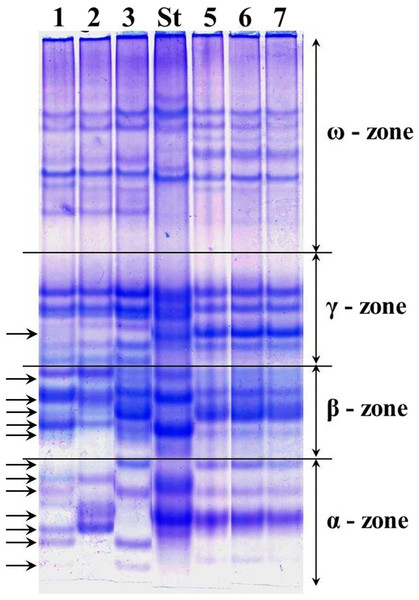
The alleles of loci Gli-1 and Gli-2 identified in the wheat germplasm collection (70 accessions) and their GGFs are presented in Supplemental Information 1. Most of the studied wheats were monomorphic (76%) while the remaining 24% accessions were polymorphic. Grains of such polymorphic wheats consisted of a mixture of genotypes, with variable alleles in one or more Gli loci. For example, several biotypes of gliadins were present in polymorphic cv. Lutescence 65 with various spectra of gliadin components in three zones, α-, β- and γ, but identical in ω-zone (Fig. 2, lanes 1–3). Plants of cv. Byrlestik were monomorphic and represent the single type of gliadin spectrum (Fig. 2, lanes 5–7). In general, the inter-cultivar polymorphic alleles of Gli encode the biosynthesis of gliadin components located in all four zones (α-, β-, γ- and ω-zones) of the gliadin spectrum on the polyacrylamide gel electrophoregram (Fig. 2).
Figure 2: Electrophoregram of the gliadin spectrum of polymorphic cv. Lutescence 65 (Lanes 1–3) in comparison to cv. Bezostaya 1 (Lane 4, used as a Standard) and monomorphic cv. Byrlestik (Lanes 5–7).
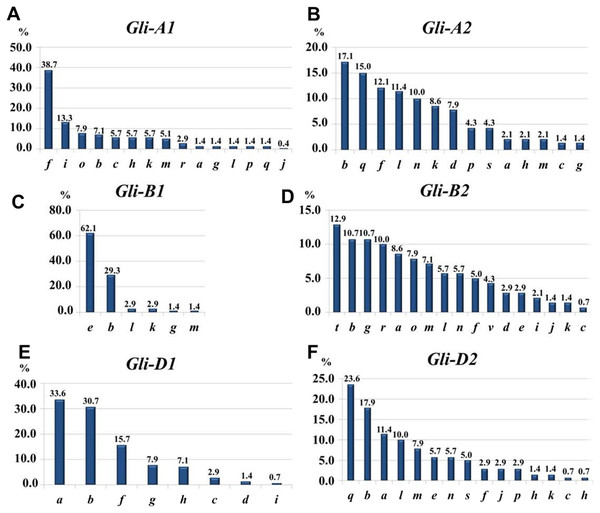
Subfractions α, β, γ and ω with polymorphic bands are indicated.At the Gli-1 locus, the highest frequencies were found in alleles Gli-A1f (38.7%), -B1e (62.1%), and -D1a (33.6%). In contrast, the level of highest frequency of alleles was smaller at the Gli-2 locus and comprising Gli-A2b (17.14%), -B2t (12.9%), and -D2q (23.6%). Therefore, the GGF of the majority of wheats bred and cultivated in Northern Kazakhstan is: f, e, a, b, t, q, based on highest frequencies of the alleles. In total, results of gliadin electrophoresis revealed six and eight alleles in Gli-B1 and Gli-D1 loci, respectively, 14 alleles in each of three loci, Gli-A1, Gli-A2 and Gli-D2, and 17 alleles in Gli-B2 locus (Fig. 3).
Figure 3: Allele frequencies in Gli loci identified in the studied collection of 70 accessions of spring bread wheat from Northern Kazakhstan.
