An integrative approach to infer systematic relationships and define species groups in the shrub frog genus Raorchestes, with description of five new species from the Western Ghats, India
- Published
- Accepted
- Received
- Academic Editor
- Diogo Provete
- Subject Areas
- Biodiversity, Ecology, Evolutionary Studies, Taxonomy, Zoology
- Keywords
- Anura, Bioacoustics, Biodiversity hotspot, Diagnostic characters, Eye colour, Integrative taxonomy, Molecular phylogeny, Morphology, Peninsular India, Rhacophoridae
- Copyright
- © 2021 Garg et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. An integrative approach to infer systematic relationships and define species groups in the shrub frog genus Raorchestes, with description of five new species from the Western Ghats, India. PeerJ 9:e10791 https://doi.org/10.7717/peerj.10791
Abstract
The genus Raorchestes is a large radiation of Old World tree frogs for which the Western Ghats in Peninsular India is the major center for origin and diversification. Extensive studies on this group during the past two decades have resolved long-standing taxonomic confusions and uncovered several new species, resulting in a four-fold increase in the number of known Raorchestes frogs from this region. Our ongoing research has revealed another five new species in the genus, formally described as Raorchestes drutaahu sp. nov., Raorchestes kakkayamensis sp. nov., Raorchestes keirasabinae sp. nov., Raorchestes sanjappai sp. nov., and Raorchestes vellikkannan sp. nov., all from the State of Kerala in southern Western Ghats. Based on new collections, we also provide insights on the taxonomic identity of three previously known taxa. Furthermore, since attempts for an up-to-date comprehensive study of this taxonomically challenging genus using multiple integrative taxonomic approaches have been lacking, here we review the systematic affinities of all known Raorchestes species and define 16 species groups based on evidence from multi-gene (2,327 bp) phylogenetic analyses, several morphological characters (including eye colouration and pattern), and acoustic parameters (temporal and spectral properties, as well as calling height). The results of our study present novel insights to facilitate a better working taxonomy for this rather speciose and morphologically conserved radiation of shrub frogs. This will further enable proper field identification, provide momentum for multi-disciplinary studies, as well as assist conservation of one of the most colourful and acoustically diverse frog groups of the Western Ghats biodiversity hotspot.
Introduction
The genus Raorchestes Biju, Shouche, Dubois, Dutta, and Bossuyt, 2010 was described to accommodate a large radiation of Asian Shrub frogs currently comprising 67 species with distributions right from the Western Ghats in Peninsular India, up to central, eastern and northeastern India, Nepal, Bangladesh, Myanmar, southern China, Thailand, Malaysia, Laos, Cambodia, and Vietnam (Biju et al., 2010; AmphibiaWeb, 2020; Frost, 2020). The genus is closely related to Pseudophilautus Laurent, 1943, another radiation of nearly 80 species chiefly restricted to Sri Lanka with only three recognised members from southern India (Biju et al., 2010; Meegaskumbura et al., 2019). Phylogenetically, the two genera have shown a sister-group relationship (e.g., Li et al., 2009; Yu et al., 2009; Biju et al., 2010; Pyron & Wiens, 2011; Vijayakumar et al., 2016) that has become debatable, especially with recent descriptions of new closely related taxa (e.g., Abraham et al., 2013; Li et al., 2013; Meegaskumbura et al., 2015, 2019; Chan, Grismer & Brown, 2018). Until a few decades ago, Raorchestes and Pseudophilautus members were included in a single genus Philautus Gistel, 1848, which has now mostly been restricted to the Sunda Shelf and Philippines (Biju et al., 2010; Li et al., 2013; Wostl et al., 2017; AmphibiaWeb, 2020; Frost, 2020). The presumed occurrence of genus Philautus in India based on literature prior to Biju et al. (2010) (such as, Dubois, 1987; Bossuyt & Dubois, 2001; Delorme et al., 2006), and the inclusion of at least six Indian taxa in Philautus thus far (Philautus dubius (Boulenger, 1882), Philautus garo (Boulenger, 1919), Philautus kempiae (Boulenger, 1919), Philautus kempii (Annandale, 1912), Philautus microdiscus (Annandale, 1912), and Philautus namdaphaensis Sarkar & Sanyal, 1985) (Frost, 2020) is erroneous and should be considered uncertain until confirmed by future evidence.
The Western Ghats mountain range in Peninsular India is a major center for the origin and diversification of Raorchestes frogs (Biju et al., 2010; Vijayakumar et al., 2016), and it is here that the genus reaches its highest diversity (~80%) (Jiang et al., 2020) with near absolute endemism. Until the end of twentieth century, the diversity of shrub frogs in the Western Ghats comprised only 10 of the presently recognised Raorchestes species, which were primarily described by colonial researchers (Jerdon, 1853; Günther, 1876; Boulenger, 1882, 1891; Annandale, 1919) followed by limited post-colonial descriptions (Rao, 1937). It was also common belief that the Western Ghats and Sri Lanka, which together form a single globally recognised biodiversity hotspot unit (Myers et al., 2000; Mittermeier et al., 2004), share several known shrub frogs (Kirtisinghe, 1957; Inger et al., 1984; Dutta & Manamendra-Arachchi, 1996). However, based on extensive field explorations in the Western Ghats, Biju (2001) not only doubted the occurrence of shared species between these regions, suggesting that several confused members likely represent undescribed taxa, but also showed the presence of an unprecedentedly high number of previously undiscovered and new tree frog taxa within the Western Ghats. At the same time, Bossuyt & Dubois (2001) taxonomically reviewed the genus Philautus sensu lato resulting in nomenclatural stability and the transfer of several species formerly attributed to various other genera such as Ixalus, Phyllomedusa, and Rhacophorus (Jerdon, 1853, 1870; Günther, 1876; Boulenger, 1882, 1891; Annandale, 1919; Ahl, 1931). Subsequent studies also provided evidence for the fact that the shrub frogs of the Western Ghats and Sri Lanka are endemic to the respective regions, with considerably high undescribed diversity in both regions (Pethiyagoda & Manamendra-Arachchi, 1998; Biju, 2001; Meegaskumbura et al., 2002; Bossuyt et al., 2004). Altogether, what transpired was a spate of new species descriptions from yet unexplored as well as previously explored regions across the Western Ghats, with an ever-increasing estimate of its known shrub frog diversity (Bossuyt, 2002; Kuramoto & Joshy, 2003; Biju & Bossuyt, 2005a, 2006, 2009; Gururaja et al., 2007; Biju et al., 2010). The recognition of Raorchestes (Biju et al., 2010) provided further stability to the generic allocations of Asian shrub frogs, with frequent new discoveries holding up the genus as one of the most actively researched anuran groups of the Western Ghats during the following decade (Zachariah et al., 2011, 2016; Seshadri, Gururaja & Aravind, 2012; Padhye et al., 2013; Vijayakumar et al., 2014, 2016; Priti et al., 2016; Gowande, Ganesh & Mirza, 2020). It is no surprise that since the turn of the century 43 new species have been formally described, resulting in a four-fold increase in the number of Raorchestes frogs known from this region within just two decades.
Despite active research and frequent descriptions of new species, there has been a lack of integrative understanding of species in this large and rather morphologically conserved group of frogs ever-since the formal description of the genus. Although integrative approaches have increasingly been employed to delimit and describe new species during the past decade (e.g., Vijayakumar et al., 2014; Priti et al., 2016; Zachariah et al., 2016), such studies largely rely on older works based on genus Philautus (e.g., Bossuyt & Dubois, 2001; Biju & Bossuyt, 2009) for comparisons with previously known taxa. Vijayakumar et al. (2014, 2016) provided comprehensive phylogenies of Western Ghats Raorchestes frogs with lineage-based grouping of species; however, the diagnosis of these phylogenetically identified species assemblages based on morphological, acoustic, or behavioral characters remains unattempted.
Vocalisation in anurans has long been a subject of interest to behavioral ecologists, evolutionary biologists, physiologists (Gerhardt & Huber, 2002; Wells, 2007), and more recently to taxonomists as discussed elaborately in a review by Köhler et al. (2017). Acoustic characters are known to be useful in identification and delimitation of species, and vocalisations all the more conspicuous since they serve as premating isolation mechanisms carrying useful evolutionary and systematic information (Ryan, 2001; Bee, Suyesh & Biju, 2013a; Köhler et al., 2017). As taxonomic studies are increasingly becoming integrative in nature, call characters have gained importance in Indian anuran systematics (e.g., Kanamadi, Kadadevaru & Schneider, 2001; Kuramoto et al., 2007; Grosjean & Dubois, 2011; Bee, Suyesh & Biju, 2013a, 2013b; Garg et al., 2017, 2019). Specifically in the case of genus Raorchestes, out of the 55 species known from Peninsular India (prior to the present study), the call structure was previously known only for eleven species, namely Raorchestes (as Philautus) tuberohumerus, Raorchestes (as Philautus) luteolus, R. kakachi, R. graminirupes, R. flaviocularis, R. chalazodes, R. honnametti, R. kollimalai, R. sanctisilvaticus, R. silentvalley, and R. lechiya (Kuramoto & Joshy, 2001; Seshadri, Gururaja & Aravind, 2012; Bee, Suyesh & Biju, 2013b; Vijayakumar et al., 2014; Priti et al., 2016; Zachariah et al., 2016; Mirza et al., 2019; Gowande, Ganesh & Mirza, 2020). Due to lack of available acoustic data for a majority of Raorchestes species, vocalisation has not been effectively utilized for integrative systematic studies on this taxonomically challenging genus, and has become imperative for strengthening our understanding of systematic relationships particularly among several morphologically cryptic species.
Several anuran studies have emphasized on the usefulness of eye colour and pattern as a character for species level identification (e.g., Duellman, 1970; Glaw & Vences, 1997; Amat, Wollenberg & Vences, 2013; Glaw et al., 2018) or study of ontogenetic colour changes (e.g., Hoffman & Blouin, 2000; Biju et al., 2013); however, the application of this trait for field identification of frogs is seldom attempted (Glaw & Vences, 1997; Stuebing & Wong, 2000). Among the ~230 known frog species of the Western Ghats, genus Raorchestes is the most remarkably diverse in terms of skin colouration as well as eye colours and patterns. This group is also notorious for lacking distinct morphological characters between closely related species and high intraspecific variability in body colour and patterns in some cases, together with the relatively small adult size of its members, which makes sole reliance on morphology-based identification and systematic studies rather challenging (Bossuyt & Dubois, 2001; Biju & Bossuyt, 2009; Vijayakumar et al., 2014). In this backdrop, eye colouration as a character for species-level identification as well as interspecific and group-level comparisons comprehensively across the genus remains overlooked, other than a few new species descriptions (e.g., Gururaja et al., 2007; Biju & Bossuyt, 2009; Vijayakumar et al., 2014; Zachariah et al., 2016).
In this study, we investigate the intrageneric systematic relationships among Raorchestes frogs and characterise the phylogenetically identified 16 major species groups (largely congruent with previous studies such as Biju & Bossuyt, 2009; Vijayakumar et al., 2014, 2016), based on morphological (including eye colouration and patterns), acoustic, and associated behavioural traits (such as calling height). Our ongoing research has also revealed another five new species in this genus, all from the State of Kerala in southern Western Ghats, which are formally described on the basis of integrative evidence. In addition, we provide remarks on the taxonomic status of certain poorly known taxa. New insights from this study aim to facilitate a better working taxonomy for this rather large and taxonomically challenging genus, as well as guide future research on ecology, biogeography, evolution, and conservation of its members.
Materials and Methods
Field sampling
Field surveys, sampling, and call recordings were carried out primarily during the breeding season of shrub frogs in the Western Ghats (May/June–September) between 2009 and 2019. Adults were found through opportunistic searches or by locating calling males. Sampled individuals were photographed in life followed by euthanisation in Tricaine methanesulphonate (MS-222). Tissue samples were extracted from the thigh muscle, preserved in absolute ethanol, and eventually stored at −20 °C for molecular studies. The specimens were fixed in 4% formalin and transferred to 70% ethanol for preservation. Type specimens are deposited in the Bombay Natural History Society (BNHS), Mumbai, and referred specimens are available at the Systematics Lab, University of Delhi (SDBDU), India. Geographical coordinates of the sampling localities were recorded using a Garmin 76CSx GPS with the WGS84 datum system. Distribution maps were prepared in QGIS version 2.6.1 (http://www.qgis.org).
Fieldwork, including collection of animals in the field, was conducted with permissions and following guidelines from the responsible authorities in the State Forest Departments (Field permits: Nos. WL12-1830/2009, WL10-2606/12, WL10-25421/2014, 67254/2001/WL5, D-22 (8)/Research/4543/2012-13; PCCF(WL)/E2/CR/13/2016–17 and WL10-43756/2015). Research received ethical approval from Department of Environmental Studies, University of Delhi (DES/1020 dated 9 February 2015), India.
Phylogenetic study
Genomic DNA was extracted from populations sampled from the State of Kerala that were suspected to represent undescribed species, using the Qiagen DNeasy blood and tissue kit (Qiagen, Valencia, CA, USA). The following six gene fragments were PCR-amplified using previously published primers: four mitochondrial—16SrRNA (Simon et al., 1994), 12SrRNA + tRNAVAL (Richards & Moore, 1996), and Cytochrome b (Che et al., 2012); two nuclear—Rhodopsin and Tyrosinase (Bossuyt & Milinkovitch, 2000). The fragments were sequenced on both strands using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) on ABI 3730 automated DNA sequencer (Applied Biosystems). Newly generated sequences were checked and assembled in ChromasPro v1.34 (Technelysium Pty Ltd., St. South Brisbane, QLD, Australia), and deposited in the National Centre for Biotechnology Information (NCBI) GenBank under accession numbers MW020034–MW020035, MW020166–MW020171 and MW023233–MW023244 (Table S1).
Further taxon sampling for phylogenetic studies was carried out by retrieving previously published DNA sequences for vouchers with maximum availability of the analysed genes and as much as possible those representing typical exemplars of all the currently recognised Raorchestes species (except R. thodai) available in the NCBI GenBank. Additionally, one member (the type species, if available) from all known rhacophorid genera and an outgroup taxon were included in the dataset (Table S1). The datasets for different gene sequences were prepared and aligned for 94 taxa using the ClustalW tool in MEGA 7.0 (Kumar, Stecher & Tamura, 2016). Alignments for coding DNA were checked by comparison with amino acid sequences; non-coding fragments were manually optimised and ambiguous sites were excluded from phylogenetic analyses. The tRNAVAL gene was also excluded from the 12S fragment, due to its non-availability for most of the Genbank sequences. The resultant character set of total 2,327 basepairs was partitioned by genes for the five studied gene fragments and the best-fit models of DNA evolution determined individually by implementing the Akaike Information Criterion in ModelTest 3.4 (Posada & Crandall, 1998) were used for analyses.
Phylogenetic inferences were made under the Maximum Likelihood (ML) criteria. ML searches were performed for 100 independent runs with GTRGAMMA model for each gene partition along with 1,000 thorough bootstrap replicates for assessing the clade support, using RAxML 7.3.0 (Stamatakis, 2006; Stamatakis et al., 2008) in raxmlGUI 1.1 (Silvestro & Michalak, 2012). Further, Bayesian analyses were performed in MrBayes 3.1.2 (Ronquist & Huelsenbeck, 2003) using the best determined model (GTR + I + G) for each gene partition, with two parallel runs of four Markov chain Monte Carlo (MCMC) chains executed for 10 million generations. Trees were sampled after every 1,000 generations and the Bayesian Posterior Probabilities (BPP) for clades were summarised after discarding the first 2.5 million generations as burn-in. Convergence of the parallel runs was confirmed by split frequency standard deviations of less than 0.01 as well as the nearing of potential scale reduction factors to 1.0 for all model parameters using Tracer v1.3 (Rambaut et al., 2014).
Morphological study
Morphological studies were carried out to compare the populations suspected to represent new species with all previously known Raorchestes species and available names, based on examination of available types and other museum specimens, original descriptions, or new topotypic material. All the Raorchestes species known from Peninsular India were also comprehensively studied in order to identify shared morphological characters for grouping of species. Sex and maturity were determined by the presence of secondary sexual characters (such as nuptial pads and vocal sacs in males) or examination of gonads. Only adult specimens were used for morphometric studies.
Measurements and associated terminologies follow Biju & Bossuyt (2009). The following measurements were taken to the nearest 0.1 mm by using a digital slide-caliper or a binocular microscope with a micrometer ocular: snout–vent length (SVL), head width (HW, at the angle of the jaws), head length (HL, from rear of mandible to tip of snout), MN (distance from the rear of the mandible to the nostril), MFE (distance from the rear of the mandible to the anterior orbital border), MBE (distance from the rear of the mandible to the posterior orbital border), snout length (SL, from tip of snout to anterior orbital border), eye length (EL, horizontal distance between bony orbital borders), inter upper eyelid width (IUE, the shortest distance between the upper eyelids), maximum upper eyelid width (UEW), internarial distance (IN), internal front of the eyes (IFE, shortest distance between the anterior orbital borders), internal back of the eyes (IBE, shortest distance between the posterior orbital borders), NS (distance from the nostril to the tip of the snout), EN (distance from the front of the eye to the nostril), TYD (greatest tympanum diameter), TYE (distance from the tympanum to the back of the eye), forearm length (FAL, from flexed elbow to base of outer palmar tubercle), hand length (HAL, from base of outer palmar tubercle to tip of third finger), FLI–IV (finger length), thigh length (TL, from vent to knee), shank length (SHL, from knee to heel), foot length (FOL, from base of inner metatarsal tubercle to tip of fourth toe), total foot length (TFOL, from heel to tip of fourth toe), FD (maximum disc width of finger), width of finger (FW, measured at the base of the disc), TD (maximum disc width of toe), width of toe (TW, measured at the base of the disc). Digit number is represented by roman numerals I–V in subscript. All measurements and photographs were taken for the right side of the specimen, except when a character was damaged, in which case the measurement was taken on the left side. All measurements provided in the taxonomy section are in millimetres.
For the convenience of discussion, Raorchestes species of the Western Ghats are grouped based on their body size as small (male SVL 17.0–25.0 mm), medium (male SVL 25.1–45.0 mm) and large (male SVL 45.1–65.0 mm). Terminologies for the snout shape follow Heyer et al. (1990). The webbing formulae follow Savage & Heyer (1967) as modified by Myers & Duellman (1982) and followed by Biju et al. (2014), and the degree of webbing relative to subarticular tubercles is described by numbering the tubercles 1–3, starting from the base. Further, the webbing is categorised as basal (slightly above or beyond the basal subarticular tubercles on all toes), small (webbing on toe IV beyond the third subarticular tubercle but below the second subarticular tubercle on either side), medium (webbing on toe IV beyond the second subarticular tubercle but below the first subarticular tubercle on either side), and large (webbing on toe IV extending beyond the first subarticular tubercle on either side), following Garg & Biju (2017). Finger and toe disc morphology types follow Biju et al. (2011).
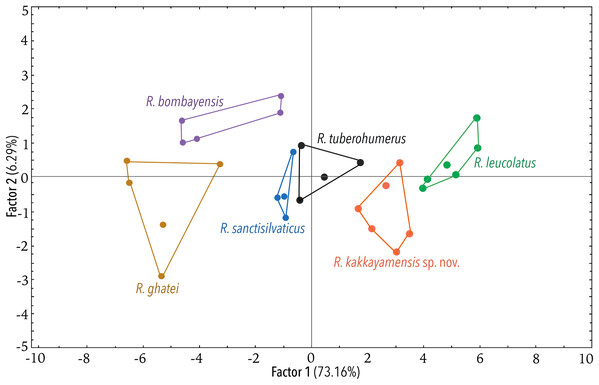
Using the statistical software Statistica v7.1 (StatSoft Inc.), Principal Component Analysis (PCA) and Discriminant Function Analysis (DFA) were performed to specifically assess the degree of morphological differentiation among the six recognised members of the Raorchestes bombayensis group. PCA was performed using 20 morphometric parameters taken from adult males. Factor scores of the first two Principal Components (PC) were observed on a scatterplot. Furthermore, sets of 20 predictor variables were generated from the PCA and all the factor scores were used as input variables for performing a DFA, in order to also determine the classification success of the studied samples.