.Levels of genetic diversity (H), intra-population diversity (μ) and frequencies of rare alleles (h) in 70 wheat accessions from Northern Kazakhstan were calculated based on allele frequencies in Gli loci from Supplemental Information 1 and are presented in Table 1A. For comparison, four other studies of wheat from Northern Kazakhstan (Supplemental Information 2) with partial overlap in the accessions studied were joined together with the current study, with the combined results for 139 wheat accessions in total from Northern Kazakhstan presented in Table 1B.
| Diversity estimates | Gliadin-coding Gli loci | |||||
|---|---|---|---|---|---|---|
| A1 | B1 | D1 | A2 | B2 | D2 | |
| A. 70 wheat accessions from Northern Kazakhstan (Supplemental Information 1) | ||||||
| H | 0.81 | 0.53 | 0.76 | 0.89 | 0.92 | 0.87 |
| μ ± Sμ | 10.43 ± 0.73 | 3.65 ± 0.35 | 6.00 ± 0.41 | 12.04 ± 0.58 | 15.13 ± 0.64 | 11.56 ± 0.63 |
| h ± Sh | 0.25 ± 0.05 | 0.39 ± 0.06 | 0.25 ± 0.05 | 0.14 ± 0.04 | 0.11 ± 0.04 | 0.17 ± 0.05 |
| B. 139 wheat accessions from Northern Kazakhstan (Supplemental Informations 1 and 2) | ||||||
| H | 0.80 | 0.45 | 0.75 | 0.90 | 0.93 | 0.89 |
| μ ± Sμ | 12.32 ± 0.71 | 4.33 ± 0.18 | 6.78 ± 0.40 | 14.44 ± 0.61 | 17.37 ± 0.57 | 13.88 ± 0.56 |
| h ± Sh | 0.32 ± 0.04 | 0.52 ± 0.04 | 0.32 ± 0.04 | 0.20 ± 0.03 | 0.13 ± 0.03 | 0.18 ± 0.03 |
Most of the results presented in Tables 1A and 1B are very similar, indicating for a representable subset of 70 wheat accessions for Northern Kazakhstan. For example, genetic diversity, H, was highest in loci Gli-B2 (0.92/0.93) and Gli-A2 (0.89/0.90), while smallest H = 0.53/0.45 were calculated for Gli-B1 in both parts of Table 1. The same trend has been found for intra-population diversity μ = 15.13∕17.37 and 12.04/14.44 for alleles of loci Gli-B2 and Gli-A2, respectively, with maximal number of the identified alleles (17 and 14 alleles, respectively). In contrast, the locus Gli-B1 had the smallest value of μ = 3.65∕4.33 with only six identified alleles as the smallest number in this study and with highest frequency of the Gli-B1e allele (Table 1, Fig. 3).
The structure of intra-population diversity can be characterised by the frequencies of rare alleles (h). A population can be estimated as ‘balanced’ if values of h are less than 0.3 and as small as possible (Zhivotovsky, 1980). Therefore, the most balanced for intra-population diversity was found for locus Gli-B2 (h = 0.11/0.13), while locus Gli-B1 had the highest value for h (0.39/0.52) due to the highest frequency of a single allele, Gli-B1e.
The highest frequencies of each gliadin allele in the combined group of 139 wheat accessions were accounted as: Gli-A1f (39.3%), -B1e (71.9%), -D1a (41.0%), -A2q (17.8%), -B2t (13.5%), and -D2q (20.4%). The GGF in the analysis of 139 wheat accession was as follows: f, e, a, q, t, q, and almost identical to those identified in the current study, with only a single difference for Gli-A2- q or -b. Therefore, the most typical GGF in wheat accessions from Northern Kazakhstan can be identified as: f, e, a, q + b, t, q.
Comparative phylogenetic analysis of the biodiversity of gliadin- coding loci in bread wheat from Northern Kazakhstan and other origins
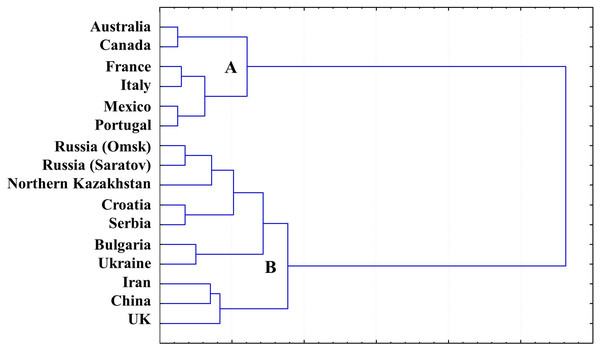
A gliadin dendrogram (Fig. 4) was established based on a cluster analysis of our combined current and previously published results of allele variation in the Gli loci and GGF, in wheat originating from Northern Kazakhstan (Supplemental Information 1 and 2) and other publicly available data for wheat from other countries (Table 2).