Eye colouration and pattern
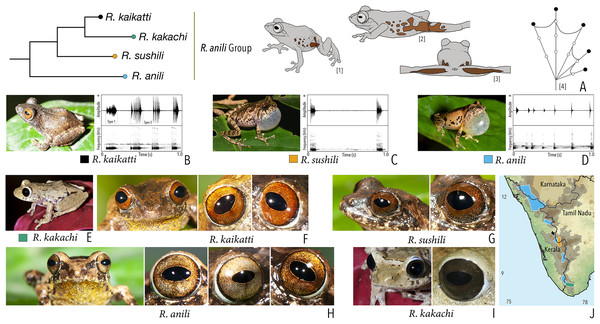
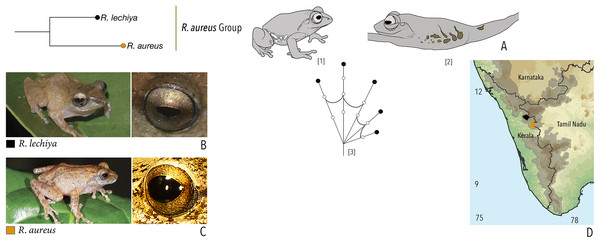
We made a dedicated effort to photograph and document the colour and pattern of the eyes of all the Raorchestes species in the Western Ghats from various angles. All the photographs were taken with the aid of external flashlight, either during the day or night. The interpretations for eye colour and pattern in this study are solely based on photographs. The possibility of variations in eye colour were also observed under captivity before photography. However, 19 randomly tested species did not show significant variation in eye colour, unlike the changes usually observed in the case of dorsal skin colouration. The following parameters were described: (1) eye colour of individual species; (2) comparisons with morphologically and phylogenetically related species. Terminologies for the eye structures (Fig. 1) are adopted and modified from Glaw & Vences (1997).
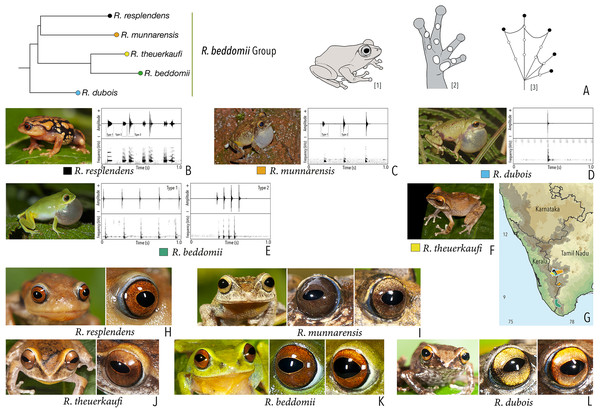
Figure 1: Terminologies for eye structure and the types of eye colours and patterns discussed in the text for Raorchestes members.
Pupil is rounded and horizontal in all known Raorchestes species. (A and B) R. resplendens eye marked with sclera, iris periphery, and iris. (C–J) Major types of eye colours and patterns. (C) Iris with horizontal and vertical bands. (D) Black iris with dense metallic silver mosaic pattern. (E) Black iris with golden yellow patches. (F) Brown iris with silver white or golden radiating lines. (G) Brown iris with metallic greenish-yellow reticulations. (H) Iris horizontally divided into light upper and dark lower halves. (I) Yellow iris with an inner reddish-brown ring. (J) Brown iris with dense golden yellow speckling. (K) Reddish-brown iris with yellowish-green spots and blotches on the palpebral membrane.Call recordings and acoustic analyses
The sound recordings of 43 species (representing 14 species groups) were made at night when the animals were actively calling (18:00–04:00 h). Calls were recorded in the field using a Sennheiser microphone ME 66 connected or MKH 416 directional microphone connected to a digital solid-state recorder, such as Marantz PMD620, Marantz PMD670, Fostex FR2LE, Zoom H6 and Zoom H4n (44.1 kHz sampling rate, 16-bit resolution) and monitored in real time using Sony MDR V500 headphones. The gain settings of the recorder were adjusted prior to each recording to avoid clipping the amplitude envelopes of recorded calls and maintained throughout to ensure a constant signal to noise ratio within a recording.
Acoustic properties were measured using Raven Pro v1.4 (Charif, Waack & Strickman, 2010). Our use of terminology to describe species-specific vocal repertoires follows our earlier reports describing the vocalizations of Pseudophilautus kani and Raorchestes graminirupes (Bee, Suyesh & Biju, 2013a, 2013b), and readers are referred to those studies for additional details not described here. Briefly, the members of genus Raorchestes produced one to three types of call, which either had pulsatile or non-pulsatile temporal structures. Calls could be produced singly or organised into longer “call groups” (a series of calls delivered in quick succession separated by a short time interval from a subsequent call group). Call groups consisted of either repetitions of the same call type or a mixture of different call types. We labelled calls as Type 1 (“Type 1” calls were the most frequently delivered call type or if two different call types were delivered together as groups then the first delivered call in a call group was named as “Type 1”) and Type 2 and Type 3 for the species producing more than one call type. For species with pulsatile calls, we analysed five temporal properties (call duration, call rise time, call fall time, number of pulses per call, and pulse rate) and one spectral property (overall dominant frequency). Three temporal properties (call duration, call rise time, and call fall time) and one spectral property (overall dominant frequency) were used for analyses for species with non-pulsatile calls. We would note that in addition to among-species variation, the temporal and spectral properties of anuran vocalisations also vary among individuals within species and within individuals, for example, as a function of differences in temperature and social context (Gerhardt & Huber, 2002). The values reported below do not take into consideration these sources of call variation, which are to be expected to operate within each species.
For visual representations of calls, oscillograms showing the amplitude versus time waveform were prepared using a time frame of 1 s for species groups (n = 8) that produce calls/call groups longer than 0.1 s and a time frame of 0.1 s for species groups (n = 6) that produce calls shorter than 0.1 s. The overall dominant frequency information for the calls of each species was obtained using Raven’s spectrogram function after selecting the entire duration of the call (1,024-point fast Fourier transform, Hann window, 50% overlap, 43.1 Hz resolution). Spectrograms were prepared for graphical representation of the call spectrum at similar time frames as the oscillograms.
New species names
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:7021B266-C54A-4E64-8645-AACCBFBB1A72. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Phylogenetic relationships
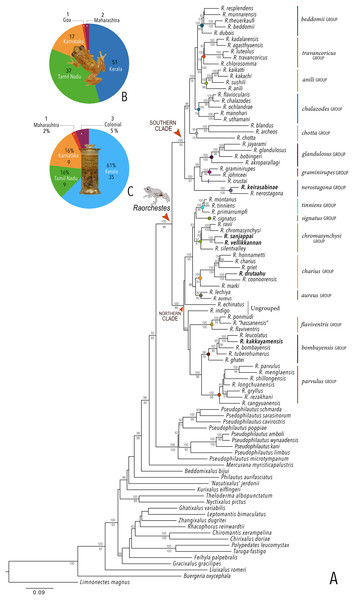
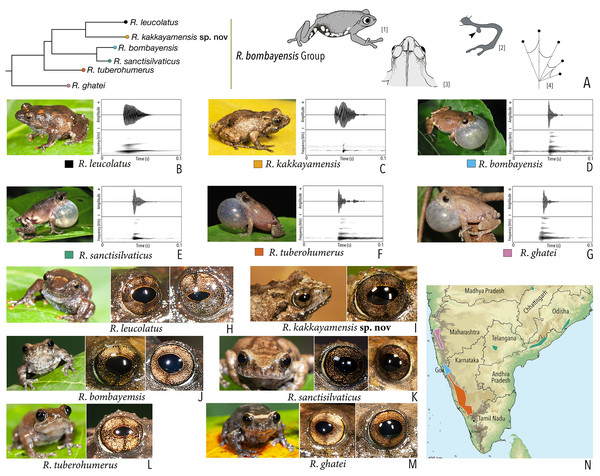
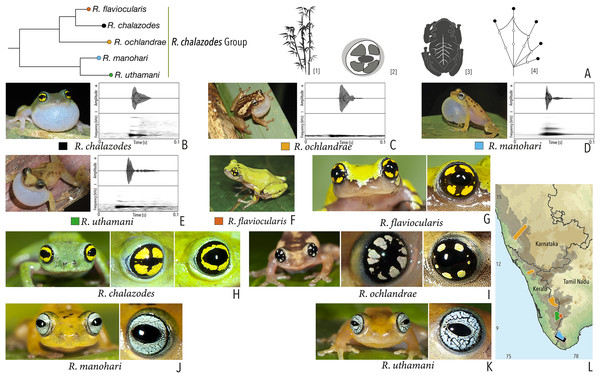
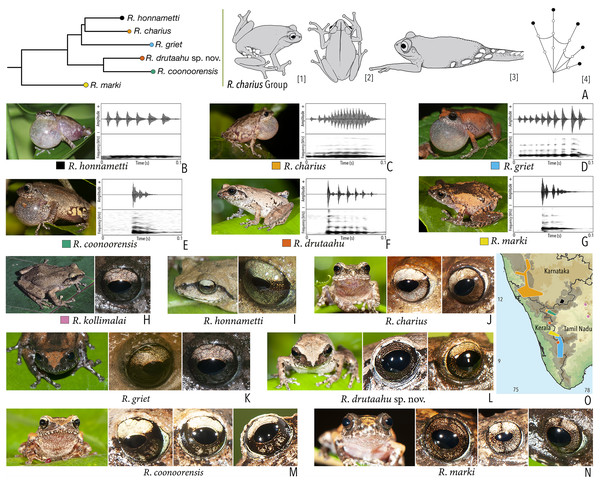
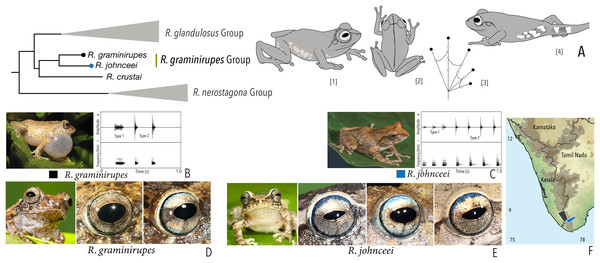
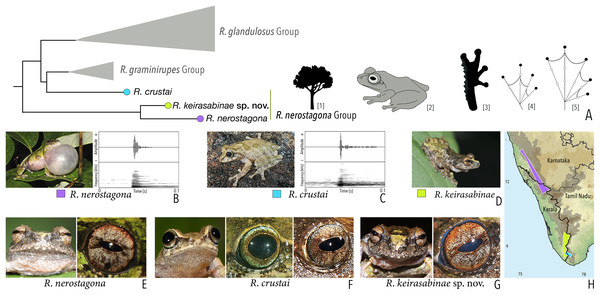
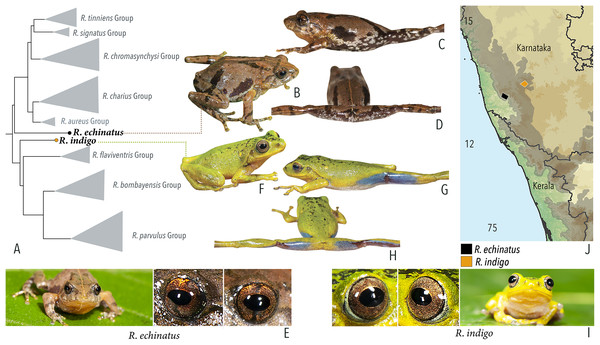
Our Maximum Likelihood (ML) and Bayesian phylogenetic analyses (Fig. 2) recovered genus Raorchestes as a well-supported monophyletic clade, showing a sister-group relationship with the genus Pseudophilautus (e.g., Li et al., 2009; Yu et al., 2009; Biju et al., 2010; Pyron & Wiens, 2011; Vijayakumar et al., 2016). The focal genus showed two major radiations, the northern clade and the southern clade (Vijayakumar et al., 2016), that were further divided into 16 major sub-clades largely congruent with Vijayakumar et al. (2014) and hereafter referred to as species groups, as indicated with modifications in Fig. 2. Most of the recognised species groups were recovered with high (BPP ≥ 95, BS ≥ 70) support, except for the Raorchestes charius group and R. aureus group that received moderate support probably due to the phylogenetic position of R. marki (included in R. charius species group in the present study; in R. aureus sub-clade as per Vijayakumar et al., 2016; unresolved in Vijayakumar et al., 2014). The relationships of three species (R. crustai, R. echinatus, and R. indigo) also remained unresolved, as shown previously by Vijayakumar et al. (2014, 2016). While R. crustai appreared to show a closer but unsupported phylogenetic affinity to members of the R. graminirupes group, we provisionally assign it to the R. nerostagona group based on additional morphological and acoustic evidence (see ‘Grouping of species using integrative approaches’), until further evidence proves otherwise. On the other hand R. echinatus and R. indigo are treated as ungrouped species (see ‘Grouping of species using integrative approaches’ for discussion on morphological affinities). Although the phylogenetic relationships at the species-level were often well-supported, several lineages were also either moderately to weakly support or remained unresolved (Fig. 2).
Figure 2: Phylogenetic relationships in the genus Raorchestes and State-wise figures for species in the Western Ghats.
(A) Maximum Likelihood phylogram, based on 2,327 bp partitioned dataset for three mitochondrial and two nuclear gene fragments from 94 taxa, showing phylogenetic relationships among 60 previously recognised and five new Raorchestes species along with representatives of other known rhacophorid genera. The focal genus Raorchestes comprises 16 major species-groups discussed in the study. New species described in the study are indicated in bold letters. The values above and below the branches indicate Bayesian Posterior Probabilities (BPP) and RAxML Bootstrap support (BS), respectively. (B) Number of Raorchestes species reported from the Indian States encompassing the Western Ghats. (C) Proportion of the currently recognised species originally described from each state. Descriptions from regions with colonial names that may include more than one State are categorised separately.In addition to the previously known Raorchestes species, our study also included five populations representing potential candidate species (Fig. 2). Our analyses concordantly supported the distinct phylogenetic position of all these lineages in four recognised species groups (one in R. bombayensis group; one in R. charius group; two in R. chromasynchysi group; and one in R. nerostagona group) with well-supported sister-group relationships. Based on additional integrative evidence, we confirm that these putative lineages represent distinct new species and are formally described below.
Description of new species
Raorchestes drutaahu sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:4B07924D-80B3-468B-8C35-C3A8C3001E23
Fast-calling Shrub Frog
(Figs. 2–3; Tables 1–3; Tables S1 and S2)
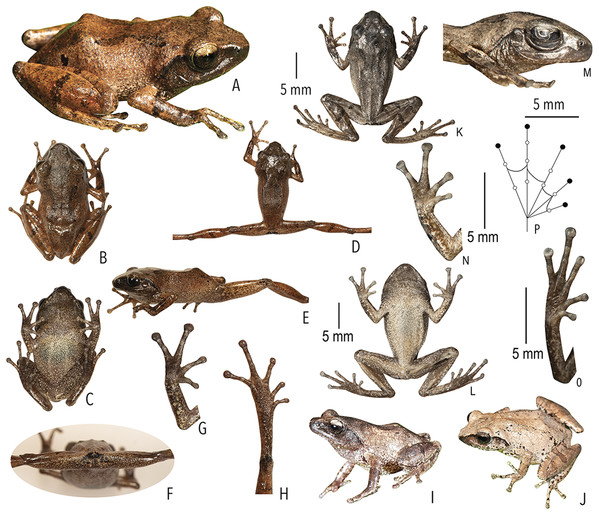
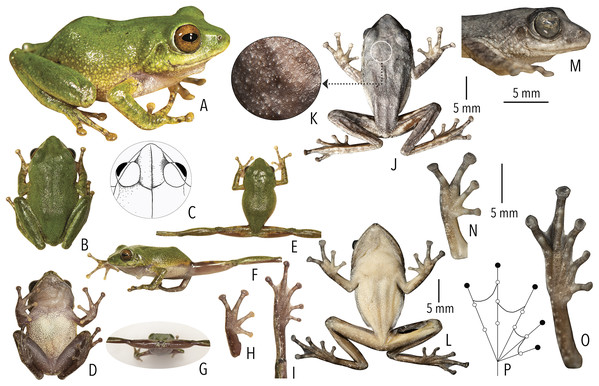
Figure 3: Type and referred specimens of Raorchestes drutaahu sp. nov.
(A–H) Holotype, in life (BNHS 6088, adult male). (A) Dorsolateral view. (B) Dorsal view. (C) Ventral view. (D) Dorsal view of body and thighs. (E) Lateral view. (F) Posterior view of thighs. (G) Ventral view of hand. (H) Ventral view of foot. (I–J) Paratypes, in life. (I) Dorsolateral view (BNHS 6089, adult male). (J) Dorsolateral view (SDBDU 2015.3025, adult female). (K–P) Holotype, in preservation (BNHS 6088, adult male). (K) Dorsal view. (L) Ventral view. (M) Lateral view of head. (N) Ventral view of hand. (O) Ventral view of foot. (P) Schematic illustration of webbing on foot.| Group/Species | Dorsum | Eye colouration and markings | |||
|---|---|---|---|---|---|
| Colour | Markings | Iris | Iris periphery | Sclera | |
| Raorchestes anili group | |||||
| Raorchestes anili | Light to dark brown | Dark brown inverted ‘V’-shaped mark | Light golden brown with reddish tinge | Dark brown | Light blue |
| Raorchestes kaikatti | Greyish or reddish-brown | With or without inconspicuous dark spots and markings | Brown or reddish-brown to orange | Dark brown | Light blue |
| Raorchestes kakachi | Light to dark brown or light grey | With or without dark irregular spots and markings | Dark brown or reddish-brown | Dark brown | Light blue |
| Raorchestes sushili | Brown, greyish or reddish-brown | Dark inverted ‘V’-shaped mark and irregular spots | Brown, copper, or reddish-brown | Dark brown | Light blue |
| Raorchestes aureus group | |||||
| Raorchestes aureus | Light brown or pale yellow | Absence of prominent markings | Golden brown | Black | Light blue |
| Raorchestes lechiya | Light brown or greyish-brown | With or without dark bands, spots or markings | Golden brown | Black | Light blue |
| Raorchestes beddomii group | |||||
| Raorchestes beddomii | Bright green or yellowish-green | Absence of prominent markings | Red, brick red, or orange | Black | Light blue |
| Raorchestes dubois | Highly variable from white, grey, green, brown, yellow to red | With or without contrasting and variable spots, streaks, bands or markings | Light golden brown with reddish tinge | Black | Scarlet blue |
| Raorchestes munnarensis | Brown to yellowish-grey | Dark inverted ‘V’ or ‘X’-shaped mark | Light brown to coffee brown | Black | Scarlet blue |
| Raorchestes resplendens | Reddish-orange interspersed with black | Multiple prominent bright orange macroglands | Red or brick red | Black | Scarlet blue |
| Raorchestes theuerkaufi | Brown or reddish brown | Irregular dark mottling and scattered patches | Light golden brown or copper | Black | Light blue |
| Raorchestes bombayensis group | |||||
| Raorchestes bombayensis | Brown or greyish-brown | With or without inverted ‘V’ or ‘X’-shaped mark | Brown with dense golden speckling, and dark brown horizontal and vertical bands | Dark brown | Light silvery blue |
| Raorchestes ghatei | Brown or greyish-brown | With or without inconspicuous dark bands (‘V’ or ‘X’-shaped), spots or markings | Brown with dense golden speckling, and dark brown horizontal and vertical bands | Dark brown | Light silvery blue |
| Raorchestes kakkayamensis sp. nov. | Brown or reddish-brown | Dark discontinuous concave bands and scattered darks streaks or markings | Brown with dense golden speckling, and dark brown horizontal and vertical bands | Dark brown | Light silvery blue |
| Raorchestes leucolatus | Brown to reddish-brown | Scattered orange spots or patches and inconspicuous dark markings | Brown with dense golden speckling, and dark brown horizontal and vertical bands | Dark brown | Light silvery blue |
| Raorchestes sanctisilvaticus | Brown or greyish-brown | With or without inverted ‘V’ or ‘X’-shaped mark | Brown with dense golden speckling, and dark brown horizontal and vertical bands | Dark brown | Light silvery blue |
| Raorchestes tuberohumerus | Light to dark brown | Faint to prominent dark X-shaped mark or irregular markings | Brown with dense golden speckling, and dark brown horizontal and vertical bands | Dark brown | Light silvery blue |
| Raorchestes chalazodes group | |||||
| Raorchestes chalazodes | Green, yellowish or bluish-green | Rarely with scattered spots | Black with a golden yellow ring that may or may not be divided by a black cross mark | Black | Scarlet blue |
| Raorchestes flaviocularis | Green or reddish green | Lichen pattern exposing reddish fleshy skin | Black with a golden yellow ring that may or may not be divided by a black cross mark | Black | Indistinct |
| Raorchestes ochlandrae | Brown or reddish-brown | Light yellow dorsolateral bands with or without elongate blotches or scattered spots | Black with light yellow patches in a radial pattern | Black | Indistinct |
| Raorchestes manohari | Bright yellow to greyish-yellow | Scattered dark brown spots | Black with dense metallic silver mosaic pattern | Black | Indistinct |
| Raorchestes uthamani | Yellow with grey or red tinge | With or without faint dark brown streaks or spots | Black with dense metallic silver mosaic pattern | Black | Indistinct |
| Raorchestes charius group | |||||
| Raorchestes charius | Brown or reddish-brown | Contrasting concave bands and irregular patches | Light or dark brown with golden tinge, horizontally divided into light upper and dark lower halves | Blackish-brown | Light grey |
| Raorchestes coonoorensis | Light brown or reddish-brown | With or without, continuous or discontinuous dark concave bands | Brown with golden tinge, horizontally divided into light upper and dark lower halves | Blackish-brown | Light blue |
| Raorchestes drutaahu sp. nov. | Light to dark brown or straw | With or without, continuous or discontinuous grey bands or stripes | Brown with golden tinge, horizontally divided into light upper and dark lower halves | Blackish-brown | Light grey |
| Raorchestes griet | Brown with grey or reddish tinge | Irregular black patches or dark concave bands | Brown with golden tinge, horizontally divided into light upper and dark lower halves | Black | Light grey |
| Raorchestes honnametti | Brown or grey | Faint to prominent contrasting concave bands | Brown with golden tinge, horizontally divided into light upper and dark lower halves | Black | Light blue |
| Raorchestes kollimalai | Light or dark brown | Faint to prominent, continuous or discontinuous contrasting concave bands | Brown with golden tinge, horizontally divided into light upper and dark lower halves | Black | Light blue |
| Raorchestes marki | Grey, brown or reddish-brown | Dark X-shaped mark or a pair of concave bands | Brown with dense golden yellow speckling, and with brown horizontal and vertical bands | Black | Light bluish-grey |
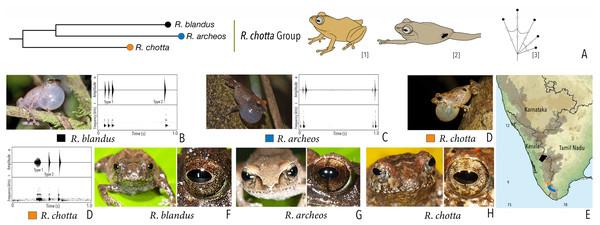
| Raorchestes chotta group | |||||
| Raorchestes archeos | Greyish, reddish or yellowish-brown | With or without dark broad median band | Golden brown, with dark vertical band | Brown | Greyish-white |
| Raorchestes blandus | Greyish to reddish-brown | Irregular dark brown and orange patches | Golden brown | Brown | Greyish-white |
| Raorchestes chotta | Brown with yellow or grey tinge | Irregular dark brown blotches or scattered dark spots | Golden brown | Brown | Greyish-white |
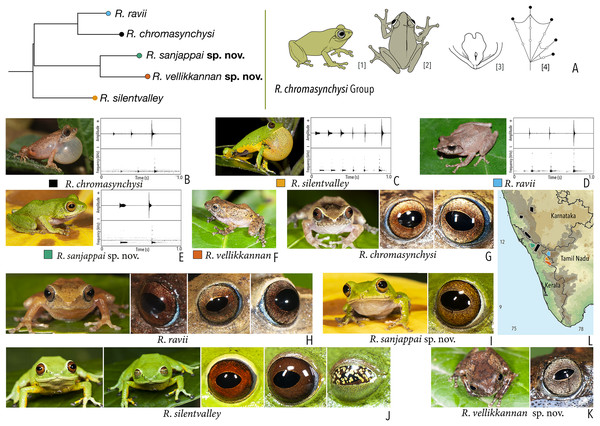
| Raorchestes chromasynchysi group | |||||
| Raorchestes chromasynchysi | Brown or Green | With or without contrasting dark bands, streaks or markings | Golden brown with reddish tinge | Blackish-brown | Scarlet blue |
| Raorchestes ravii | Brown or orangish-brown | With or without dark median band, faint X-shaped mark or scattered spots | Golden brown | Dark brown | Scarlet blue |
| Raorchestes sanjappai sp. nov. | Green | Without prominent dark markings, occasionally with markings | Reddish-brown | Dark brown | Light blue |
| Raorchestes silentvalley | Green | With or without yellow or bluish-black spots | Brown or dark red | Blackish-brown | Light blue |
| Raorchestes vellikkannan sp. nov. | Brown or pale yellow | Dark brown ‘X’-shaped mark and scattered spots | Silver grey with minute brown speckling | Dark brown | Light blue |
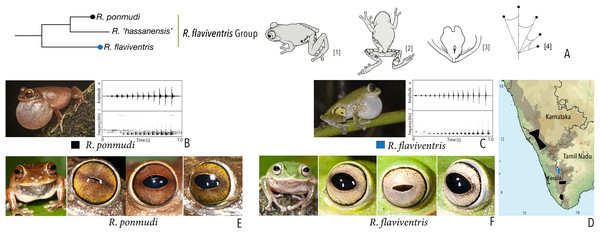
| Raorchestes flaviventris group | |||||
| Raorchestes flaviventris | Green or yellowish-green | With or without scattered pale yellow, golden yellow, or white spots | Creamy white with minute brown speckles | Dark brown | Bluish-grey |
| Raorchestes ponmudi | Brown, reddish-brown or light to dark grey | Dark brown concave bands or X-shaped mark, with or without few scattered white blotches and minute black spots | Light brown or golden brown with minute dark speckles | Dark brown | Bluish-grey |
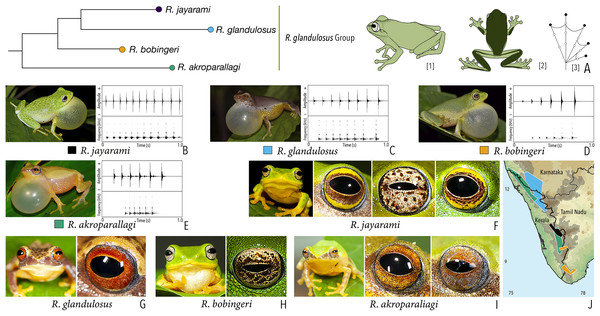
| Raorchestes glandulosus group | |||||
| Raorchestes akroparallagi | Shades of green, yellow, brown, reddish-brown, or light grey | With or without yellow dorsolateral streaks, scattered small grey or large blackish-brown spots | Light brown to reddish-brown with golden speckles | Blackish-brown | Light blue |
| Raorchestes bobingeri | Green | Without prominent markings | Light yellow or greyish-yellow with an inner brown ring or irregular spots | Blackish-brown | Light blue |
| Raorchestes glandulosus | Green, greenish-yellow, brown, reddish-brown, or purplish | With or without uniformly scattered contrasting spots and reticulations | Bright to dark red, or reddish-brown with golden speckles | Blackish-brown | Light blue |
| Raorchestes jayarami | Green, bluish-green or yellow | With or without uniformly scattered contrasting spots or irregular streaks | Bright yellow or greyish-yellow with an inner reddish-brown ring or spots | Blackish-brown | Light blue |
| Raorchestes graminirupes group | |||||
| Raorchestes graminirupes | Brown or yellow with grey or reddish tinge | Dark irregular patches, streaks, or longitudinal bands | Greyish-brown with dense metallic silver or light brown speckles, with scarlet blue ring | Black | Light silvery blue |
| Raorchestes johnceei | Brown, grey, pale yellow, or reddish | Dark irregular patches, broad median band, pair of continuous or discontinuous concave bands or inverted V-shape mark | Light greyish-brown with dense metallic silver or light brown speckles, with scarlet blue ring | Black | Light silvery blue |
| Raorchestes nerostagona group | |||||
| Raorchestes crustai | Brown, greyish-brown or green | Irregular dark brown or greenish-brown blotches, continuous or discontinuous concave bands, or inverted V-shape mark | Light greyish-brown | Dark brown | Scarlet blue |
| Raorchestes keirasabinae sp. nov. | Brown, greyish-brown or greenish-brown | Irregular brown, black and green patches | Reddish-grey with faint or prominent horizontal brown band | Dark brown | Scarlet blue |
| Raorchestes nerostagona | Brown or greyish-green | Irregular dark green, reddish-brown, or bluish-black patches of various size | Reddish-grey with faint or prominent horizontal brown band | Dark brown | Scarlet blue |
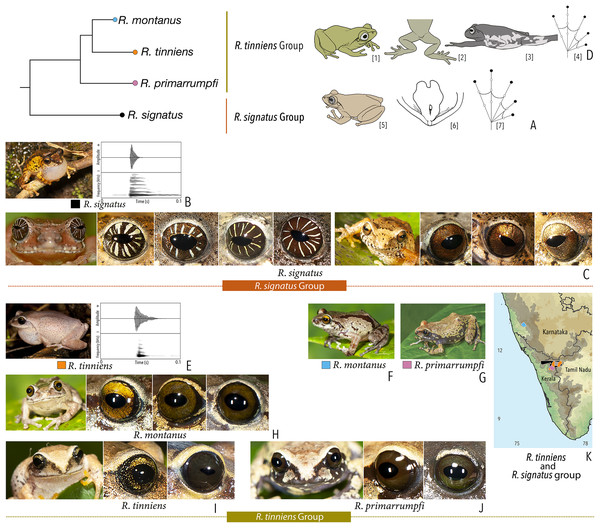
| Raorchestes signatus group | |||||
| Raorchestes signatus | Brown, grey, or red | With or without, faint to prominent, dark ‘X’-shape or inverted V-shape mark, contrasting broad median band, or scattered dark spots and patches | Reddish-brown with or without silver white or golden radiating lines and golden speckling | Without prominent ring | Greyish-brown |
| Raorchestes tinniens group | |||||
| Raorchestes montanus | Light brown to chocolate brown, pinkish or reddish-brown, with metallic tinge in all morphs | With or without dark streaks, concave bands, X-shape mark, or variable mosaic patterns | Dark brown or golden brown | Black | Ash grey |
| Raorchestes primarrumpfi | Brown, grey, reddish-brown, or greenish-brown, with metallic tinge in all morphs | With or without dark irregular patches or contrasting yellowish spots | Dark brown or greyish-brown | Black | Ash grey |
| Raorchestes tinniens | Brown, grey, reddish-brown, or greenish-brown, with metallic tinge in all morphs | With or without dark irregular patches or contrasting yellowish spots | Dark brown with golden brown speckling | Black | Ash grey |
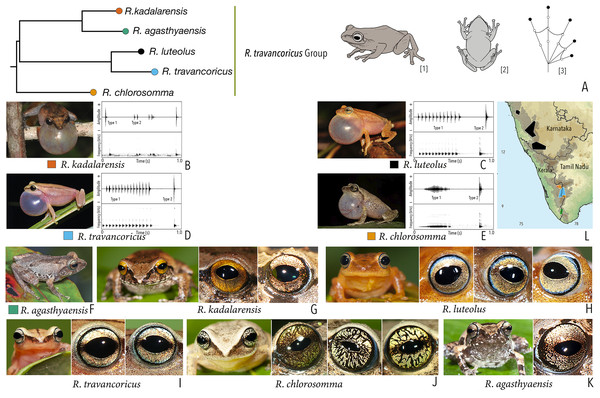
| Raorchestes travancoricus group | |||||
| Raorchestes agasthyaensis | Brown, greyish-brown, or reddish-brown | Dark inverted V-shape mark with scattered dark patches or dark brown broad median band | Dark brown with dense golden speckling, and horizontally divided into light upper and dark lower halves | Dark brown | Ash grey |
| Raorchestes chlorosomma | Brown or grey | Continuous or discontinuous dark concave bands or irregular streaks, or dark broad median band | Metallic greyish-green or greenish-yellow with dark brown reticulations | Dark brown | Scarlet blue |
| Raorchestes kadalarensis | Brown, greyish-brown, reddish-brown, or bluish-brown | Dark inverted V-shape mark | Dark brown with dense golden speckling, and horizontally divided into light upper and dark lower halves | Dark brown | Ash grey |
| Raorchestes luteolus | Yellow, reddish-yellow, or yellowish-brown | Faint, continuous or discontinuous, dark longitudinal lines or without prominent markings | Golden yellow or light grey with brown speckling with a cobalt blue outsider ring | Black or bluish-black | Indistinct |
| Raorchestes travancoricus | Red or reddish-brown | Prominent longitudinal dark lines | Golden yellow or light grey with brown speckling with a cobalt blue outsider ring | Black or bluish-black | Indistinct |
| Ungrouped species | |||||
| Raorchestes indigo | Green or greenish-yellow | With or without irregular and scattered black, yellow or bluish-black spots | Golden brown | Dark brown | Light blue |
| Raorchestes echinatus | Brown, greyish-brown, or reddish-brown | Thin middorsal line, irregular dark streaks and scattered minute dark spots | Golden brown with dark vertical band | Dark brown | Indistinct |
Note:
Two species are not assigned to any species group. Values are calculated for a typical single call of each species. Broad calling height categories: Ground (ground and associated grass): 0–0.5 m; Low (low bushes and shrubs): 0.5–1.5 m; Medium 1 (high shrubs): 1.5–4 m; Medium 2 (lower canopy): 4–7 m; Canopy (high canopy): 7 m and above (up to 40 m).
| Group/species | Call delivery pattern | Call type | Temporal properties | Spectral properties | Calling height (meters) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temporal structure | Call duration (ms) | Call rise time (ms) | Call fall time (ms) | Pulses/call | Pulse rate (Pulses/sec) | Broad frequency peaks | Dominant frequency (kHz) | ||||
| Raorchestes anili group | 1–2 types | Pulsatile | 51.2–980.4 | Nil–960.2 | Nil–48.8 | 5–22 | 6.3–250.8 | Single | 2.4–2.9 | 1.5–5 | |
| R. anili | Delivered in groups | Type 1 | Pulsatile, widely spaced pulses | 980.4 | 960.2 | 20.2 | 7 | 6.3 | Single | 2.9 | 1.5–5 |
| R. kaikatti | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Pulsatile, more closely packed pulses than Type 2 | 102.7 | 67.5 | Not significant | 22 | 250.8 | Single | 2.4 | 1.5–5 |
| Type 2 | Pulsatile, more widely packed pulses than Type 1 | 68.9 | Not significant | 14.4 | 5 | 89.3 | Single | 2.4 | |||
| R. sushili | Uniform intervals, delivered in groups | Type 1 | Pulsatile, closely spaced pulses | 51.2 | Not significant | 48.8 | 11 | 242.8 | Single | 2.6 | 1.5–5 |
| R. kakachi | not studied | 1.5–3 | |||||||||
| Raorchestes beddomii group | 1–3 types | Pulsatile or Non-pulsatile | 13.2–498.2 | 1.0–475.3 | Nil–49.2 | 1–15 | Nil–406.5 | 1–3 | 2.2–2.7 | 0–20 | |
| R. beddomii | Delivered in groups (only Type 2 call) |
Type 1 | Pulsatile | 150.0 | 140.2 | 11.2 | 4 | 29.2 | Single | 2.6 | 0.5–2 |
| Type 2 | Non-pulsatile | 13.2 | 1.1 | 12.0 | 1 | – | Single | 2.6 | |||
| R. dubois | Generally uniform intervals, not delivered in groups | Type 1 | Non-pulsatile | 10.6 | 1.0 | 8.3 | 1 | – | Single | 2.7 | 0–2 |
| R. munnarensis | Delivered in groups, lack any fixed call order | Type 1 | Pulsatile, pulses more widely spaced than Type 2 | 498.2 | 475.3 | Not significant | 3 | 6.4 | Single | 2.2 | 1.5–20 |
| Type 2 | Pulsatile, pulses more closely packed than Type 1 | 22.8 | 7.7 | 14.4 | 5 | 406.5 | Single | 2.2 | |||
| R. resplendens | Delivered in groups | Type 1 | Non-pulsatile | 51.3 | 16.7 | 49.2 | 1 | – | Three | 2.5 | |
| Type 2 | Pulsatile | 28.7 | 1.5 | 27.1 | 7 | 269.2 | Two | 2.7 | 0–1 | ||
| Type 3 | Non-pulsatile | 64.7 | 41.6 | 24.7 | 1 | – | Four | 2.7 | |||
| R. theuerkaufi | Not studied | 0.5–3 | |||||||||
| Raorchestes bombayensis group | 1 type | Non-pulsatile | 11.6–29.4 | 1.2–9.2 | 9.5–25.4 | 1 | – | Single | 2.9–4.1 | 0.5–5 | |
| R. bombayensis | Not delivered in groups | Type 1 | Generally single pulse | 11.6 | 1.2 | 9.5 | 1 | – | Single | 3.1 | 2–5 |
| R. ghatei | Not delivered in groups | Type 1 | Generally single pulse | 17.7 | 1.5 | 16.0 | 1 | – | Single | 2.9 | 1–4 |
| R. kakkayamensis sp. nov. | Not delivered in groups | Type 1 | Generally single pulse | 25.3 | 9.2 | 15.9 | 1 | – | Single | 3.8 | 1–3 |
| R. leucolatus | Not delivered in groups | Type 1 | Generally single pulse | 29.4 | 2.7 | 25.4 | 1 | – | Single | 4.1 | 1–4 |
| R. sanctisilvaticus | Not delivered in groups | Type 1 | Generally single pulse | 12.2 | 1.3 | 10.8 | 1 | – | Single | 3.1 | 1–3 |
| R. tuberohumerus | Not delivered in groups | Type 1 | Generally single pulse | 14.6 | 1.3 | 13.3 | 1 | – | Single | 3.3 | 0.5–2 |
| Raorchestes chalazodes group | 1 type | Non-pulsatile | 18.4–36.0 | 1.2–4.1 | 11.0–32.3 | 1 | – | Single | 2.7–3.6 | 1–7 | |
| R. chalazodes | Rapidly delivered in long call groups | Type 1 | Non-pulsatile | 18.4 | 2.9 | 15.2 | 1 | – | Single | 2.7 | 1.5–7 |
| R. ochlandrae | Rapidly delivered in long call groups | Type 1 | Non-pulsatile | 25.3 | 4.1 | 20.9 | 1 | – | Single | 2.7 | 1.5–7 |
| R. manohari | Rapidly delivered in long call groups | Type 1 | Non-pulsatile | 12.3 | 1.2 | 11.0 | 1 | – | Single | 3.6 | 1–7 |
| R. uthamani | Rapidly delivered in long call groups | Type 1 | Non-pulsatile | 36.0 | 3.2 | 32.3 | 1 | – | Single | 3.4 | 1–7 |
| R. flaviocularis | Not studied | 1.5–7 | |||||||||
| Raorchestes charius group | 1 type | Pulsatile | 24.6–92.6 | 1.2–59.4 | 16.2–49.1 | 3–17 | 89.3–266.6 | Single | 2.4–4.1 | 0–4 | |