Figure 4: Gliadin dendrogram showing the allele diversity in Gli loci of bread wheat from Northern Kazakhstan and other countries.
| Countries/regions | Gliadin coding loci Gli | References | |||||
|---|---|---|---|---|---|---|---|
| A1 | B1 | D1 | A2 | B2 | D2 | ||
| Australia | g | b | f | c | c | w | Metakovsky et al. (2018) |
| Canada | m | d | j + a | m | c | h + m | |
| France | af + c | b + f | b | m | o + c | h | |
| Italy | a | g | k | g + o | o | a | |
| Mexico | o + a | d + b | b + a | f | c | m + j | |
| Portugal | a | c + l | b | f | c | c + j | |
| Russia (Saratov) | f | e | a | q | s | e | |
| Bulgaria | b + a | b | b | b + g | b | b | |
| Croatia | a + b | e | a | e | e | m + a | |
| Serbia | a + b | b + l | b | b + g | b | b + a | |
| Ukraine | b | b | g | f + b | b | e | |
| Russia (Omsk) | f | e | a | q | b | a | Novoselskaya-Dragovich, Fisenko & Puhalskii (2013) |
| Iran | f | f | b | g + l | o | a + n + h | Salavati et al. (2008) |
| China | o + a | l + e | a + f | g + f | I + h | b + a | Novoselskaya-Dragovich et al. (2011) |
| UK | f | f | b | l | g | a | Chernakov & Metakovsky (1994) |
| Northern Kazakhstan | f | e | a | q | t | q | Current study |
Two major Clades (designated as A and B) were found in GGF, with strong separation of the analysed accessions. Wheat genotypes from Australia, America and Western Europe form Clade A, while the more diverse Clade B includes accessions with Gli alleles mostly distributed in Eastern Europe and Asia, with the exception of the UK. As expected, all wheat cultivars from Northern Kazakhstan had GGF most closely related to Russian wheats, particularly those developed in the two big Breeding Research Institutes in Saratov and Omsk, in the European and Siberian part of Russia, respectively. These regions are very close to Northern Kazakhstan geographically (Fig. 1), and also have a long history of exchange of wheat germplasms within the former Soviet Union.
Discussion
The presented study is an important part of the breeding program for seed quality in wheat, to illustrate breeder selections for wheat genotypes with various combinations of gliadin alleles. The received results can be used as the basis of a breeding strategy for wheat genotype selection with preferred GGF and favourable combinations of Gli alleles. In the current study, genetic origin, gliadin characteristics and the value of breeding for alleles in each of gliadin-coding loci in 70 wheat accessions will be discussed in separate sub-sections.
Locus Gli-A1
Fifteen Gli-A1 alleles were identified in the current study in wheat cultivars from Northern Kazakhstan, out of 29 alleles published in the recent Catalogue of gliadin-coding genes (Metakovsky et al., 2018). The highest frequency (0.39) was found in genotypes with allele f. The wide-spread occurrence of the allele f in 27 wheat accessions from Northern Kazakhstan out of the 70 studied seems to be related to introgression of the following high grain quality cultivars: Cesium 111 (f), Albidum 24 (f) and Saratovskaya 29 (j + f) in the early stages of the wheat breeding process in Kazakhstan (Metakovsky et al., 2006).
The possible origin and spread of other Gli-A1 alleles, i, o and b, is likely also related to wheat cultivars from Russia. For example, a series of cultivars entitled Omskaya 20, 22 and 23, originating from the forest-steppe zone of South-Western Siberia, with allele Gli-A1o, seems to be used in the exchange breeding process (Metakovsky et al., 2006). This conclusion is similar to those in our previous published results using a different set of wheat cultivars from Northern Kazakhstan (Utebayev et al., 2016).