| R. charius | Uniform intervals, not delivered in groups | Type 1 | Pulsatile, relatively short and closely packed pulses | 92.6 | 49.2 | 37.2 | 17 | 226.6 | Single | 2.4 | 0–1.5 |
| R. griet | Uniform intervals, not delivered in groups | Type 1 | Pulsatile, relatively short and closely packed pulses | 75.6 | 59.4 | 16.2 | 10 | 151.3 | Single | 3.5 | 0.5–1.5 |
| R. honnametti | Uniform intervals, not delivered in groups | Type 1 | Pulsatile, relatively short and closely packed pulses | 68.6 | 13.3 | 44.2 | 6 | 89.3 | Single | 2.6 | 0.5–1.5 |
| R. coonoorensis | Not delivered in groups | Type 1 | Pulsatile, relatively short and closely packed pulses | 24.6 | 1.2 | 22.2 | 3 | 200 | Single | 2.9 | 0.5–1.5 |
| R. drutaahu sp. nov. | Not delivered in groups | Type 1 | Pulsatile, relatively short and closely packed pulses | 50.6 | 1.2 | 49.1 | 6 | 134.5 | Single | 3.6 | 0.5–1.5 |
| R. kollimalai | Not studied | 1–4 | |||||||||
| R. marki | Not delivered in groups | Type 1 | Pulsatile, closely packed pulses | 36.7 | 1.3 | 32.6 | 4 | 266.6 | Single | 4.1 | 1.5–3 |
| Raorchestes chotta group | 1–2 types | Pulsatile or Non-pulsatile | 17.1–71.2 | 1.1–38.3 | 15.0–18.8 | 1–19 | Nil–382.1 | Single | 3.1–3.6 | 0–4 | |
| R. archeos | Uniform intervals, delivered in groups | Type 1 | Pulsatile, relatively short and closely packed pulses | 19.7 | 1.2 | 17.2 | 6 | 382.1 | Single | 3.1 | 0–2 |
| R. blandus | Delivered in groups (only Type 1 call), lack any fixed call order |
Type 1 | Pulsatile | 17.1 | 1.5 | 15.8 | 3 | 370.3 | Single | 3.5 | 0.5–4 |
| Type 2 | Non-pulsatile | 20.1 | 1.4 | 18.8 | 1 | – | Single | 3.5 | |||
| R. chotta | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Pulsatile | 71.2 | 38.3 | 18.6 | 19 | 283.5 | Single | 3.6 | 0–2 |
| Type 2 | Non-pulsatile | 17.2 | 1.1 | 15.0 | 1 | – | Single | 3.6 | |||
| Raorchestes chromasynchysi group | 1 type | Pulsatile | 411.2–716.0 | 345.2–693.8 | Nil–35.1 | 2–6 | 2.7–7.2 | Single | 2.2–2.5 | 0.5–6 | |
| R. chromasynchysi | Not delivered in groups | Type 1 | Pulsatile, widely spaced pulses | 381.4 | 345.2 | Not significant | 3 | 5.8 | Single | 2.5 | 0.5–6 |
| R. ravii | Not delivered in groups | Type 1 | Pulsatile, widely spaced pulses | 499.2 | 483.1 | 16.1 | 3 | 4.2 | Single | 0.5–5 | |
| R. sanjappai sp. nov. | Not delivered in groups | Type 1 | Pulsatile, widely spaced pulses | 411.2 | 376.2 | 35.1 | 2 | 2.7 | Single | 2.4 | 0.5–3 |
| R. silentvalley | Uniform intervals, not delivered in groups | Type 1 | Pulsatile, widely spaced pulses | 716.0 | 693.8 | Not significant | 6 | 7.2 | Single | 2.2 | 1–4 |
| R. vellikkannan sp. nov. | Not studied | 1–4 | |||||||||
| Raorchestes flaviventris group | 1 type | Pulsatile | 480.0–721.7 | 458.7–712.4 | – | 12–15 | 21.1–26.4 | Single | 1.7–1.9 | 1–7 | |
| R. flaviventris | Uniform intervals, not delivered in groups | Type 1 | Pulsatile | 721.7 | 712.4 | Not significant | 15 | 21.1 | Single | 1.9 | 1–6 |
| R. ponmudi | Uniform intervals, not delivered in groups | Type 1 | Pulsatile | 480.0 | 458.7 | Not significant | 12 | 26.4 | Single | 1.7 | 1–7 |
| Raorchestes glandulosus group | 1 type | Pulsatile | 445.6–813.3 | 429.4–711.2 | Nil–99.1 | 6–11 | 10.9–13.7 | Single | 2.7–3.6 | 1–8 | |
| R. akroparallagi | Uniform intervals, not delivered in groups | Type 1 | Pulsatile | 445.6 | 429.4 | Not significant | 6 | 13.7 | Single | 3.4 | 1–4 |
| R. bobingeri | Uniform intervals, not delivered in groups | Type 1 | Pulsatile | 565.6 | 550.1 | Not significant | 6 | 10.9 | Single | 3.6 | 1.5–7 |
| R. glandulosus | Uniform intervals, not delivered in groups | Type 1 | Pulsatile | 609.6 | 588.1 | Not significant | 8 | 11.7 | Single | 2.7 | 1.5–8 |
| R. jayarami | Uniform intervals, not delivered in groups | Type 1 | Pulsatile | 813.3 | 711.2 | 99.1 | 11 | 12.5 | Single | 2.9 | 1.5–6 |
| Raorchestes graminirupes group | 1–2 types | Pulsatile | 27.8–91.3 | Nil–50.7 | 15.9–88.7 | 3–18 | 114.8–222.5 | Single | 2.1–2.8 | 0–7 | |
| R. graminirupes | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Pulsatile | 91.3 | 1.7 | 88.7 | 18 | 222.5 | Single | 2.7 | 0–3 |
| Type 2 | Pulsatile | 27.8 | 1.0 | 26.8 | 5 | 203.3 | Single | 2.8 | |||
| R. johnceei | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Pulsatile | 66.3 | 50.7 | 15.9 | 8 | 114.8 | Single | 2.1 | 0.5–7 |
| Type 2 | Pulsatile | 28.8 | not significant | 28.0 | 3 | 171.4 | Single | 2.2 | |||
| Raorchestes nerostagona group | 1 type | Non-pulsatile | 13.3–23.3 | 1.2–2.2 | 12.0–20.2 | Nil | Nil | Single | 2.0–2.2 | 3–40 | |
| R. crustai | Not delivered in groups | Type 1 | Non-pulsatile | 13.3 | 1.2 | 12.0 | 1 | – | Single | 2.2 | 3–20 |
| R. nerostagona | Not delivered in groups | Type 1 | Non-pulsatile | 23.3 | 2.2 | 20.2 | 1 | – | Single | 2.0 | 5–40 |
| R. keirasabinae sp. nov. | Not studied | 5–30 | |||||||||
| Raorchestes signatus group | 1 type | Non-pulsatile | 20.2 | 2.0 | 18.2 | Nil | Nil | Single | 2.1 | 0.5–10 | |
| R. signatus | Uniform intervals, delivered in groups | Type 1 | Non-pulsatile | 20.2 | 2.0 | 18.2 | 1 | – | Single | 2.1 | 0.5–10 |
| Raorchestes tinniens group | 1 type | Non-pulsatile | 8.5 | 1.1 | 6.5 | Nil | Nil | Single | 2.6 | 0–2 | |
| R. tinniens | Uniformly intervals, delivered in groups | Type 1 | Non-pulsatile | 8.5 | 1.1 | 6.5 | 1 | – | Single | 2.6 | 0–1.5 |
| R. montanus | Not studied | 0–2 | |||||||||
| R. primarrumpfi | Not studied | 0–1.5 | |||||||||
| Raorchestes travancoricus group | 2 types | Pulsatile or Non-pulsatile | 15.6–474.5 | 1.6–355.7 | 13.0–116.8 | 3–47 | 31.9–238.1 | Single | 2.2–3.4 | 0–4 | |
| R. agasthyaensis | Not studied | 0–2 | |||||||||
| R. chlorosomma | Delivered in groups, lack any fixed call order | Type 1 | Pulsatile, closely packed pulses | 246.2 | 109.5 | 106.6 | 47 | 195.8 | Single | 2.2 | 1.5–4 |
| Type 2 | Pulsatile, closely packed pulses | 30.2 | 2.0 | 27.3 | 5 | 238.1 | Single | 2.2 | |||
| R. kadalarensis | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Non-pulsatile | 15.6 | 1.6 | 13.0 | 1 | – | Single | 3.4 | 0–2 |
| Type 2 | Pulsatile | 52.6 | 33.7 | 18.9 | 3 | 66.9 | Single | 3.4 | |||
| R. luteolus | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Pulsatile | 390.5 | 270.5 | 88.6 | 12 | 31.9 | Single | 2.7 | 0.5–1.5 |
| Type 2 | Non-pulsatile | 27.2 | 1.6 | 25.6 | 1 | – | Single | 2.5 | |||
| R. travancoricus | Delivered in groups, fixed call order (Type 1 followed by Type 2) | Type 1 | Pulsatile | 474.5 | 355.7 | 116.8 | 15 | 33.1 | Single | 3.3 | 0.5–1.5 |
| Type 2 | Non-pulsatile | 17.2 | 1.8 | 15.3 | 1 | – | Single | 3.3 | |||
| Raorchestes aureus group | 1 type | Pulsatile or Non-pulsatile | 180–320 | NA | NA | 1–8 | NA | Single | 2.6–3.0 | 0.5–2 | |
| R. aureus* | Not studied | Type 1 | Non-pulsatile | 180 | NA | NA | 1 | NA | Single | 3.0 | 0.5–2 |
| R. lechiya* | Not studied | Type 1 | Pulsatile | 320 | NA | NA | 8 (average) | NA | Single | 2.6 | 0.5–2 |
| *Call properties based on data available in Zachariah et al., 2016 | |||||||||||
| Ungrouped species | |||||||||||
| R. indigo | Not studied | 0.5–3 | |||||||||
| R. echinatus | Not studied | 0–1 | |||||||||
| State/District | Locality | Species studied |
|---|---|---|
| Tamil Nadu | ||
| Coimbatore | Grass Hills | R. dubois, R. flaviventris, R. griet, R. resplendens, R. sushili |
| Sholayar | R. akroparallagi, R. anili, R. blandus, R ochlandrae, R. sushili | |
| Valparai | R. akroparallagi, R. beddomii, R. flaviventris, R. griet, R. jayarami, R. ochlandrae, R. sushili, R uthamani | |
| Dindigal Anna | Kodaikanal | R. dubois |
| Kanyakumari | Kiriparai | R. akroparallagi |
| Namakkal | Kolli Hills | R. kollimalai |
| Nilgiris | Avalanche | R. signatus, R. tinniens |
| Bangitapal | R. primarrumpfi, R. signatus, R silentvalley | |
| Coonoor | R. charius, R.coonoorensis, R. signatus, R. tinniens | |
| Kotagiri | R. coonoorensis, R. signatus, R. tinniens | |
| Mukurthi | R. lechiya, R. primarrumpfi, R. signatus, R. silentvalley, R. tinniens | |
| Naduvattam | R. charius, R. coonoorensis, R. ravii, R. signatus, R. tinniens | |
| Ooty | R. signatus, R. tinniens | |
| Parsons Valley | R. signatus, R. tinniens | |
| Pykara | R. coonoorensis, R. signatus, R. tinniens | |
| Salem | Yercaud | R. kollimalai |
| Theni | Bodinayakkanur | R. travancoricus |
| Meghamalai | R. beddomii, R. chlorosomma, R. dubois, R. flaviocularis, R griet, R. cf. kaikatti, R. munnarensis, R. travancoricus | |
| Tirunelveli | Kakachi |
R. agasthyaensis, R. bobingeri, R. chalazodes, R. crustai, R. graminirupes, R. johnceei, R. kakachi, R. manohari |
| Kannikatti | R. akroparallagi, R. archeos | |
| Kodayar |
R. agasthyaensis, R. bobingeri, R. chalazodes, R. crustai, R. graminirupes, R. johnceei, R. kakachi, R. manohari |
|
| Sengaltheri | R. bobingeri, R. johnceei | |
| Singampatti | R. beddomii | |
| Kerala | ||
| Idukki | Chinnar | R. chlorosomma, R dubois, R griet, R. jayarami, R. kadalarensis, R. munnarensis, R. resplendens |
| Devikulam | R. beddomii, R chlorosomma, R. dubois, R. griet, R. jayarami, R. kadalarensis, R. munnarensis | |
| Eravangalar | R. dubois, R. flaviocularis, R uthamani | |
| Eravikulam National Park | R. beddomii, R. chlorosomma, R. dubois, R. flaviventris, R griet, R. kadalarensis, R. munnarensis, R. ochlandrae, R. resplendens, R. sushili | |
| Kozhikana | R. akroparallagi, R. anili | |
| Kadalar | R. chlorosomma, R. dubois, R. drutaahu sp. nov., R. flaviventris, R. jayarami, R. kadalarensis, R. keirasabinae sp. nov., R. munnarensis, R. ochlandrae, R. sushili, R. theuerkaufi | |
| Mathikettan | R. beddomii, R. chlorosomma, R. jayarami, R munnarensis, R. sushili | |
| Mattupetti |
R. beddomii, R. chlorosomma, R. griet, R. jayarami, R. kadalarensis, R. munnarensis |
|
| Meesapulimala | R dubois, R. resplendens | |
| Munnar |
R. beddomii, R. cf. bobingeri, kadalarensis, R. munnarensis, R. chlorosomma, R. griet, R. resplendens, R. kadalarensis |
|
| Painav | R. akroparallagi | |
| Thekkady | R. anili, R. keirasabinae sp. nov. | |
| Upper Manalar |
R. beddomii, R. chlorosomma, R. dubois, R. flaviocularis, R. cf. kaikatti, R. munnarensis, R. travancoricus, R. uthamani |
|
| Vandiperiyar |
R. anili, R. griet, R. keirasabinae sp. nov., R. ponmudi, R. travancoricus |
|
| Vagamon | R. akroparallagi, R. anili, R. cf. bobingeri, R. griet, R. keirasabinae sp. nov., R. ponmudi, R. travancoricus | |
| Vaguvarai | R. beddomii, R chlorosomma, R. dubois, R griet, R munnarensis | |
| Vattavada | R. beddomii, R dubois, R. griet | |
| Kannur | Paithal Mala | R. charius, R luteolus, R. tuberohumerus |
| Aralam | R. akroparallagi | |
| Kasargod | Anakallu | R. akroparallagi |
| Ranipuram | R. anili, R. charius, R. luteolus, R. ponmudi, R. tuberohumerus | |
| Kollam | Shendurney |
R. agasthyaensis, R. akroparallagi, R. anili, R. archeos, R. bobingeri, R. beddomii, R. chalazodes, R. chotta, R. crustai, R. johnceei, R. kakachi, R. keirasabinae sp. nov., R. manohari, R. ponmudi |
| Thenmala | R akroparallagi | |
| Kozhikode | Kakkayam | R. akroparallagi, R. anili, R glandulosus, R. kakkayamensis sp. nov., R. ochlandrae, R. ponmudi |
| Palakkad | Nelliyampathi | R. jayarami, R. kaikatti, R. marki, R ochlandrae |
| Parambikulam | R. akroparallagi, R. anili, R. blandus, R. ochlandrae, R. sushili | |
| Silent Valley National Park |
R. anili, R charius, R. glandulosus, R. lechiya, R. signatus, R. silentvalley, R. tinniens, R. vellikannan sp. nov. |
|
| Siruvani |
R. anili, R. aureus, R. drutaahu sp. nov., R. leucolatus, R. vellikannan sp. nov. |
|
| Pathanamthitta | Gavi | R. akroparallagi, R. anili, R. keirasabinae sp. nov, R. ponmudi, R. uthamani |
| Pamba | R. akroparallagi | |
| Thrissur | Chimmini | R. kaikatti, R. keirasabinae sp. nov., R ochlandrae |
| Malakkappara–Sholayar | R. akroparallagi, R. anili, R. blandus, R. ochlandrae, R. sushili | |
| Vazhachal | R. akroparallagi, R. anili, R. blandus, R. ochlandrae | |
| Thiruvananthapuram | Athirimala | R. agasthyaensis, R. archeos, R. beddomii, R. chalazodes, R. crustai, R. graminirupes, R. johnceei, R. kakachi, R. manohari |
| Bonacaud | R. akroparallagi, R. anili, R. chotta, R. keirasabinae sp. nov., R. ponmudi | |
| Chathankod–Makki | R. akroparallagi, R. keirasabinae sp. nov. | |
| Pandipath |
R. agasthyaensis, R. archeos, R. bobingeri, R. beddomii, R. chalazodes, R. crustai, R. graminirupes, R. johnceei, R. manohari |
|
| Ponkalapara | R. archeos, R. bobingeri, R. beddomii, R. graminirupes | |
| Ponmudi |
R. akroparallagi, R. anili, R. archeos, R. bobingeri, R. chotta, R. graminirupes, R. keirasabinae sp. nov., R. ponmudi |
|
| Wayanad | Banasura |
R chromasynchysi, R. glandulosus, R. ochlandrae, R tuberohumerus |
| Kalpetta |
R. akroparallagi, R. anili, R. nerostagona, R. ponmudi, R. tuberohumerus |
|
| Kurichiyarmala | R. anili, R charius, R. chromasynchysi, R. glandulosus, R. ponmudi | |
| Mananthavady |
R. akroparallagi, R. anili, R. glandulosus, R. nerostagona, R. ponmudi, R. tuberohumerus |
|
| Muthanga | R. anili, R. tuberohumerus | |
| Periya | R. akroparallagi, R. anili, R. ponmudi, R. sanjappai sp. nov. | |
| Pozhuthana | R. akroparallagi, R. anili, R. ochlandrae, R. ponmudi | |
| Sultan Bathery | R. akroparallagi, R. anili, R. glandulosus, R. nerostagona, R. ponmudi | |
| Thirunelly |
R. anili, R. charius, R. chromasynchysi, R. ochlandrae, R. tuberohumerus |
|
| Vellarimala | R. charius, R. glandulosus, R. signatus | |
| Vythiri | R akroparallagi, R. anili, R. nerostagona, R. ochlandrae, R. ponmudi, R. tuberohumerus | |
| Karnataka | ||
| Belgaum | Londa | R. bombayensis |
| Chamrajnagar | BR Hills | R. honnametti |
| Chikmagalur | Baba Budangiri | R. charius, R. chromasynchysi, R. echinatus |
| Balehoonoor | R. luteolus, R. tuberohumerus | |
| Bygoor | R. luteolus, R. tuberohumerus | |
| Chikmagalur | R. charius, R. tuberohumerus, R. luteolus | |
| Kemmangundi | R. charius, R. chromasynchysi, R. ochlandrae | |
| Kudremukh NP | R. charius, R. chromasynchysi, R. indigo, R. luteolus, R. montanus, R. ochlandrae, R. tuberohumerus | |
| Mudigere | R. luteolus, R. tuberohumerus | |
| Muthodi | R. charius, R. chromasynchysi, R. luteolus, R. ochlandrae, R. tuberohumerus | |
| Dakshin Kannada | Beluvai | R. akroparallagi |
| Mangalore | R. akroparallagi | |
| Punacha | R. akroparallagi | |
| Hassan | Kempholay | R. luteolus, R. tuberohumerus |
| Kottigehara | R. charius, R. tuberohumerus | |
| Sakleshpur | R. luteolus, R. tuberohumerus | |
| Kodagu | Bhagamandala | R. anili, R. ponmudi |
| Madikeri | R. anili, R. charius, R. glandulosus, R. luteolus, R. nerostagona, R. ponmudi, R. tuberohumerus | |
| Nishanimotta | R. chromasynchysi | |
| Thalakaveri | R. chromasynchysi, R. tuberohumerus | |
| Yevakapadi | R. anili, R. charius, R. chromasynchysi, R. glandulosus, R. luteolus, R. ponmudi, R. tuberohumerus | |
| Shimoga | Agumbe | R. tuberohumerus, R. nerostagona |
| Jog falls | R. luteolus, R. tuberohumerus | |
| Uttara Kannada | Castle Rock | R. bombayensis |
| Mavingundi | R. luteolus | |
| Goa | ||
| South Goa | Netravali | R. bombayensis |
| Maharashtra | ||
| Pune | Bhimashankar | R. ghatei |
| Lavasa | R. ghatei | |
| Raigad | Matheran | R. ghatei |
| Phansad | R. bombayensis | |
| Satara | Kaas | R. ghatei |
| Mahabaleshwar | R. ghatei | |
| Sawantadi | Amboli | R. bombayensis |
| Andhra Pradesh | ||
| East Godavari | Maredumilli | R. sanctisilvaticus |
| Vishakhapatnam | Peddavalasa | R. sanctisilvaticus |
| Chintapalli | R. sanctisilvaticus | |
| Borra Caves | R. sanctisilvaticus | |
| Araku Valley | R. sanctisilvaticus | |
| Odisha | ||
| Khurda | Barbara | R. sanctisilvaticus |
| Mayurbhanj | Similipal | R. sanctisilvaticus |
| Madhya Pradesh | ||
| Anuppur | Amarkantak | R. sanctisilvaticus |
Etymology. The species name is derived from Sanskrit ‘druta’ (meaning fast) and ‘ahu’ (meaning call), referring to the fast-pulsatile calls of the new species. The species epithet drutaahu is treated as an invariable noun in apposition to the generic name.
Holotype. BNHS 6088, an adult male, from Kadalar (10.1311° N, 77.0005° E, 1,430 m asl), Munnar, Idukki district, Kerala State, India, collected by SDB and SG on 17 August 2014. Paratypes. BNHS 6089, an adult male, collected by SDB and SG, along with the holotype; BNHS 6090, an adult male, collected by RS and SDB, from the holotype locality on 10 August 2012; and BNHS 6091, an adult male, from Siruvani (10.9587° N, 76.6667° E, 1,048 m asl), Palakkad district, Kerala State, India, collected by SDB, SG, and RS on 07 July 2015. Referred specimen. SDBDU 2015.3025, an adult female, from Siruvani, Palakkad district, Kerala State, India, collected by SDB, SG, and RS on 07 July 2015.
Phylogenetic relationship. Raorchestes drutaahu sp. nov. is a member of the Raorchestes charius group and shows a well-supported sister-group relationship with R. coonoorensis (Fig. 2). For the mitochondrial 16S rRNA, Raorchestes drutaahu is divergent from other members of the group as: 4.5–7.9% from R. charius; 4.3–5.2% from R. coonoorensis; 4.3–6.6% from R. griet; and 4.6–5.6% from R. honnametti.
Morphological diagnosis and comparison. Raorchestes drutaahu sp. nov. can be distinguished from other known congeners, except members of the Raorchestes charius group, by the combination of following morphological characters: a small-sized species (male SVL 20–23 mm); outline of the snout rounded to sub-ovoid in ventral view; tympanum distinct, nearly half of the eye diameter; dorsum light to dark brown or straw coloured with horny spinules and ridges; dorsum with two faint to prominent shaped concave bands, extending from behind the eye to nearly the vent; lateral surfaces of the head dark brown from tip of the snout and along the margins of the eye and supratympanic fold; a pair of black irregular shaped spots near the groin on either side of posterior dorsum; flank and groin grey or light brown without contrasting colour blotches or markings; posterior surface of thighs dark to light brown without prominent markings; iris brown with a golden tinge, horizontally divided into light upper and dark lower halves; foot webbing small, below the second subarticular tubercle on either side of toe IV (Fig. 3).