Locus Gli-B1
Very limited genetic diversity was found in the Gli-B1 locus, where the single allele e showed the absolute highest frequency at 62% (Fig. 3). It is important to note that this allele is quite widely distributed, especially in southern regions of European Russia (Novoselskaya-Dragovich et al., 2003) as well as in South-Eastern and South-Western Siberia, which are close and directly neighbouring to Northern Kazakhstan, respectively (Nikolaev, Pukhal’sky & Upelniek, 2009). The occurrence and quite frequent distribution of the allele Gli-B1e may be directly related to the actively-used popular Russian drought tolerant cultivars with elite grain quality from the Saratov region: Albidum 43 (f, e, a, q, o, a), Lutescence 62 (j, e, a, q, o, a), and Saratovskaya 29 (j+f, e, a, q + s, q + s, e) (Metakovsky et al., 2006). However, these cultivars had some disadvantages, particularly a sensitivity to a range of diseases (Morgounov, Rosseeva & Koyshibayev, 2007).
The second allele, Gli-B1b, with three-fold less frequency (29%) has a much wider distribution among wheat cultivars from Scandinavian countries to Australia (Metakovsky et al., 2018) and may therefore indicate the wide adaptability of genotypes with this allele. It is very likely that the Gli-B1b allele is originated from historic and classical winter wheat cultivars bred in the former Soviet Union, Besostaya 1 (b, b, b, b, b, b) and Mironovskaya 808 (f, b, g, n, m, e) (Metakovsky et al., 2006).
Locus Gli-D1
There were eight alleles identified in this locus in the studied wheat accessions, which is exactly half of all that were published in the recent Catalogue of gliadin-coding loci (Metakovsky et al., 2018). Three alleles showed the highest range of frequencies: Gli-D1a, 0.34; -b, 0.31; and -f, 0.16 (Fig. 3). However, the spectrum of genetic diversity in the present study slightly differed from our paper published earlier with another set of bread wheat accessions from Northern Kazakhstan, where only four Gli-D1 alleles were identified with the following frequencies: allele a, 44.2%; and each of alleles f and i, 23.3%, respectively (Utebayev et al., 2016).
Similar to those indicated for other alleles above, Russian wheat cultivars were widely used in the initial breeding programs in Northern Kazakhstan. Therefore, it is very likely that the most commonly distributed allele, a, is originated from one or several cultivars, particularly Albidum 43, Lutescence 62, or Saratovskaya 29 (Chernakov & Metakovsky, 1994; Nikolaev, Pukhal’sky & Upelniek, 2009). Additionally, this allele, Gli-D1a, had quite high frequencies among wheat cultivars in Southern Kazakhstan, with a very different environment, but the origin of the allele a from the former Soviet Union wheat germplasm genepool is not in doubt (Absattarova, 2002). This statement is in complete consensus with data for GGF in Kazakh wheats published in a recent review (Metakovsky et al., 2018). The comparison of world-wide distribution of Gli allele a among wheat genotypes bred and grown in Croatia, Finland and Spain (Sontag-Strohm, 1997; Metakovsky et al., 2018), indicated for a possible association between allele Gli-D1 a with adaptability of wheat plants to various environments.
It is important to note that two Gli-D1 alleles, a and f, encode the synthesis of almost identical spectra of gliadin components. The only additional gliadin component present with smaller size in the γ-zone of protein electrophoresis was recorded in wheat genotypes Gli-D1 with allele a but not with allele f. Therefore, it is hypothesised that wheat genotypes Gli-D1a and -f can have very similar gliadin gene nucleotide sequences (Chebotar et al., 2012).
The moderately distributed allele b is also very likely to have originated from foreign wheat accessions introgressed earlier in the Kazakh breeding program. However, it is interesting that the Gli-D1b allele originates from a very different genepool of winter wheat, rather than spring wheat. This statement is based on published data showing a quite high distribution of the allele b among winter wheat, but not in spring wheat, in the former Soviet Union (Kozub et al., 2009; Novoselskaya-Dragovich et al., 2015). Therefore, we can speculate that the possible introgression of the Gli-D1b allele from winter wheat can indicate for the wide adaptability of wheat genotypes, regardless of their responses to cold and vernalisation.