Within the Raorchestes charius group, R. drutaahu sp. nov. is more closely related to R. charius, R. coonoorensis, and R. honnametti. However, R. drutaahu sp. nov. can be differentiated from these three species by its groin with faint white blotches (vs. groin light brown with pale yellow or greyish blotches in R. coonoorensis; groin deep brown with yellow blotches in R. charius; groin light brown with minute white marbling in R. honnametti); a pair of black irregular shaped spots on either side of the posterior dorsum near the groin (vs. absent); and relatively reduced webbing on foot, third toe webbing just above the first subarticular tubercle on the outside (vs. more extensive, nearly up to the second subarticular tubercle). Specifically, it also differs from R. charius and R. honnametti by its relatively smaller adult size, male SVL 20–23 mm, female SVL 24.5 mm (vs. larger, male SVL 26–35 mm in R. charius; and male SVL 23–28 mm in R. honnametti). Further, it differs from R. griet by relatively larger adult size, male SVL 20–23 mm, female SVL 24.5 mm (vs. smaller, male SVL 18–22 mm, female SVL 22 mm); and more extensive webbing on foot, well beyond the first subarticular tubercle on either side of toe IV (vs. reduced, up to or slightly above the first subarticular tubercle on either side). Raorchestes drutaahu sp. nov. also differs from the most recently described species R. kollimalai by relatively smaller adult size, male SVL 20–23 mm, female SVL 24.5 mm (vs. larger, male SVL 25.8–29.7 mm); head nearly as wide as long (vs. wider than long); shank nearly equal or shorter than thigh (vs. longer) (Gowande, Ganesh & Mirza, 2020); and webbing between toes III and IV rudimentary, well below the first subarticular tubercle on either side (vs. nearly up to the first subarticular tubercle).
We specifically also compare Raorchestes drutaahu sp. nov. with the type series of R. ravii (see taxonomic remarks for that species), and show that it differs due to the presence of black irregular shaped spots on either side of the posterior dorsum near the groin (vs. absent); head nearly as wide as long, HW/HL ratio 0.99–1.01 (vs. wider than long, HW/HL ratio 1.35–1.36); snout rounded to sub-ovoid (vs. pointed); tympanum distinct (vs. indistinct); shank nearly equal or shorter than thigh, SHL/TL ratio 0.95–1.0 (vs. shank longer than thigh, SHL/TL ratio 1.06–1.07); relatively reduced foot webbing, fourth toe webbing below the second subarticular tubercle on either side, I1–2+II2+–3III3––31/2IV3+ –2+V (vs. more extensive, up to or above the second subarticular tubercle on either side of toe IV, I1+–2+II2+–3+III2–3+IV3––2V; iris brown with golden tinge, horizontally divided into light upper and dark lower halves (vs. uniformly golden brown or reddish-brown); sclera light grey (vs. scarlet blue, based on the holotype photograph); and short pulsatile male advertisement calls with closely packed pulses and relatively faster pulse rate, characteristic for members of the R. charius group (vs. widely spaced pulses, characteristic for members of the R. chromasynchysi group).
Description of holotype (measurements in mm). Small-sized adult male (SVL 21.9) with a slender body; head nearly as long as wide (HL 7.8; HW 7.7; MN 6.8; MFE 5.1; MBE 2.8); outline of the snout rounded to sub-ovoid in dorsal and ventral view, rounded in lateral view; snout length (SL 3.4) longer than horizontal diameter of eye (EL 2.6); loreal region acutely flat with rounded canthus rostralis; distance between posterior margins of eyes (IBE 6.7) 1.7 times the distance between anterior margins of eyes (IFE 4.0); tympanum rather distinct (TYD 1.2), 46.2% of eye diameter (EL 2.6); supratympanic fold rather distinct; tongue with a lingual papilla. Forearm (FAL 4.9) shorter than hand (HAL 5.7); fingers without lateral dermal fringe; webbing absent; subarticular tubercles rather prominent, rounded, single, III2 and IV2 weakly-developed; prepollex rather indistinct; palmar tubercle small, rounded; supernumerary tubercles present; nuptial pad present, smooth. Hindlimbs moderately long, thigh (TL 10.5) nearly equal to shank (SHL 10.4) and longer than foot (FOL 7.9); distance from heel to tip of toe IV (TFOL 14.0); foot webbing small: I1–2+II2+–3III3––31/2IV3+–2+V, below the second subarticular tubercle on either side of toe IV; dermal fringe along toe V absent; subarticular tubercles rather prominent, rounded, simple, IV2 and V2 weakly-developed; supernumerary tubercles present (Fig. 3).
Skin of snout and between eyes shagreened with fine scattered and various sized granular projections; a faint horny ridge extending from the tip of the snout to the vent; a weakly-developed horny ridge between the eyes, arranged in a triangle directed posteriorly; lateral surfaces of head shagreened; dorsal surface of limbs shagreened to sparsely granular. Ventral skin on throat shagreened to granular; chest, belly, and posterior surface of thighs granular.
Colour of holotype. In life. Dorsum brown; a faint dark grey stripe between the eyes; dorsum with two dark brown )( shaped concave bands, extending from behind the eyes to the level of the groin; a pair of black irregular shaped spots on either side of the posterior dorsum near the groin; lateral surfaces of head dark brown; lateral abdominal surfaces lighter than dorsum; groin light greyish-brown without blotches; anterior and posterior surface of thighs brown with dark greyish-brown mottling; dark blackish-brown markings around the cloacal opening; fore and hind limbs (including fingers and toes) brown or light brown with a few scattered dark brown cross-bands; iris brown with golden tinge, upper half lighter than lower half. Ventral surfaces light brown with minute dark brown speckling; hand and foot greyish-brown (Fig. 3). In preservation. Dorsum dark grey with blackish-brown )( shaped concave bands; lateral surfaces of head blackish-brown; lateral abdominal surfaces greyish with small off-white spots; groin grey with light grey blotches; posterior surface of thighs light brown with scattered creamish mottling; limbs with dark cross-bands. Ventral surface of throat creamish-white with dense minute dark brown speckles; chest, belly, fore and hind limbs creamish-white with scattered dark brown speckles; limbs greyish-brown with creamish-white mottling (Fig. 3).
Variations. Morphometric data from five specimens, including the holotype, is given in Table S2. The dorsal colouration and markings are variable in life: BNHS 6089 and BNHS 6090: dark blackish-brown markings around the cloacal opening, surrounded with white patches; BNHS 6091: dorsum greyish-brown with prominent dark brown markings; stripe between eyes dark brown. BNHS 6089 and SDBDU 2015.3025: dorsum light greyish-brown to straw coloured with faint and irregular dark brown dorsal markings.
Vocalisation. Raorchestes drutaahu sp. nov. males produce a single type of call. Calls are not delivered in groups and have a pulsatile temporal structure, with relatively short and closely packed pulses. A typical call shows a duration of 50.6 ms; the amplitude envelope being characterised by a rise time of 1.2 ms and fall time of 49.1 ms; with six pulses delivered at a rate of 134.5 pulses/second; and the spectrum showing a single broad peak with mean dominant frequency of 3.6 kHz. For comparison see Table 2 and the group definition, including the oscillogram and spectrogram figures cited therein.
Distribution and natural history. Raorchestes drutaahu sp. nov. is endemic to the Western Ghats and currently known only from elevations ranging between 1,000 to 1,450 m asl at two localities: Kadalar in Idukki district (south of Palghat gap) and Siruvani in Palakkad district (north of Palghat gap). The species has been observed in forest areas, either on grassland-shola fringes or fragmented forest patches near plantations. Individuals were located on leaves of short shrubs at heights of 0.5–1.5 m.
Raorchestes kakkayamensis sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:BD7A17C6-A1E2-421A-B0E8-2336931CD63F
Kakkayam Shrub Frog
(Figs. 2, 4 and 5; Tables 1–3; Tables S1–S5)
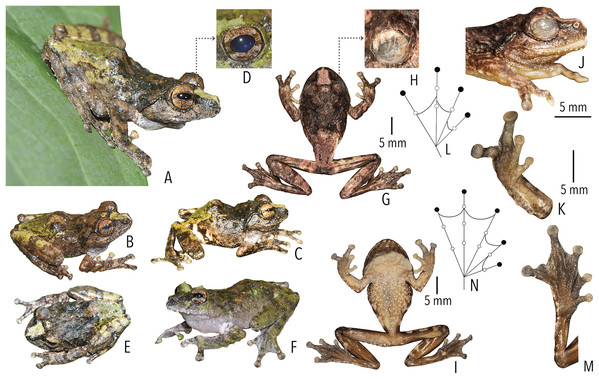
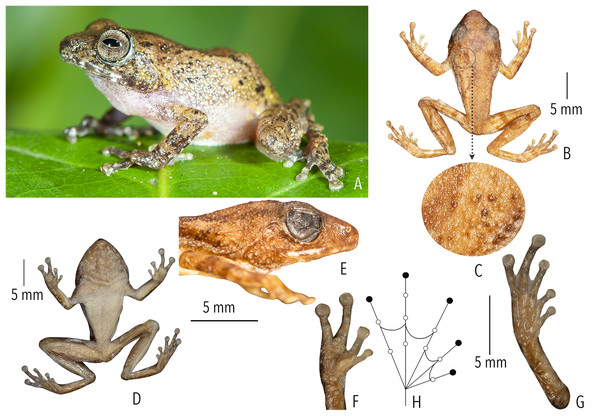
Figure 4: Holotype (BNHS 6092, adult male) of Raorchestes kakkayamensis sp. nov.
(A–H) In life. (A) Dorsolateral view. (B) Dorsal view. (C) Ventral view. (D) Dorsal view of body and thighs. (E) Lateral view. (F) Posterior view of thighs. (G) Ventral view of hand. (H) Ventral view of foot. (I–N) In preservation. (I) Dorsal view. (J) Ventral view. (K) Lateral view of head. (L) Ventral view of hand. (M) Ventral view of foot. (N) Schematic illustration of webbing on foot.Figure 5: Projection of the first two Principal Component (PC) factor planes explaining 79.45% of the total variation among six species of the Raorchestes bombayensis group and showing morphometric distinctness of the new species, R. kakkayamensis.
Etymology. The species is named after the place Kakkayam, where the type series was collected.
Holotype. BNHS 6092, an adult male, from Kakkayam (11.5542° N, 75.9196° E, 750 m asl), Kozhikode district, Kerala State, India, collected by SDB and RS in June 2018. Paratypes. BNHS 6093–6096, four adult males, collected along with holotype. Referred specimen. SDBDU 2019.3423, an adult male, from the holotype locality, collected by RS and SD on 28 July 2018.
Phylogenetic relationship. Raorchestes kakkayamensis sp. nov. is a member of the Raorchestes bombayensis group and shows a well-supported sister-group relationship with R. leucolatus (Fig. 2). For the mitochondrial 16S rRNA, Raorchestes kakkayamensis is divergent from other members of the group as: 3.2–4.6% from R. bombayensis; 5.3–5.9% from R. ghatei; 2.3–2.4% from R. leucolatus; 4.0–4.5% from R. sanctisilvaticus; and 3.1–3.6% from R. tuberohumerus.
Morphological diagnosis and comparison. Raorchestes kakkayamensis sp. nov. can be distinguished from other known congeners, except members of the Raorchestes bombayensis group, by the combination of the following morphological characters: small-sized species (male SVL 17–19 mm, female SVL 24 mm); a knobbed bony projection on humerus in males (visible externally in preserved specimens); lateral surfaces of abdomen and groin with contrasting white blotches on grey to dark grey background; presence of horny spinules and horny ridges on dorsal skin; and finger and toe discs yellowish-brown (Fig. 4).
Within the Raorchestes bombayensis group, Raorchestes kakkayamensis sp. nov. differs from R. bombayensis and R. ghatei by its smaller adult size, male SVL 17–19 mm (vs. male SVL > 19 mm); differs from R. tuberohumerus by its relatively smaller adult size, male SVL 17–19 mm (vs. larger, male SVL 18–22 mm); flank and groin with white blotches on grey to dark grey background (vs. yellow blotches); and finger and toe discs yellowish-brown (vs. grey to brown); and differs from R. leucolatus by its head longer than wide, HW/HL ratio 0.86–0.95 (vs. wider than long, HW/HL ratio 1.20–1.33); thigh relatively longer than shank, TL/SHL ratio 1.03–1.12 (vs. nearly equal, TL/SHL ratio 0.99–1.04); supernumerary tubercles prominent on toes II–V (vs. weakly-developed); and finger and toe discs yellowish-brown (vs. orange or orangish-red).
Morphometrically, the new species Raorchestes kakkayamensis was also well differentiated from the other recognised members of the Raorchestes bombayensis group, that is R. bombayensis, R. ghatei, R. leucolatus, R. sanctisilvaticus, and R. tuberohumerus. All the six species formed distinct clusters when projected on the first two PCA factor planes that had eigenvalues >1.0 and explained 79.44% of variation among the species (Fig. 5). The PCA factor loadings representing the composition of PCA factors, and the parameters correlated with each PCA factor are shown in Table S3. PCA Factor 1 loaded heavily on all the morphometric parameters (total 20) except FWIII, while PCA Factor 2 loaded heavily on two morphometric parameters, FWIII and TWIV. Furthermore, our DFA resulted in 100% classification success with all the individual samples being classified into their respective species (Table S5). The coefficients of canonical discriminant function representing the composition of standardized canonical discriminant scores are shown in Table S4. All the five discriminant function roots showed eigenvalues >1.0 and explained 100% of the variations among these species. The PCA and DFA results provide additional evidence for morphometric differentiation of Raorchestes kakkayamensis sp. nov. from its closest congeners.
Description of holotype (measurements in mm). Small-sized adult male (SVL 18.8) with a slender body; head longer than wide (HL 7.0; HW 6.5; MN 5.0; MFE 5.1; MBE 1.9); outline of the snout nearly sub-ovoid in dorsal and ventral view, acute in lateral view; snout length (SL 2.8) longer than horizontal diameter of eye (EL 2.5); loreal region acute with indistinct canthus rostralis; distance between posterior margins of eyes (IBE 6.1) twice the distance between anterior margins of eyes (IFE 3.1); tympanum (TYD 1.0) 40% of eye diameter (EL 2.5); supratympanic fold rather indistinct; tongue with a lingual papilla. Forearm (FAL 3.9) shorter than hand (HAL 4.9); fingers without lateral dermal fringe; webbing absent; subarticular tubercles rather prominent, rounded, single, III2 and IV2 weakly-developed; prepollex rather indistinct; palmar tubercle small, rounded; supernumerary tubercles absent; nuptial pad present, smooth. Hindlimbs moderately long, thigh (TL 9.2) longer than shank (SHL 8.9) and foot (FOL 7.3); distance from heel to tip of toe IV (TFOL 11.8); foot webbing small: I2–2II2–3III21/4–31/2IV3+–2V, below the second subarticular tubercle on either side of toe IV; dermal fringe along toe V absent; subarticular tubercles rather prominent, rounded, simple, IV2 and V2 weakly-developed; supernumerary tubercles absent (Fig. 4).
Skin of snout and between eyes shagreened; upper eyelids and lateral surfaces of head shagreened to sparsely glandular; anterior and posterior part of back shagreened with sparsely scattered spinular projections; lateral surfaces of abdomen with scattered granulations. Ventral surface of throat and chest shagreened to granular; belly and posterior surface of thighs granular (Fig. 4).
Colour of holotype. In life. Dorsum brown to reddish-brown with a pair of dark discontinuous concave bands extending from behind the eye to the level of the groin, with a thin mid-dorsal line; a dark brown coloured horizontal band between the upper eyelids; snout lighter in colour than the dorsum; lateral surfaces of head darker than dorsal colouration; lateral abdominal surfaces light brown with small off-white spots; groin and anterior surface of thighs dark brown with distinct white blotches; posterior surface of thighs light and dark brown with scattered white spots; limbs with dark brown cross-bands; finger and toe discs yellowish-brown; iris brown with dense golden yellow speckling and faint dark brown horizontal and vertical bands. Ventral surface of throat blackish-brown with scattered white speckles; chest, and belly, light brown with dense dark brown speckling and irregular white spots; fore and hind limbs dark brown with white to ash blue spots and minute light brown speckling (Fig. 4). In preservation. Dorsum greyish-brown with blackish-brown discontinuous concave bands extending from behind the eye to the level of the groin, with a thin light grey mid-dorsal line; snout light grey; lateral surfaces of head darker than dorsal colouration; lateral abdominal surfaces greyish-brown with small off-white spots; groin and anterior surface of thighs dark brown with distinct white blotches; posterior surface of thighs light and greyish-brown with scattered white spots; limbs with faint or prominent dark greyish-brown cross-bands. Ventral surface of throat dark brown with scattered white speckles, especially along the margins of lower jaw; chest, belly, fore and hind limbs light brown with white spots and minute dark brown speckling (Fig. 4).
Variations. Morphometric data from six specimens, including the holotype, is given in Table S2. The dorsal colouration and markings are slightly variable from light to dark brown or reddish-brown, but there is a uniform triangular light brown colouration from the snout tip to the anterior margins of the eyes. BNHS 6096: less prominent dorsal markings and granulations.
Vocalisation. Raorchestes kakkayamensis sp. nov. males produce a single type of call. The calls generally have a single pulse and are not delivered in groups. A typical call has duration of 25.3 ms, the amplitude envelope being characterised by a rise time of 9.2 ms and fall time of 15.9 ms, with a dominant frequency of 3.8 kHz. For comparison see Table 2 and the group definition, including the oscillogram and spectrogram figures cited therein.
Distribution and natural history. Raorchestes kakkayamensis sp. nov. is endemic to the Western Ghats and currently known only from its type locality (Kakkayam) in Kozhikode district, north of Palghat gap in Kerala State. The species was observed inside a primary forest patch and adjoining secondary forest areas at an elevation of 750 m asl. Individuals were located on the ground leaf litter or found perching on vegetation 1–3 m high.
Raorchestes keirasabinae sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:39BCB0CE-166E-4C90-AE1E-564BD626ABA9
Keira’s Shrub Frog
(Figs. 2 and 6; Tables 1–3; Tables S1 and S2)
Figure 6: Raorchestes keirasabinae sp. nov.
(A–F) In life. (A–C) Holotype (BNHS 6097, adult male), dorsolateral view. (D) Enlarged view of spinular projections on the upper eyelid of the holotype. (E) Dorsal view (not collected). (F) Frontolateral view (not collected). (G–N) Holotype (BNHS 6097, adult male), in preservation. (G) Dorsal view. (H) Enlarged view of spinular projections on the upper eyelid. (I) Ventral view. (J) Lateral view of head. (K) Ventral view of hand. (L) Schematic illustration of webbing on hand. (M) Ventral view of foot. (N) Schematic illustration of webbing on foot.Etymology. The species is named after a young nature lover Keira Sabin, in appreciation of the long-time support and commitment of the Andrew Sabin Family Foundation towards amphibian research and conservation around the world. The species epithet keirasabinae is treated as a noun in the genitive case.
Holotype. BNHS 6097, an adult male, from Chathankod-Makki (8.6723° N, 77.1301° E, 230 m asl), Thiruvananthapuram district, Kerala State, India, collected by SDB and SG on 29 April 2015. Paratype. BNHS 6098, an adult male, from Ponmudi (8.75° N, 77.13° E, 980 m asl), Thiruvananthapuram district, Kerala State, India, collected by SDB in May 2006. Referred specimen. SDBDU 2019.3450, an adult male, from Vallakadavu (9.5281° N, 77.1144° E, 834 m asl), Periyar Tiger Reserve, Idukki district, Kerala State, India, collected by SD on 27 July 2016.
Phylogenetic relationship. Raorchestes keirasabinae sp. nov. is a member of the Raorchestes nerostagona group and shows a well-supported sister-group relationship with R. nerostagona (Fig. 2). For the mitochondrial 16S rRNA, Raorchestes keirasabinae sp. nov. is divergent from other members of the group as: 6.1–6.6% from R. crustai and 3.1–3.7% from R. nerostagona.
Morphological diagnosis and comparison. Raorchestes keirasabinae sp. nov. can be distinguished from other known congeners, except members of the Raorchestes nerostagona group, by the combination of the following morphological characters: medium-sized (male SVL 29–31 mm) predominantly canopy dwelling species; snout vertical in lateral view; webbed fingers; nearly fully webbed toes; and a distinct dermal fringe along the outer margins of the fore and hind limbs (Fig. 6). Further, its distribution is reportedly restricted to regions south of Palghat gap in the Western Ghats, whereas its sister species R. nerostagona is found north of Palghat gap.
Within the Raorchestes nerostagona group, Raorchestes keirasabinae sp. nov. differs from the closely related R. nerostagona by relatively reduced webbing between fingers, I1–2+II2–3−III21/2–2IV (vs. more extensive, I1– 2+II2–21/2III2–2−IV) as well as toes, I1–2II1+–2III1+–2IV2–1+V (vs. more extensive, I1–2II1–2−III1–2−IV2–1V); and its snout length nearly equal to the eye diameter, SL/EL ratio 1.0–1.02 (vs. snout relatively longer than eye, SL/EL ratio 1.32–1.47). Further, Raorchestes keirasabinae sp. nov. differs from R. crustai by the outline of its snout nearly rounded in dorsal and ventral view (vs. nearly pointed), vertical in lateral view (vs. obtuse); well-developed dermal fringe along the outer margins of the fore and hind limbs (vs. weakly-developed); nearly fully webbed toes, I1–2II1+–2III1+–2IV2–1+V (vs. relatively reduced webbing between toes, I1–2+II2–3−III2–3IV2–12/3V); and dorsal skin granular with prominent spinular projections on lateral surfaces of head, between eyes, on upper eyelids, and on dorsum and flanks (vs. dorsal skin granular with a few spinular projections on upper eyelids).