Locus Gli-A2
The Gli-2 gene is much more diverse in wheat, where the smallest number of alleles were recorded in Gli-A2 and accounted for 14 (Fig. 3) of the 39 registered in the recent Catalogue of Gli alleles (Metakovsky et al., 2018). The most commonly distributed alleles among the studied wheat cultivars from Northern Kazakhstan were: Gli-A2b (17.1%), -f (12.1%), and -q (15.0%). The first allele b was very typical for wheat cultivars from very diverse geographical regions and had similarities to wheats from the UK, Eastern Europe and the Krasnodar region in the southern part of Russia (Metakovsky et al., 2018). Winter wheat germplasm accessions also had about 22% of the allele Gli-A2b (Novoselskaya-Dragovich et al., 2015), and this allele is particularly spread among wheat cultivars with high tolerance to cold temperatures (Markarova, 2015). This leads us to the conclusion that the Gli-A2b allele may be associated with genotypes with high adaptability to unfavourable conditions for plant growth.
The allele Gli-A2f was present in wheat cultivars originating from the Saratov region, Russia (Novoselskaya-Dragovich, Fisenko & Puhalskii, 2013) and in some winter wheat cultivars (Novoselskaya-Dragovich et al., 2015) but is known to show the highest frequencies in spring wheat from Mexico and Portugal (Metakovsky et al., 2018).
The third allele, Gli-A2q, was very likely introgressed and spread widely in wheat cultivars in Northern Kazakshtan from germplasm originating from the nearby Russian regions of Saratov and Omsk (Novoselskaya-Dragovich, Fisenko & Puhalskii, 2013). For example, cv. Lutescence 62 was widely used for hybridisations in Kazakhstan with GGF (j, e, a, q, o, a) from the Saratov Breeding Institute, and it was consequently bred during individual selection of plants of the original historical cv. Poltavka (f + j, e, a, q+ k, o, a + e) (Rutz, 2005; Metakovsky et al., 2006). The influence of the wheat genepool originating from the Saratov region on the wheat breeding program in Northern Kazakhstan was described in the genetic polymorphism of Gli alleles in papers published a relatively long time ago (Sozinov, Metakovsky & Koval, 1986; Metakovsky et al., 1988). However, among Kazakh wheat cultivars with elite quality of grain, only the allele Gli-A2q had the highest frequency of distribution, indicating for a possible genetic association with high grain quality (Dobrotvorskaya et al., 2009).
Locus Gli-B2
Seventeen out of 45 Gli alleles described in recent Catalogues (Metakovsky et al., 2018) were identified and analysed in the current study. The highest frequency was found for the allele Gli-B2t, 12.8%, followed by 10.7% for alleles -b and -g, respectively. The origin of the first allele t remains unclear because it was registered as a minor Gli allele in some modern wheat cultivars from the Omsk Breeding Station, Russia (Chernakov & Metakovsky, 1994). We can propose that the origin of the allele Gli-B2t is likely related to the old Russian cv. Cesium 111 used for hybridisations with GGF (f, m, i, j, t, i) and published earlier (Metakovsky et al., 2006; Morgounov, Rosseeva & Koyshibayev, 2007).
The occurrence and distribution of allele Gli-B2b is definitely related to the use and introgression of wheat accessions from Eastern Europe and Russia, where this allele was exclusively present (Metakovsky et al., 2018). In contrast, the Gli allele g very likely originates from one of the wide geographically dispersed countries such as the Scandinavian group (Metakovsky et al., 2018), the UK (Chernakov & Metakovsky, 1994), France (Metakovsky & Branlard, 1998), and China (Novoselskaya-Dragovich et al., 2011).