Description of holotype (measurements in mm). Medium-sized adult male (SVL 29.6) with a slender body; head wider than long (HL 11.3; HW 12.1; MN 9.7; MFE 7.8; MBE 4.1); outline of the snout rounded in dorsal and ventral view, vertical in lateral view; snout length (SL 4.5) nearly equal to the horizontal diameter of eye (EL 4.4); loreal region obtusely concave, canthus rostralis sharp; tympanum (TYD 1.4) 31.8% of eye diameter (EL 4.4); supratympanic fold rather indistinct; tongue emarginate with a lingual papilla. Forearm (FAL 5.8) shorter than hand (HAL 8.9); fingers without prominent lateral dermal fringe; webbing present, I1–2+II2–3–III21/2–2IV; subarticular tubercles rather prominent, rounded, III1 and IV1 double, III2 and IV2 weakly-developed; prepollex rather distinct and oval; supernumerary tubercles present. Hindlimbs moderately long, thigh (TL 15.7) longer than shank (SHL 14.5) and foot (FOL 12.9); distance from heel to tip of toe IV (TFOL 20.3); foot webbing: I1–2II1+–2III1+–2IV2–1+V; dermal fringe along toe V present with serrated margins, ending with a well-developed spinular projection on the heel; subarticular tubercles rather prominent, rounded, simple, IV1 and V1 weakly-developed; supernumerary tubercles absent, foot ventral side granular (Fig. 6).
Dorsal skin on snout shagreened to granular; lateral surfaces of head, between eyes, and upper eyelids, glandular with short spinular projections; anterior and posterior parts of dorsum prominently glandular with spinular projections; dorsal surfaces of fore and hind limbs shagreened with some scattered granules; a distinct dermal fringe along the outer margin of the fore and hind limbs, ending with a well-developed spinular projection on the heel and elbow. Ventral surfaces of throat, chest, belly, and posterior part of thighs glandular (Fig. 6).
Colour of holotype. In life. Anterior part of dorsum brown with black and dark brown patches, posterior half green with grey patches; snout light green dorsally; lateral surfaces of head dark greyish-brown; dorsal surface of fore and hind limbs light greyish-brown with irregular dark grey and light green cross-bands; lateral surfaces of belly with brown and grey mottling; groin and anterior parts of thigh brown with white patches; posterior margins of thigh and shank chocolate dark brown; hand and foot brownish-grey with green tinge; iris reddish-grey with faint horizontal brown band. Ventral surfaces off-white, with variable amounts of brown or grey spots forming a vermiculated pattern; throat darker compared to the belly, with dark grey margins and bands along the lips (Fig. 6). In preservation. Anterior part of dorsum dark greyish-brown; posterior half light brown with light pink tinge; snout light pinkish-grey; lateral surfaces of head light brown; dorsal surface of fore and hind limbs light pinkish-brown with dark brown cross-bands; lateral surfaces of belly light brown vermiculated with cream white; groin brown with white patches; posterior margins of thigh and shank brown. Ventral surfaces light greyish-brown with white and brown spots; throat darker brown compared to the belly, with dark brown margins and bands along the lips (Fig. 6).
Variations. Morphometric data from three specimens, including the holotype, is given in Table S2. This species can have variable dorsal colour and markings that can be adapted according to its surroundings, possibly helping in camouflage. Spinular projections on the dorsal surfaces and flanks are more prominent in life.
Distribution and natural history. Raorchestes keirasabinae sp. nov. is endemic to the Western Ghats and currently known from elevations of 100–1,000 m asl south of Palghat gap. It has been observed at Agasthyamalai Biosphere Reserve (Chathankod-Makki, Ponmudi, Peppara Wildlife Sanctuary, and Shendurney Wildlife Sanctuary) in Thiruvananthapuram and Kollam districts, and Periyar Tiger Reserve in Idukki district of Kerala State. Since the species inhabits the highest canopy layers and cannot be located easily, it could have a wider geographical range in the Western Ghats regions south of Palghat gap, both in Kerala and the adjoining State of Tamil Nadu. The vocalisations of this species have not been recorded and analysed.
Raorchestes sanjappai sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:924C6AC3-6407-4CB5-B00C-0619C6645D97
Sanjappa’s Shrub Frog
(Figs. 2 and 7; Tables 1–3; Tables S1 and S2)
Figure 7: Holotype (BNHS 6099, adult male) of Raorchestes sanjappai sp. nov.
(A–I) In life. (A) Dorsolateral view. (B) Dorsal view. (C) Illustration of the mid-dorsal horny ridge extending from the snout tip to the vent. (D) Ventral view. (E) Dorsal view of body and thighs. (F) Lateral view. (G) Posterior view of thighs. (H) Ventral view of hand. (I) Ventral view of foot. (J–P) In preservation. (J) Dorsal view. (K) Enlarged view of horny spinules on the dorsal skin. (L) Ventral view. (M) Lateral view of head. (N) Ventral view of hand. (O) Ventral view of foot. (P) Schematic illustration of webbing on foot.Etymology. The species is named after Dr. M. Sanjappa, a renowned Indian Botanist and former Director of the Botanical Survey of India. The species name is in appreciation of his taxonomic contributions as well as generous support to SDB during the initial phases of his research career. The species epithet sanjappai is treated as a noun in the genitive case.
Holotype. BNHS 6099, an adult male, from Periya (11.8342° N, 75.8574° E, 750 m asl), Wayanad district, Kerala State, India, collected by RS and SDB on 10 June 2015. Paratype. BNHS 6100, an adult male, from the holotype locality, collected by RS and SDB on 29 July 2013. Referred specimen. SDBDU 2019.3440, an adult male, collected by RS and SD, from the holotype locality on 30 July 2018.
Phylogenetic relationship. Raorchestes sanjappai sp. nov. is a member of the Raorchestes chromasynchysi group and shows a sister-group relationship with R. vellikkannan sp. nov. (Fig. 2). For the mitochondrial 16S rRNA, Raorchestes sanjappai is divergent from other members of the group as: 4.6–5.5% from R. chromasynchysi; 5.9% from R. ravii; 5.1–5.7% from R. silentvalley; and 3.1–3.3% from R. vellikkannan sp. nov.
Morphological diagnosis and comparison. Raorchestes sanjappai sp. nov. can be distinguished from other known congeners, except members of the Raorchestes chromasynchysi group, by the combination of the following morphological characters: small-sized species (male SVL 22–24 mm); uniform green dorsal colouration; dorsal skin shagreened to sparsely granular with horny spinular projections and a horny ridge extending from the snout tip to the vent; tongue with papillae; flanks and groin light brown to yellowish; and foot webbing moderate, below the third subarticular tubercle on either side of toe IV (Fig. 7).
Within the Raorchestes chromasynchysi group, Raorchestes sanjappai sp. nov. differs from R. chromasynchysi in having a rather indistinct tympanum (vs. distinct); head wider than long, HW/HL ratio 1.10–1.12 (vs. nearly equal, HW/HL ratio 0.97–1.06); snout longer than eye diameter, SL/EL ratio 1.17–1.42 (vs. nearly equal, SL/EL ratio 0.94–1.03); and relatively more extensive webbing on foot, extending up to the third subarticular tubercle on the outside of toe IV, I2–2II2–3III2––3IV2–2–V (vs. reduced, just above the second subarticular tubercle, I1+–2+II2–3III2––3–IV3––2–V). It differs from R. vellikkannan sp. nov. by head wider than long, HW/HL ratio 1.10–1.12 (vs. nearly equal, HW/HL ratio 0.99–1.0); foot webbing moderate, extending up to the third subarticular tubercle on the outer side of toe IV, I2–2II2–3III2––3IV2–2–V (vs. relatively reduced, below the second subarticular tubercle on either side of toe IV, I2–2+II2–3III2–31/3IV31/2–2+V); and dorsal skin shagreened to sparsely granular with relatively less prominent spinules (vs. prominently granular with more sharply pointed spinules). It differs from R. silentvalley by relatively reduced webbing on foot, not extending beyond the third subarticular tubercle on either side of toe IV, I2–2II2–3III2––3IV2–2–V (vs. more extensive, above the third subarticular tubercle on either side of toe IV, I1+–2+II2–3III1+–2–IV2––1+V); lateral surfaces of abdomen and groin light brown to yellowish (vs. lateral surfaces of abdomen bright yellow and groin dark purplish-blue; and ventral surfaces of palm and feet, including the digit tips, light grey (vs. purplish-black). It differs from R. ravii by snout sub-ovoid in in ventral view (vs. pointed); head wider than long, HW/HL ratio 1.10–1.12 mm (vs. nearly equal, HW/HL ratio 0.99–1.01); thigh nearly equal to shank, TL/SHL 0.98 (vs. relatively shorter, TL/SHL 0.93–0.95); and relatively more extensive webbing on foot, outer side of IV toe webbing reach up to third subarticular tubercle, I2–2II2–3III2––3IV2–2–V (vs. reduced, just above the second subarticular tubercle, I2–2II2–3III2––31/2IV3––2–V).
Description of holotype (measurements in mm). Small-sized adult male (SVL 23.9) with a slender body; head wider than long (HW 9.1; HL 8.6; MN 7.5; MFE 6.0; MBE 3.2); the outline of snout sub-ovoid in dorsal and ventral views, rounded in lateral view; snout length (SL 3.7) longer than horizontal diameter of eye (EL 2.6); loreal region acute with rounded canthus rostralis; tympanum rather indistinct; supratympanic fold rather distinct; tongue emarginate with a lingual papilla. Forearm (FAL 5.0) shorter than hand (HAL 6.8); fingers without lateral dermal fringe; webbing absent; subarticular tubercles rather prominent, rounded, III1 and IV1 weakly-developed; prepollex rather distinct and oval; supernumerary tubercles absent. Hindlimbs moderately long, thigh (TL 12.0) shorter than shank (SHL 12.2) and longer than foot (FOL 9.0); distance from heel to tip of toe IV (TFOL 15.1); foot webbing moderate: I2–2II2–3III2––3IV2–2–V; dermal fringe absent; subarticular tubercles rather prominent, rounded, simple, IV1 and V1 weakly-developed; supernumerary tubercles absent (Fig. 7).
Dorsal skin shagreened to sparsely granular with horny spinules; a mid-dorsal horny ridge extending from the snout tip to the vent; lateral surfaces of head relatively more granular in comparison to the dorsum; lateral abdominal surfaces relatively less granular compared to the dorsum; limbs shagreened to sparsely granular. Ventral skin on throat shagreened to sparsely granular; chest, belly and posterior surface of thighs granular; limbs shagreened (Fig. 7).
Colour of holotype. In life. Dorsum uniformly green, without prominent dorsal markings; dorsal colouration extends onto the dorsal surface of fore and hind limbs, and loreal and tympanic regions; lateral abdomen surfaces light brown to yellowish; groin light brown with yellow markings (but not blotches); posterior part of thighs light to dark brown, with light greenish spots on the anal region; iris reddish-brown. Ventral surface of throat light flesh grey with minute dark spots; chest and belly greyish-white; limbs greyish-brown; hand and foot dark grey or blackish (Fig. 7). In preservation. Dorsum bluish-grey without prominent dorsal markings; lateral abdomen surfaces light grey; groin light brown; posterior part of thighs dark brown. Ventral surfaces off-white without prominent markings, other than minute black spots on throat; limbs off-white to greyish-brown with darker spots towards the margins (Fig. 7).
Variations. Morphometric data from three specimens, including the holotype, are given in Table S2.
Vocalisation. Raorchestes sanjappai sp. nov. males produce a single type of call. Calls are not delivered in groups and have a pulsatile temporal structure with widely spaced pulses. A typical call has a duration of 411.2 ms, the call envelope being characterised by a rise time of 376.2 ms and fall time of 35.1 ms, with two pulses delivered at a rate of 2.7 pulses/second, and an overall dominant frequency of 2.4 kHz. For comparison see Table 2 and the group definition, including the oscillogram and spectrogram figures cited therein.
Distribution and natural history. Raorchestes sanjappai sp. nov. is endemic to the Western Ghats and currently known only from an altitude of about 750 m asl at its type locality (Periya) that lies north of Palghat gap in the Wayanad district of Kerala State. The species was observed inside secondary forests and individuals were located on vegetation up to 3 m high during the breeding season.
Raorchestes vellikkannan sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:3E3E9E2A-E489-4AAD-8975-275BF0008CCB
Silver-eyed Shrub Frog
(Figs. 2 and 8; Tables 1–3; Tables S1 and S2)
Figure 8: Holotype (BNHS 6101, adult male) of Raorchestes vellikkannan sp. nov.
(A) Dorsolateral view in life. (B–H) In preservation. (B) Dorsal view. (C) Enlarged view of the dorsal skin texture. (D) Ventral view. (E) Lateral view of head. (F) Ventral view of hand. (G) Ventral view of foot. (H) Schematic illustration of webbing on foot.Etymology. The species name is derived from Malayalam (the language of Kerala State where the type series were collected) ‘velli’ (meaning silver) and ‘kannu’ (meaning eye) referring to the silver colour of the iris in this species. The species epithet vellikkannan is treated as an invariable noun in apposition to the generic name.
Holotype. BNHS 6101, an adult male, from Singappara (10.9794° N, 76.615° E, 856 m asl), Siruvani, Palakkad district, Kerala State, India, collected by SDB, SG, and RS on 06 July 2015. Referred specimen. SDBDU 2015.3019, an adult male, collected along with the holotype.
Phylogenetic relationship. Raorchestes vellikkannan sp. nov. is a member of the Raorchestes chromasynchysi group and shows a sister-group relationship with Raorchestes sanjappai sp. nov. (Fig. 2). For the mitochondrial 16S rRNA, Raorchestes vellikkannan sp. nov. is divergent from other members of the group as: 4.6–6.2% from R. chromasynchysi; 5.7–6.8% from R. ravii; 3.1–3.3% from R. sanjappai sp. nov.; and 4.7–5.3% from R. silentvalley.
Morphological diagnosis and comparison. Raorchestes vellikkannan sp. nov. can be distinguished from other known congeners, except members of the Raorchestes chromasynchysi group, by the combination of the following morphological characters: small-sized species (male SVL 22.2–22.9 mm); dorsum yellowish-brown; dorsal skin having prominent granular projections with sharply pointed spinules; dorsum with discontinuous or weakly-developed longitudinal mid-dorsal horny ridge; tongue with papillae; foot webbing small, below the second subarticular tubercle on either side of toe IV, I2–2+II2–3III2–31/3IV31/2–2+V (Fig. 8).
Within the Raorchestes chromasynchysi group, Raorchestes vellikkannan sp. nov. differs from R. chromasynchysi, R. ravii, R. sanjappai sp. nov., and R. silentvalley by relatively reduced webbing on foot, below the second subarticular tubercle on either side of toe IV, I2–2+II2–3III2–31/3IV31/2–2+V (vs. above the second subarticular tubercle on either side of toe IV, I2–2II2–3III2––3–IV3––2–V in R. chromasynchysi; above the second subarticular tubercle on the outer side of toe IV, I2–2II2–3III2––31/2IV3––2–V in R. ravii; up to the third subarticular tubercle on the outer side of toe IV, I2–2II2–3III2––3IV2–2–V in R. sanjappai sp. nov.; and above the third subarticular tubercle on either side of toe IV, I1+–2+II2– 3III1+–2–IV2––1+V in R. silentvalley). Specifically, it also differs from R. chromasynchysi by the outline of its snout rounded to sub-ovoid in dorsal and ventral views (vs. nearly pointed); groin and anterior part of thighs light brown (vs. dark brown with yellow blotches); thigh nearly equal to shank, TL/SHL ratio 0.99–1.0 (vs. thigh relatively shorter than shank TL/SHL ratio 0.89–0.98). Further, it differs from R. silentvalley by dorsum predominantly greyish-brown (vs. light or dark green); lateral surfaces of abdomen and groin brown (vs. lateral surfaces of abdomen bright yellow and groin dark blue); ventral surfaces off-white or light grey (vs. yellow); and ventral surfaces of palm and feet light grey (vs. purplish-black). It differs from R. ravii by snout rounded to sub-ovoid in ventral view (vs. pointed); snout length nearly equal to the eye diameter, SL/EL ratio 1.0–1.03 (vs. snout longer than eye diameter, SL/EL ratio 1.20–1.42); and thigh nearly equal to shank, TL/SHL ratio 0.99–1.0 (vs. thigh shorter than shank, TL/SHL ratio 0.93–0.95).
For more differences with R. sanjappai sp. nov., see the comparison section of that species.
Description of holotype (measurements in mm). Small-sized adult male (SVL 22.9) with a slender body; head width equal to its length (HW 8.6; HL 8.6; MN 7.7; MFE 5.5; MBE 3.2); outline of the snout rounded to sub-ovoid in dorsal and ventral view, acute in lateral view; snout length (SL 3.2) nearly equal to the horizontal diameter of eye (EL 3.1); loreal region acute with rounded canthus rostralis; tympanum rather indistinct; supratympanic fold rather distinct; tongue emarginate with a lingual papilla. Forearm (FAL 5.2) shorter than hand (HAL 6.6); fingers without lateral dermal fringe; webbing absent; subarticular tubercles rather prominent, rounded, III1 and IV1 weakly-developed; prepollex rather distinct and oval; supernumerary tubercles absent. Hindlimbs moderately long, thigh (TL 12.1) nearly equal to shank (SHL 12.2) and longer than foot (FOL 8.4); distance from heel to tip of toe IV (TFOL 15.1); foot webbing small: I2–2+II2–3III2–31/3IV31/2–2+V; dermal fringe absent; subarticular tubercles rather prominent, rounded, simple, IV1 and V1 weakly-developed; supernumerary tubercles absent (Fig. 8).
Dorsal skin shagreened to prominently granular with sharply pointed horny spinules; a weakly-developed and discontinuous mid-dorsal horny ridge; lateral surfaces of the head relatively more granular in comparison to the dorsum; lateral abdominal surfaces shagreened with scattered granular projections; limbs shagreened to sparsely granular. Ventral skin on throat shagreened; chest, belly, and posterior surface of thighs granular; limbs shagreened (Fig. 8).
Colour of holotype. In life. Dorsum light brown coloured with a yellowish tinge; limbs slightly lighter than dorsal colouration; dorsum with a dark brown ‘X’ shaped marking and scattered brown spots; loreal and tympanic regions darker than dorsum with more brownish spots; lateral abdomen surfaces light yellowish-brown; groin yellowish-brown without prominent markings, with a few irregular dark spots; posterior part of thighs light to dark chocolate brown; limbs light yellowish-brown with dark brown cross-bands; iris silver grey with minute brown speckling. Ventral surface of throat greyish-white with minute brown spots; chest and belly off-white; limbs light greyish-brown; hand and foot dark grey (Fig. 8). In preservation. Dorsum light brown with a faint dark ‘X’ mark; upper eyelids light greyish-brown; an inverted light brown triangle placed just below the level of eye; lateral abdominal surfaces light grey; groin greyish-brown; posterior part of thighs light brown; limbs light greyish-brown with dark grey cross-bands. Ventral surfaces off-white without prominent markings, other than minute blackish-brown speckles on throat and limbs (Fig. 8).
Distribution and natural history. Raorchestes vellikkannan sp. nov. is endemic to the Western Ghats and currently known only from its type locality (Singappara, Siruvani) and surrounding regions of the Silent Valley National Park in Palakkad district of Kerala State, north of Palghat gap. This species was observed inside primary forests and individuals were found on vegetation up to 4 m high during the breeding season. The vocalisations of this species have not been recorded and analysed.
Taxonomic remarks on three known taxa
Raorchestes ravii Zachariah, Dinesh, Kunhikrishnan, Das, Raju, Radhakrishnan, Palot, and Kalesh, 2011
This taxon was described based on two specimens from “Naduvattam (11° 23′ N 76°34′E; 1,890 m.a.s.l), Nilgiri district, Tamil Nadu, India” (Zachariah et al., 2011). However, subsequent studies could not unambiguously report new collections of this species from its type locality. Vijayakumar et al. (2014) assigned some of their collections from an unknown locality in the Western Ghats (CESF 1154 and CESF 1672) as “Raorchestes aff. ravii”, which were shown to have a sister-group relationship with R. chromasynchysi. However, Zachariah et al. (2016) indicated another population from Vijayakumar et al. (2014)’s study, CESF 469 from an unknown locality in the Western Ghats originally identified as “R. aff. coonoorensis”, to be “R. ravii” in their phylogenetic analyses without any discussion. Here we report new collections (SDBDU 2013.2410–2411) from the type locality Naduvattam, which match with the description and type of R. ravii. These are found to be genetically close to “Raorchestes aff. ravii” (Vijayakumar et al., 2014) (1–1.5% divergence for 16S) and R. chromasynchysi (1–2.4% divergence for 16S).