Locus Gli-D2
The sixth and last gliadin-coding locus, Gli-D2, was present with 14 alleles. The three most widely distributed alleles were q, b and a, with corresponding percentage of frequencies: 23.5%, 17.8% and 11.4%, respectively. In the comparison with gliadin allele distributions, Gli-D2b was originated from Russian wheat germplasm (Metakovsky et al., 2018). Both q and a alleles were widely distributed in local wheats from Northern Kazakhstan, and regarding our previous study, allele Gli-D2a was for the first time found in three Kazakh wheat cultivars, Milturum 45, Tzelinogradka and Snegurka (Utebayev et al., 2016). These three cultivars were included in wheat breeding in Northern Kazakhstan as genetic donors, and the first two of them (Milturum 45 and Tzelinogradka) were bred from original, old and polymorphic cv. Cesium 111 with GGF—f, m, i, j, t, a+ e (Metakovsky et al., 2006). It is more likely that modern Kazakh wheat genotypes with allele Gli-D2a had a pedigree progenitor from one of the biotypes of cv. Cesium 111. Less likely, but still possible, is that the origin of the a allele is from other countries where it was found, such as Croatia, Germany, France, Holland, Italy, Scandinavian countries, Spain or the UK (Metakovsky et al., 2018), indicating for a possible wide interest for wheat breeding programs.
Comparison of genetic diversity between Gli-1 and Gli-2 alleles
In both our current and previous study (Utebayev et al., 2016), the three most popular and widely distributed modern spring bread wheat cultivars from Northern Kazakhstan with elite grain quality have the following GGF: Akmola 2 (g, e, a, i, e, s), Astana (g + j, e, f + i, p, h, b), and Karabalykskaya 90 (i + m + f, e, a + g, q + l, v, a) (Supplemental Information 2). These cultivars have a similar composition of alleles in the gene Gli-1, with three homeologous loci (-A1, -B1 and -D1) to wheat cultivars with very high grain quality from the Russian Breeding Institutes, Saratov and Omsk. Therefore, it was hypothesised that allele compositions in each of three loci of Gli-1 were directly related to grain and baked bread quality and its improvement (Li et al., 2009; Novoselskaya-Dragovich, Fisenko & Puhalskii, 2013). In contrast, allele compositions in the second gene Gli-2 with three homeologous loci (-A2, -B2 and -D2) located in chromosome group 6, were genetically associated with possible adaptation of plants to a dry environment (Novoselskaya-Dragovich, Fisenko & Puhalskii, 2013).
Such a conclusion, made from the comparison between Gli-1 and Gli-2 genes, may explain how non-pedigree related wheat cultivars from various geographic regions with a different climate have very similar or identical compositions of Gli-1 alleles. This is because one of the main targets of wheat breeding is the production of wheat with elite quality of grain and baked bread, where genetic diversity for allele composition in Gli-1 is much smaller than in Gli-2: 14, 6 and 8 alleles for Gli-A1, -B1 and -D1; and 14, 17 and 14 alleles for Gli-A2, -B2 and -D2, respectively (Fig. 3). It is possible that a single perfect pedigree genotype with excellent grain quality was used as a progenitor in many modern wheat cultivars, providing limited genetic variability in allele composition of Gli-1. In contrast, the Gli-2 gene, with much wider variability in allele compositions, was more likely involved in plant adaptation to a dry environment. Because such environments are quite variable in different countries and geographic regions, it may be reflected in and explain the higher variability in allele diversity in Gli-2. The presented results reflect the efforts of wheat breeders over many years of artificial selection based on phenotyping variability in grain quality and tolerance to dry environments, as apparent in the results of genetic diversity in both gliadin-coding genes based on gliadin analyses.
Conclusions
Genetic diversity in the alleles of gliadin-coding genes Gli-1 and Gli-2 was studied, and gliadin genetic formulas were established following the results of gliadin electrophoresis in a set of 70 spring bread wheat cultivars from Northern Kazakhstan. The Gli alleles with highest frequencies in the studied wheat material were identified as follows: Gli-A1f (39.3%), -B1e (71.9%), -D1a (41.0%), -A2q (17.8%), -B2t (13.5%), and -D2q (20.4%). This allele combination of both Gli genes was the most widely distributed in Northern Kazakhstan, and genotypes with such gliadin formula can be used as prospective breeding material for elite grain quality and better adaptability to the dry environment of the Northern Kazakhstan region and for wheat breeding under similar conditions.
Supplemental Information
Gliadin genetic formulas (Current study)
Gliadin genetic formulas of bread wheat from Northern Kazakhstan (Current study).
Gliadin genetic formulas (Previous studies)
Gliadin genetic formulas of bread wheat from Northern Kazakhstan (Previous studies).