Our morphological examination of the type specimens of R. ravii and comparison with R. chromasynchysi, as also indicated by Vijayakumar et al. (2014), suggests that R. ravii is a member of the Raorchestes chromasynchysi group (see ‘Grouping of species using integrative approaches’) due to the combination of characters such as small adult size (male SVL 20–23 mm); pointed snout; dorsal skin with horny spinules; presence of horny ridge between the eyes and another extending from the snout tip to the vent (mentioned as absent by Zachariah et al., 2011; but observed to be present on the holotype); tongue with papillae (mentioned as absent by Zachariah et al., 2011; but observed to be present on the holotype); iris golden brown, horizontally not divided into lighter upper and darker lower halves; and foot webbing extending up to or above the second subarticular tubercle on either side of toe IV. Furthermore, R. chromasynchysi that has been reported from Naduvattam is a confusing frog with highly variable colouration and markings, including brown morphs that show the presence of dark X or H-shaped marks on the dorsum, brown band connecting the eyes, lateral surfaces of head and supratympanic region darker brown, flanks and groin without contrasting blotches, and limbs irregularly barred. The original description of R. ravii also mentions that its advertisement call is “a distinct bell like musical note similar to water drops falling into water”, which can be acoustically interpreted as a pulsatile call delivered with widely spaced pulses, a characteristic feature of members of the R. chromasynchysi group. Hence, based on phylogenetic, morphological, and acoustic evidence, we consider R. ravii to be a member of the R. chromasynchysi group.
Raorchestes sanctisilvaticus (Das & Chanda, 1997)
Recently three taxa, Philautus sanctisilvaticus Das & Chanda, 1997 (=Raorchestes sanctisilvaticus), Philautus similipalensis Dutta, 2003 (=Raorchestes similipalensis) and Philautus terebrans Das & Chanda, 1998 (=Raorchestes terebrans), were shown to be conspecific due to overlapping morphological characters and shallow genetic divergence (Mirza et al., 2019). Consequently, the first available name, Philautus sanctisilvaticus Das & Chanda, 1997 (=Raorchestes sanctisilvaticus) was recognised as the valid name, with which the latter two taxa (Philautus similipalensis and P. terebrans) were synonymised.
A further comparative study of Raorchestes sanctisilvaticus (from its type locality, along with typical and additional populations referring to its two synonyms) and R. bombayensis was herein carried out. Based on available and additional new morphological, phylogenetic, and acoustic evidence, we find that R. sanctisilvaticus is closely related to, and could possibly be considered conspecific with, R. bombayensis. Morphologically, the foot webbing of R. similipalensis and R. terebrans differs from that in R. sanctisilvaticus, whereas some populations of R. sanctisilvaticus have a relatively less mottled ventral skin (vs. prominently mottled in some populations of R. bombayensis). Both these morphological characters differ feebly and are also observed to be variable among the various different populations referring to these four species.
Acoustically, the non-pulsatile call of R. sanctisilvaticus is very similar to that of R. bombayensis and R. similipalensis, while the call of R. terebrans is slightly longer in duration, 12.2 ms (vs. 11.6 ms, 12.1 ms and 16.1 ms, respectively). The dominant frequency of calls of all the four species is similar (approximately 3 kHz).
Furthermore, the interspecific divergence for the mitochondrial 16S between R. bombayensis (from northern Karnataka, Goa, and Maharashtra) and R. sanctisilvaticus (from Madhya Pradesh, Odisha, Chhattisgarh, and Andhra Pradesh) is 0.8–1.5%, which overlaps with the intraspecific divergence among different populations of R. bombayensis (up to 0.8%) and R. sanctisilvaticus (up to 0.8%).
Hence, since all these populations (for which various different names are currently available) neither have considerable genetic divergences nor can they be reliably distinguished from each other based on morphology or calls, and further in the light of recent taxonomic actions by Mirza et al. (2019), the most parsimonious taxonomic resolution would be to consider them as a single species, for which the earliest available name Ixalus bombayensis Annandale, 1919 (=Raorchestes bombayensis) should be applied, with Philautus sanctisilvaticus Das & Chanda, 1997, Philautus similipalensis Dutta, 2003 (=Raorchestes sanctisilvaticus) and Philautus terebrans Das & Chanda, 1998 (=Raorchestes sanctisilvaticus) as its junior subjective synonyms. However, we currently refrain from any taxonomic action in this regard, pending further population level studies with comprehensive sampling across the entire known range of R. bombayensis and this taxon.
Raorchestes thodai Zachariah, Dinesh, Kunhikrishnan, Das, Raju, Radhakrishnan, Palot, and Kalesh, 2011
This species was described based on two specimens from “Ooty (Udhagamandalam) town (11° 24′ N; 76° 40′ E; 1980 m.a.s.l), Nilgiris district, Tamil Nadu, India” primarily based on iris, flanks, and groin colouration. However, subsequent studies could not gather new collections conspecific to this species from its type locality (Vijayakumar et al., 2014). During our herpetological surveys in the Nilgiris over the past decade, we have not encountered any new samples referable to this species. Furthermore, the level of morphological differentiation between R. thodai and the closely allied R. signatus remains unclear due to several overlapping characters, such as dorsal colouration and marking as well as presence or absence of radiating lines on iris, which are observed to be highly variable even among individuals of the same population of R. signatus, thereby not permitting a distinction between the two taxa. Hence, R. thodai Zachariah, Dinesh, Kunhikrishnan, Das, Raju, Radhakrishnan, Palot, and Kalesh, 2011 is likely to be a junior subjective synonym of R. signatus (Boulenger, 1882).
Grouping of species using integrative approaches
Genus: Raorchestes Biju, Shouche, Dubois, Dutta, and Bossuyt, 2010
Proposed common name: Rao’s Oriental Shrub Frogs
Raorchestes anili group
Figure 9: Raorchestes anili species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 22–27 mm, female SVL 25–36 mm); (2) flank and groin with dark brown blotches; (3) anterior and posterior surfaces of thigh and inner side of shank dark brown; (4) foot webbing moderate, up to the third subarticular tubercle on either side of toe IV. (B–D) Male calls in three studied species of the group: (B) Adult male, followed by a call oscillogram (above) and spectrogram (below) for R. kaikatti (1 s section showing a single Type 1 and three Type 2 calls, delivered in groups). (C and D) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. sushili (1 s section showing two calls, delivered in groups) and R. anili (1 s section showing a single call). (E) Adult male of R. kakachi (call not studied). (F–I) Eye colour and pattern in four members of the group. (J) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(E). Photo of calling male in (9D) by Karthik A. K.Members included. Raorchestes anili, R. kaikatti, R. kakachi, and R. sushili.
Group definition. Phylogenetic. The Raorchestes anili group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. anili, R. kaikatti, R. kakachi, and R. sushili, but none of the other currently recognised Raorchestes species (Fig. 2). This group is analogous to the Anili clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium-sized adults (male SVL 22–27 mm, female SVL 25–36 mm); dorsum with shades of grey to brown, predominantly with dark irregular markings; canthus rostralis sharp or rounded; flank and groin light to dark brown, with white blotches; anterior and posterior surfaces of thigh and inner side of shank dark brown, with dark contrasting blotches or bands; lateral surfaces granular with white or light brown spots; foot webbing moderate, not extending beyond the third subarticular tubercle on either side of toe IV (Fig. 9A). Eye colouration and pattern. Iris colour varies from light to dark brown or reddish-brown, occasionally with golden tinge (as in R. anili); iris periphery dark brown; sclera light silvery-blue (Figs. 9F–9I; Table 1). Acoustic. Three members of the group for which calls are studied (R. anili, R. kaikatti, and R. sushili) produce one (R. anili and R. sushili) or two (R. kaikatti) types of calls. Calls of all the three species have a pulsatile temporal structure. The overall dominant frequency of the calls ranges from 2.4 to 2.9 kHz (Figs. 9B–9D). Calling height. Members of this group usually call from heights of 1.5–5 m on higher shrubs or in the lower tree canopy (Table 2). Geographical. This group is currently restricted to Western Ghats regions in Karnataka, Kerala, and Tamil Nadu States (Fig. 9J; Table 3).
Species level acoustic comparison. Within the R. anili group, the calls of R. anili, R. sushili, and the Type 1 call of R. kaikatti have distinct call structures and can be differentiated by their call duration and pulse rate. Calls of R. anili differ from those of R. sushili and R. kaikatti due to their longer duration, 980.4 ms (vs. 51.2 ms and 102.7 ms, respectively), and a relatively slow pulse rate with delivery at 6.3 pulses/s (vs. relatively similar in the other two species, 242.8 pulses/s and 280.8 pulses/s, respectively). The dominant frequencies of the calls of R. sushili and R. kaikatti are relatively similar (2.6 kHz and 2.4 kHz, respectively) and somewhat lower compared to R. anili (2.9 kHz) (Figs. 9B–9D; Table 2).
Raorchestes aureus group
Figure 10: Raorchestes aureus species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 24–25 mm, female SVL 29–30 mm); (2) lateral abdominal surfaces and groin with irregular mottling, spots, or patches; (3) foot webbing small, not extending beyond the second subarticular tubercle on either side of toe IV. (B and C) Adult individuals and eye colour and pattern in two members of the group. (D) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B) and (C).Members included. Raorchestes aureus and R. lechiya.
Group definition. Phylogenetic. The Raorchestes aureus group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. aureus and R. lechiya, but none of the other currently recognised Raorchestes species (Fig. 2). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium-sized adults (male SVL 24–25 mm, female SVL 29 mm); dorsum with shades of grey or brown to light yellow, with or without dark irregular markings; dorsal skin shagreened to granular bearing spinular projections; snout sub-elliptical in dorsal and ventral view, rounded in lateral view; lateral abdominal surfaces and groin with irregular mottling, spots, or patches; ventral surfaces with faint to prominent mottling or vermiculation; foot webbing small, not extending beyond the second subarticular tubercle on either side of toe IV (Fig. 10A). Eye colouration and pattern. Iris colour golden brown; iris periphery black; sclera light silvery-blue (Figs. 10B and 10C; Table 1). Acoustic. Members of this group (R. aureus and R. lechiya) produce a single type of call (Zachariah et al., 2016). The calls are non-pulsatile in R. aureus while they have a pulsatile temporal structure in R. lechiya. The overall dominant frequency of the calls in these species range from 2.6–3.0 kHz (Zachariah et al., 2016) (Table 2). Calling height. Members of this group usually call from heights of 0.5–2 m on low bushes or higher shrubs (Table 2). Geographical. This group is currently restricted to Western Ghats regions north of Palghat gap in Kerala and Tamil Nadu States (Fig. 10D; Table 3).
Raorchestes beddomii group
Figure 11: Raorchestes beddomii species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 17–35 mm, female SVL 21–39 mm); (2) prominent subarticular tubercles on hand and foot; (3) foot webbing moderate, up to the third subarticular tubercle on either side of toe IV. (B–E) Male calls in members of the group: (B) Adult male, followed by a call oscillogram (above) and spectrogram (below) for R. resplendens (1 s section showing a single Type 1 call, four Type 2 calls, and two Type 3 calls, delivered in groups). (C) Calling individual, followed by a call oscillogram (above) and spectrogram (below) for R. munnarensis (1 s section showing a single Type 1 call and two Type 2 calls, delivered in groups). (D) Calling individual, followed by a call oscillogram (above) and spectrogram (below) for R. dubois (1 s section showing a single call). (E) Calling individual, followed by call oscillograms (above) and spectrograms (below) for the two call types in R. beddomii, Type 1 (1 s section showing a single call) and Type 2 (1 s section of a call group showing five calls). (F) Adult male of R. theuerkaufi (call not studied). (G) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(F). (H–L) Eye colour and pattern in five members of the group.Members included. Raorchestes beddomii, R. dubois, R. resplendens, R. munnarensis, and R. theuerkaufi.
Group definition. Phylogenetic. The Raorchestes beddomii group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. beddomii, R. dubois, R. resplendens, R. munnarensis, and R. theuerkaufi, but none of the other currently recognised Raorchestes species (Fig. 2). This group is analogous to the Beddomii clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to large-sized adults (male SVL 17–35 mm, female SVL 21–39 mm); subarticular tubercles rather prominent both on hands and feet; foot webbing small to moderate, not extending beyond the third subarticular tubercle on either side of toe IV (Fig. 11A). Eye colouration and pattern. Iris light to dark red, except light golden brown to dark brown with or without reddish tinge in R. dubois and R. munnarensis; iris periphery black, sclera light blue to scarlet blue (Figs. 11H–11L; Table 1). Acoustic. Four members of the group for which calls are studied (R. beddomii, R. dubois, R. resplendens, and R. munnarensis) produce one (R. dubois), two (R. beddomii and R. munnarensis), or three (R. resplendens) types of call. The most common call type (Type 1) are non-pulsatile in R. resplendens and R. dubois, while they have a pulsatile temporal structure in R. beddomii and R. munnarensis. The overall dominant frequency of calls in these species range from 2.2 to 2.7 kHz (Figs. 11B–11E; Table 2). Calling height. Members of this group usually call from ground level, low bushes and shrubs, to tree canopy layers of up to 20 m high (Table 2). Geographical. This group is currently restricted to the Western Ghats regions south of Palghat gap in the States of Kerala and Tamil Nadu (Fig. 11G; Table 3).
Species level acoustic comparison. Within the R. beddomii group, the four species have considerably different call structures. The Type 1 non-pulsatile call in R. resplendens (51.3 ms) is relatively longer than that in R. dubois (10.6 ms). Whereas the Type 1 pulsatile in R. munnarensis is longer (498.2 ms) and delivered at a slower pulse rate (6.4 pulses/s) compared to R. beddomii, which produces a call that is 150 ms in duration and delivered at 29.2 pulses/s. The dominant frequency of Type 1 calls of three species, R. beddomii, R. resplendens and R. dubois, are similar (2.5–2.7 kHz), while that of R. munnarensis is lower (2.2 kHz) (Figs. 11B–11E; Table 2).
Raorchestes bombayensis group
Figure 12: Raorchestes bombayensis species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 17–28 mm, female SVL 18–32 mm); lateral surfaces of abdomen, groin, and posterior part of thighs marbled with contrasting white or yellow blotches on dark brown or black background; (2) a knobbed bony projection on humerus in males; (3) presence of horny spinules and/or horny ridges on dorsal skin; (4) foot webbing small to moderate, not extending beyond the third subarticular tubercle on either side of toe IV. (B–G) Male calls in members of the group: (B and C) Adult males, followed by call oscillograms (above) and spectrograms (below) for R. leucolatus and R. kakkayamensis sp. nov. (0.1 s sections showing a single call for each species). (D, E, F and G) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. bombayensis, R. tuberohumerus, R. ghatei, and R. sanctisilvaticus (0.1 s sections showing a single call for each species). (H–M) Eye colour and pattern in six members of the group. (N) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label (B)–(G). Photo of calling male in (G) by Amit Sayyed.Members included. Raorchestes bombayensis, R. ghatei, R. kakkayamensis sp. nov., R. leucolatus, R. sanctisilvaticus, and R. tuberohumerus.
Group definition. Phylogenetic. The Raorchestes bombayensis group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. bombayensis, R. ghatei, R. kakkayamensis sp. nov., R. leucolatus, R. sanctisilvaticus, and R. tuberohumerus, but none of the other currently recognised Raorchestes species (Fig. 2). This group is analogous to the Bombayensis clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium-sized adults (male SVL 17–28 mm, female SVL 18–32 mm); dorsum predominantly brown; a knobbed bony projection on humerus in males (visible externally in preserved specimens); lateral surfaces of abdomen marbled with contrasting white or yellow blotches on brown or black background; presence of horny spinules and/or horny ridges on dorsal skin; foot webbing small to moderate, not extending beyond the first subarticular tubercle on either side of toe IV (Fig. 12A). Eye colouration and pattern. All members of the group have a brown iris with dense golden speckling and dark brown horizontal and vertical bands; iris periphery dark brown; sclera light silvery blue (Figs. 12H–12M; Table 1). Acoustic. Six members of the group for which calls are studied (R. bombayensis, R. ghatei, R. kakkayamensis sp. nov., R. leucolatus, R. sanctisilvaticus, and R. tuberohumerus) produce a single type of call with non-pulsatile temporal structure. The overall dominant frequency of the calls for these species ranges from 2.9 to 4.1 kHz (Figs. 12B–12F; Table 2). Calling height. Members of this group usually call from heights of 0.5–5 m on low bushes or higher shrubs (Table 2). Geographical. Members of this group are widely distributed right from the Western Ghats regions north of Palghat gap in the States of Kerala, Karnataka, Maharashtra, and Goa, up to the Eastern Ghats (Andhra Pradesh, Telangana, and Odisha) and Central India (Madhya Pradesh and Chhattisgarh) (Fig. 12N; Table 3).
Species level acoustic comparison. All members of the R. bombayensis group have similar call structure but R. kakkayamensis sp. nov. and R. leucolatus produce relatively longer calls, 25.3 ms and 29.4 ms, respectively (vs. 11.6 ms in R. bombayensis, 12.2 ms in R. sanctisilvaticus, 17.7 ms in R. tuberohumerus, and R. ghatei in 14.6 ms). The call of R. kakkayamensis sp. nov. has a longer rise time (9.2 ms) compared to R. leucolatus (2.7 ms), whereas the other members show very short rise time ≤1.5 ms. The call of R. leucolatus also has a relatively higher dominant frequency (4.1 kHz) compared to R. kakkayamensis sp. nov. (3.8 kHz), while other members, R. bombayensis (3.1 kHz), R. sanctisilvaticus (3.1 kHz), R. tuberohumerus (3.3 kHz), and R. ghatei (2.9 kHz) have a lower dominant frequency in comparison to R. kakkayamensis sp. nov. (Figs. 12B–12G; Table 2).
Raorchestes chalazodes group
Figure 13: Raorchestes chalazodes species group.
(A) Phylogenetic relationships and major diagnostic characters for members of the group: (1) largely associated with bamboo reeds in forested areas, especially for breeding; (2) iris black with a golden yellow ring that may or may not be divided into patches by a black cross mark or radiating pattern, or dense metallic silver with fine black reticulations forming a mosaic pattern; (3) central part of belly translucent with venations; (4) foot webbing moderate, extending up to the third subarticular tubercle on either side of toe IV. (B–E) Male calls in members of the group: (B, C, D and E) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. chalazodes, R. ochlandrae, R. manohari, and R. uthamani (0.1 s sections showing a single call for each species). (F) Adult male of R. flaviocularis (call not studied). (G–K) Eye colour and pattern in five members of the group. (L) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(F). Photo of calling male in (C) by Gururaja K. V.Members included. Raorchestes chalazodes, R. flaviocularis, R. ochlandrae, R. manohari, and R. uthamani.
Group definition. Phylogenetic. The Raorchestes chalazodes group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. chalazodes, R. flaviocularis, R. ochlandrae, R. manohari, and R. uthamani, but none of the other currently recognised Raorchestes species (Fig. 2). This group is analogous to the Chalazodes clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium-sized adults (male SVL 17–29 mm, female SVL 28–37 mm); snout rounded to semi-circular in ventral view; dorsum uniformly green, yellow, or brown with scattered spots, longitudinal markings or dorsolateral bands; distinctly patterned iris, black or dark brown with golden yellow patches, or silvery white with thin black reticulations; presence of lingual papillae; subarticular tubercles rather prominent on both hands and feet; moderate webbing on feet; and central part of belly translucent with venations (Fig. 13A). Eye colouration and pattern. Members of this group have a distinct eye colour and pattern, with blackish-brown iris having a golden yellow ring that may or may not be divided by a black cross mark or a radial pattern (R. chalazodes, R. flaviocularis, and R. ochlandrae) or iris brown with dense metallic silver mosaic pattern (R. manohari and R. uthamani); iris periphery brown; sclera scarlet blue or indistinct (Figs. 13H–13K; Table 1). Acoustic. Four members of the group for which calls are studied (R. chalazodes, R. ochlandrae, R. manohari, and R. uthamani) produce a single type of call with very similar call structure. The calls have a non-pulsatile temporal structure with duration typically ranging between 12.3 and 36 ms. The calls are generally delivered rapidly in long call groups. The overall dominant frequency of the calls ranges from 2.7 to 3.6 kHz (Figs. 13B–13H; Table 2). Calling height. Members of this group usually call from heights of 1–7 m on low to medium-sized reeds and high bamboo tree canopy (Table 2). Geographical. This group is currently restricted to the Western Ghats regions in southern Karnataka, Kerala, and Tamil Nadu States (Fig. 13L; Table 3). Habitat preference. Another unique characteristic of this group is the habitat association of its members largely with bamboo reeds in forested areas.
Species level acoustic comparison. Within the R. chalazodes group, the four species have very similar call structures consisting of non-pulsatile calls, each having a short rise time and relatively longer fall time, that are organised into long call groups of rapidly produced calls. The call duration of these species varies, with R. uthamani showing a longer call (36 ms) compared to R. chalazodes (18.4 ms), R. ochlandrae (25.3 ms), and R. manohari (12.3 ms). The dominant frequency of the calls of two species, R. chalazodes and R. ochlandrae, is similar (2.7 kHz and 2.7 kHz, respectively), while the calls of R. manohari and R. uthamani are both produced at a higher dominant frequency (3.6 kHz and 3.4 kHz, respectively) (Figs. 13B–13H; Table 2).
Raorchestes charius group
Figure 14: Raorchestes charius species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 25–35 mm; female SVL 25–39 mm); (2) horny ridges between the eyes, arranged in a triangle directed posteriorly; (3) groin light or dark brown predominantly with white or yellow blotches; (4) foot webbing small, below the second subarticular tubercle on either side of toe IV. (B–G) Male calls in members of the group: (B, C, D and E) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. honnametti, R. charius, R. griet, and R. coonoorensis (0.1 s sections showing a single call for each species). (F and G) Adult male, followed by a call oscillogram (above) and spectrogram (below) for R. drutaahu sp. nov. and R. marki (0.1 s section showing a single call for each species). (H) Adult male of R. kollimalai (call not studied). (H–N) Eye colour and pattern in seven members of the group. (O) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(H). Photo of calling male in (B) by Yatin Kalki.Members included. Raorchestes charius, R. coonoorensis, Raorchestes drutaahu sp. nov., R. griet, R. honnametti, R. kollimalai, and R. marki.
Group definition. Phylogenetic. The Raorchestes charius group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. charius, R. coonoorensis, R. drutaahu sp. nov., R. griet, R. honnametti, R. marki (Fig. 2), and R. kollimalai (Gowande, Ganesh & Mirza, 2020), but none of the other currently recognised Raorchestes species. This group is analogous to the Charius and Coonoorensis clades of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium-sized adults (male SVL 25–35 mm, female SVL 25–39 mm); dorsum light to dark brown usually with a prominent thin mid-dorsal line; a prominent horny ridge extending from the tip of snout to the vent, and a horny ridge between the eyes, arranged in a triangle directed posteriorly; dorsal skin with spinules and/or glandular projections; groin light or dark brown with white or yellow blotches, except R. drutaahu sp. nov.; foot webbing small, below the second subarticular tubercle on either side of toe IV (Fig. 14A). Eye colouration and pattern. This group has a distinct eye colour and pattern and all the members have a light to dark brown iris with golden tinge or dense golden speckling, and iris horizontally divided into light upper and dark lower halves; iris periphery blackish-brown or black; sclera light blue to light bluish-grey (Figs. 14H–14M; Table 1). Acoustic. Six known members of the group for which calls are studied (R. charius, R. coonoorensis, R. drutaahu sp. nov., R. griet, R. honnametti, and R. marki) produce a single type of call that is short and has a pulsatile temporal structure. The calls also have closely packed pulses and a relatively fast pulse rate. The overall dominant frequency of the calls ranges from 2.4 to 3.6 kHz (Figs. 14B–14G; Table 2). Calling height. Members of this group usually call from ground level, low bushes and shrubs, and higher shrubs of up to 4 m high (Table 2). Geographical. Members of this group are currently restricted to the States of Karnataka, Kerala, and Tamil Nadu, both in the Western Ghats and Eastern Ghats regions (Fig. 14N; Table 3).
Species level acoustic comparison. Within the R. charius group, all members produce calls with an overall similar structure. R. charius, R. griet and R. honnametti produce relatively longer calls (92.6 ms, 75.6 ms and 68.6 ms, respectively) compared to R. coonoorensis, R. marki and R. drutaahu sp. nov. (24.6 ms, 36.7 ms and 50.6 ms, respectively). The calls of former three species also have a longer call rise time (49.2 ms, 59.4 ms and 13.3 ms, respectively) compared to the latter three (1.2 ms, 1.3 ms and 1.2 ms, respectively). The pulse rate of all five species also varies considerably. Raorchestes drutaahu sp. nov., R. honnametti and R. griet produce calls with relatively slower pulse rate (134.5 pulses/s, 89.3 pulses/s and 151.3 pulses/s, respectively) compared to the calls of R. marki, R. charius and R. coonorensis (266.6 pulses/s, 226.6 pulses/s and 200 pulses/s, respectively). Raorchestes drutaahu sp. nov., R. griet and R. marki have a relatively higher dominant frequency (3.6 kHz, 3.5 kHz and 4.1 kHz, respectively) compared to R. charius, R. honnametti, and R. coonoorensis (2.4 kHz, 2.6 kHz and 2.9 kHz, respectively) (Figs. 14B–14G; Table 2).
Raorchestes chotta group
Figure 15: Raorchestes chotta species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 16–22 mm, female SVL 19–28 mm); (2) two black or dark brown spots on either side of the lumbar region; (3) foot webbing small, not extending beyond the second subarticular tubercle on either side of toe IV. (B–D) Male calls in members of the group: (B, C and D) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. blandus (1 s section showing three Type 1 calls delivered in group and a single Type 2 call), R. archeos (1 s section showing four calls, delivered in groups), and R. chotta (1 s section showing a single Type 1 calls and two Type 2 calls, delivered in group). (E) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(D). (F–H) Eye colour and pattern in three members of the group.Members included. Raorchestes archeos, R. blandus, and R. chotta.
Group definition. Phylogenetic. The Raorchestes chotta group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. archeos, R. chotta, and R. blandus, but none of the other currently recognised Raorchestes species. (Fig. 2). This group is analogous to the Chotta clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small-sized adults (male SVL 16–22 mm, female SVL 19–28 mm); dorsum predominantly light to dark brown; spinular projections on upper eyelids; two black or dark brown spots on either side of the lumbar region; and foot webbing small, not extending beyond the second subarticular tubercle on either side of toe IV (Fig. 15A). Eye colouration and pattern. Iris golden brown in all three members of the group, with vertical band (in R. archeos); iris periphery brown, thin and discontinuous; sclera greyish-white (Figs. 15F–15H; Table 1). Acoustic. Members of this group produce one (R. archeos) or two (R. chotta and R. blandus) types of call. The most common call type (Type 1) is pulsatile for all the three members, whereas the Type 2 call having a single pulse is found only in R. chotta and R. blandus. The overall dominant frequency of Type 1 calls range from 3.1 to 3.6 kHz (Figs. 15B–15D; Table 2). Calling height. Members of this group usually call from ground level, low bushes and shrubs, and higher shrubs of up to 4 m high (Table 2). Geographical. This group is currently restricted to the Western Ghats regions in the States of Karnataka, Kerala, and Tamil Nadu (Fig. 15E; Table 3).
Species level acoustic comparison. Within the R. chotta group, the Type 1 calls of R. chotta, R. archeos, and R. blandus have a similar structure but can be differentiated by their duration and pulse rate. The Type 1 calls of R. chotta are longer in duration (71.2 ms) and delivered at a rate of 283.5 pulses/s compared to the calls of R. archeos and R. blandus (19.7 ms and 17.1 ms, respectively), which are delivered at a faster rate (382.1 pulses/s and 370.3 pulses/s, respectively). The dominant frequencies of the calls of R. chotta and R. blandus are similar and of higher frequency (3.6 kHz and 3.5 kHz, respectively) compared to R. archeos (3.1 kHz) (Figs. 15B–15D; Table 2).
Raorchestes chromasynchysi group
Figure 16: Raorchestes chromasynchysi species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 20–35 mm, female SVL 27–37 mm); (2) dorsum with a horny ridge extending from the snout tip to the vent; (3) presence of lingual papillae; (4) foot webbing moderate to large, up to or well beyond the second subarticular tubercle on either side of toe IV. (B and C) Male calls in members of the group: Calling individual, followed by a call oscillogram (above) and spectrogram (below) for R. chromasynchysi and R. silentvalley (1 s section showing a single call for each species). (D and E) Adult male, followed by a call oscillogram (above) and spectrogram (below) for R. ravii and R. sanjappai sp. nov. (0.1 s section showing a single call for each species). (F) Adult male of R. vellikkannan sp. nov. (call not studied). (G–K) Eye colour and pattern in five members of the group. (L) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(F).Members included. Raorchestes chromasynchysi, R. ravii, R. sanjappai sp. nov., R. silentvalley, and R. vellikkannan sp. nov.
Group definition. Phylogenetic. The Raorchestes chromasynchysi group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. chromasynchysi, R. ravii, R. sanjappai sp. nov., R. silentvalley, and R. vellikkannan sp. nov., but none of the other currently recognised Raorchestes species. (Fig. 2). This group is analogous to the Chromasynchysi clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium sized shrub frogs (male SVL 20–35 mm, female SVL 27–37 mm); snout sub-elliptical to pointed; dorsum with a mid-dorsal horny ridge extending from the snout tip to the vent; tongue with papillae; foot webbing moderate to large, up to or well beyond the second subarticular tubercle on either side of toe IV (Fig. 16A). Eye colouration and pattern. Members of this group predominantly have a brown, golden brown, reddish-brown, to dark red iris, except silver grey with minute brown speckling in R. vellikkannan sp. nov.; iris periphery dark brown to blackish-brown; sclera blue or scarlet blue. One member, R. silentvalley, is also known to possess distinct yellowish-green spots and blotches on the palpebral membrane (Figs. 16G–16K; Table 1). Acoustic. Four members of the group for which calls are studied (R. chromasynchysi, R. sanjappai sp. nov., R. ravii, and R. silentvalley) produce a single type of call. The calls have a pulsatile temporal structure and are not delivered in call groups. Typically, the call duration ranges between 300 and 800 ms and the overall dominant frequency of Type 1 call ranges from 2.2 to 2.7 kHz (Figs. 16B–16E; Table 2). Calling height. Members of this group usually call from heights of 0.5–6 m on low bushes and shrubs, higher shrubs, and lower to higher tree canopy (Table 2). Geographical. Members of this group are currently restricted to the Western Ghats regions in Karnataka, Kerala, and Tamil Nadu States (Fig. 16L; Table 3).
Species level acoustic comparison. Within the R. chromasynchysi group, all the four studied species have a similar call structure. The calls have a long call rise time but lack any significant fall time. Raorchestes silentvalley produces calls of relatively longer duration of 716 ms (vs. 499.2 ms in R. ravii, 411.2 ms in R. sanjappai sp. nov., and 381.4 ms in R. chromasynchysi). R. sanjappai sp. nov. calls have a relatively slower pulse rate of 2.7 pulses/s (vs. 4.2 pulses/s in R. ravii, 5.8 pulses/s in R. chromasynchysi, and 7.2 pulses/s in R. silentvalley). The dominant frequencies of R. sanjappai sp. nov. and R. silentvalley calls are similar (2.4 kHz and 2.2 kHz, respectively) and slightly higher than that of R. silentvalley (2.5 kHz) and R. ravii (2.7 kHz) (Figs. 16B–16E; Table 2).
Raorchestes flaviventris group
Figure 17: Raorchestes flaviventris species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 30–39 mm, female SVL 48–50 mm); (2) snout semi-circular or rounded, and prominent markings or reticulations on groin and thighs; (3) presence of lingual papillae; (4) foot webbing large, not beyond the third subarticular tubercle on toe IV. (B and C) Male calls in members of the group: (B and C) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. ponmudi and R. flaviventris (1 s sections showing a single call for each species). (D) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B) and (C). (E and F) Eye colour and pattern in two members of the group.Members included. Raorchestes flaviventris and R. ponmudi.
Group definition. Phylogenetic. The Raorchestes flaviventris group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. flaviventris and R. ponmudi, but none of the other currently recognised Raorchestes species. Another taxon R. ‘hassanensis’ (currently under the junior objective synonymy of R. flaviventris) is suggested as a phylogenetically nested member of this group (Vijayakumar et al., 2014) (Fig. 2). This group is analogous to the Hassanensis clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: medium to large-sized shrub frogs (male SVL 30–39 mm, female SVL 48–50 mm) and the largest members of the genus; head wider than long; snout semi-circular or rounded; eyes protruding; prominent markings or reticulations on groin and thighs; lingual papillae present; and foot webbing moderate, not beyond the third subarticular tubercle on either side of toe IV (Fig. 17A). Eye colouration and pattern. Iris creamy white, light brown, to golden brown with minute brown speckling; iris periphery dark brown; sclera bluish-grey (Figs. 17E–17F; Table 1). Acoustic. Two members of the group for which calls are studied (R. flaviventris and R. ponmudi) produce a single type of call with very similar call structure. The calls have a pulsatile temporal structure and typically range between 400 and 800 ms, have a long call rise time, and lack any significant fall time. The overall dominant frequency of calls of these two species ranges from 1.7 to 1.9 kHz (Figs. 17B and 17C; Table 2). Calling height. Members of this group usually call from heights of 1–7 m on low bushes and shrubs, higher shrubs, and lower to higher tree canopy (Table 2). Geographical. Members of this group are currently restricted to the Western Ghats regions in Karnataka, Kerala, and Tamil Nadu States (Fig. 17D; Table 3).
Species level acoustic comparison. Within the R. flaviventris group, species have a very similar call structure. However, the calls of R. flaviventris and R. ponmudi vary in duration (712.7 ms and 458.7 ms, respectively) and pulse rate (21.1 pulses/s and 26.4 pulses/s, respectively). The dominant frequency of their calls is similar (1.9 kHz and 1.7 kHz, respectively) (Figs. 17B and 17C; Table 2).
Raorchestes glandulosus group
Figure 18: Raorchestes glandulosus species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 19–30 mm, female SVL 25–33 mm) and predominantly green shrub frogs; (2) dorsal colouration not completely extending on to the limbs and lateral surfaces; (3) foot webbing moderate, not beyond the third subarticular tubercle on either side of toe IV. (B–E) Male calls in members of the group: (B, C, D and E) Adult males, followed by call oscillograms (above) and spectrograms (below) for R. jayarami, R. glandulosus, R. bobingeri, and R. akroparallagi (1 s sections showing a single call for each species). (F–I) Eye colour and pattern in four members of the group. (J) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(E).Members included. Raorchestes akroparallagi, R. bobingeri, R. glandulosus, and R. jayarami.
Group definition. Phylogenetic. The Raorchestes glandulosus group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. akroparallagi, R. bobingeri, R. glandulosus, and R. jayarami, but none of the other currently recognised Raorchestes species. (Fig. 2). This group is analogous to the Glandulosus clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to large-sized predominantly green shrub frogs (male SVL 19–30 mm, female SVL 25–33 mm); dorsal colouration not completely extending on to the limbs; loreal region vertical; foot webbing moderate, not extending beyond the third subarticular tubercle on either side of toe IV (Fig. 18A). Eye colouration and pattern. Members of this group have two distinct iris colours and patterns: bright to dark red, reddish-brown, or light brown with golden speckles in R. glandulosus and R. akroparallagi; and bright yellow, greyish-yellow, or light yellow with an inner reddish-brown or brown continuous ring or irregular spots/reticulations in R. bobingeri and R. jayarami; iris periphery brownish-black (scarlet blue in subadults of R. glandulosus); sclera light blue (Figs. 18F–18I; Table 1). Acoustic. All members of this group produce a single type of call with very similar structure; the calls are pulsatile and typically range between 500 and 900 ms; calls typically have a long rise time and lack any significant fall time; the overall dominant frequency ranges from 2.7 to 3.6 kHz (Figs. 18B–18E; Table 2). Calling height. Members of this group usually call from heights of 1–8 m on low bushes and shrubs, higher shrubs, and lower to higher tree canopy (Table 2). Geographical. This group is currently restricted to the Western Ghats regions in the States of Karnataka, Kerala, and Tamil Nadu (Fig. 18J; Table 3).
Species level acoustic comparison. Within the R. glandulosus group, the calls of the species show very similar call structure and differ primarily in their pulse rate, which ranges between 10.9 and 13.7 pulses/second. R. jayarami, shows the longest call (813.3 ms) with a higher number of pulses (11 pulses) compared to R. glandulosus (609.6 ms, eight pulses), R. akroparallagi (445.6 ms, six pulses), and R. bobingeri (565.6 ms, six pulses). The dominant frequencies of the calls are similar for R. jayarami and R. glandulosus (2.7 kHz and 2.9 kHz, respectively), while that of R. akroparallagi and R. bobingeri are similar to each other but relatively higher (3.4 kHz and 3.6 kHz, respectively) (Figs. 18B–18E; Table 2).
Raorchestes graminirupes group
Figure 19: Raorchestes graminirupes species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 21–30 mm, female SVL 28–42 mm) and lateral abdominal surfaces green or light brown with yellow or light brown blotches; (2) triangular horny ridge present between the eyes, and a longitudinal mid-dorsal ridge extending from the snout to almost the vent; (3) groin and thighs green or light brown with yellow or light brown blotches; (4) foot webbing moderate, below the third subarticular tubercle on either side of toe IV. (B and C) Male calls in members of the group: (B) Calling individual, followed by a call oscillogram (above) and spectrogram (below) for R. graminirupes (1 s section showing a single Type 1 call and two Type 2 calls, delivered in group). (C) Adult male, followed by a call oscillogram (above) and spectrogram (below) for R. johnceei (1 s section showing three Type 1 calls and four Type 2 calls, delivered in group). (D and E) Eye colour and pattern in two members of the group. (F) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B) and (C).Members included. Raorchestes graminirupes and R. johnceei.
Group definition. Phylogenetic. The Raorchestes graminirupes group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. graminirupes and R. johnceei, but none of the other currently recognised Raorchestes species. (Figs. 2 and 19A). This group is analogous to the Graminirupes clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to large-sized adults (male SVL 21–30 mm, female SVL 28–42 mm); pointed snout; tympanum rather indistinct, strong tympanic fold; triangular horny ridge present between the eyes, and a longitudinal mid-dorsal ridge extending from the snout tip to almost the vent; and lateral abdominal surfaces, groin, and anterior thighs green or light brown with yellow or light brown blotches; foot webbing moderate, below the third subarticular tubercle on either side of toe IV (Fig. 19A). Eye colouration and pattern. Members of this group have a distinct greyish-brown iris with dense metallic silver or light brown speckles; iris periphery black with a scarlet blue inner ring (blue ring more extended in R. johnceei compared to R. graminirupes); sclera light silvery blue. (Figs. 19D and 19E; Table 1). Acoustic. Both members of the group produce two types of call; both the call types have a pulsatile temporal structure, are delivered in call groups, and have a fixed call order (Figs. 19B and 19C; Table 2). Calling height. Members of this group usually call from ground level, low bushes and shrubs, higher shrubs, lower and higher tree canopy up to 8 m high (Table 2). Geographical. This group is currently restricted to the south of Palghat gap in the Western Ghats, in Kerala and Tamil Nadu States (Fig. 19F; Table 3).
Species level acoustic comparison. Within the R. graminirupes group, the calls of the two species differ in their temporal as well as spectral properties. The Type 1 calls of R. graminirupes are longer (91.3 ms) and have more pulses (18 pulses) that are delivered at a rate of 222.5 pulses/s compared to the calls of R. johnceei, which are shorter (66.3 ms) and have fewer pulses (eight pulses) produced at a much slower rate (114.8 pules/s). The dominant frequency is higher in R. graminirupes compared with that in R. johnceei (2.7 kHz and 2.1 kHz, respectively) (Figs. 19B and 19C; Table 2).
Raorchestes nerostagona group
Figure 20: Raorchestes nerostagona species group.
(A) Phylogenetic relationships and major diagnostic characters for members of the group: (1) predominantly canopy dwelling species, (2) adult size range (male SVL 25–35 mm; female SVL 43 mm); (3) presence of weakly to well-developed dermal fringe along the outer margin of the fore and hind limbs; (4) presence of webbing between fingers (except in R. crustai); (5) foot webbing moderate to large, up to or above the second subarticular tubercle on either side of toe IV. (B and C) Male calls in members of the group: (B) Calling individual, followed by a call oscillogram (above) and spectrogram (below) for R. nerostagona (0.1 s section showing a single call). (C) Adult male, followed by a call oscillogram (above) and spectrogram (below) for R. crustai (0.1 s section showing a single call). (D) Adult male of R. keirasabinae sp. nov. (call not studied). (E–G) Eye colour and pattern in three members of the group. (H) Geographical distribution of members of the group; species range colours on map correspond to the square colours indicated alongside each species label in (B)–(D).Members included. Raorchestes keirasabinae sp. nov., R. nerostagona, and provisionally R. crustai.
Group definition. Phylogenetic. The Raorchestes nerostagona group can be characterised as the most inclusive clade and a Western Ghats radiation that contains Raorchestes keirasabinae sp. nov. and R. nerostagona (Fig. 2), analogous to the Nerostagona clade of Vijayakumar et al. (2014). Another species, R. crustai, that shows an unresolved relationship within the clade comprised of the R. graminirupes group, the R. glandulosus group, and the R. nerostagona group is provisionally included in the R. nerostagona group. Morphological. Members of this group can be diagnosed based on the combination of the following characters: medium to large-sized adults (male SVL 25–35 mm, female SVL 43 mm); presence or absence of webbing between fingers (except in R. crustai); presence of weakly to well-developed dermal fringe along the outer margin of the fore and hind limbs; and foot webbing moderate to large, up to or above the second subarticular tubercle on either side of toe IV (Fig. 20A). Eye colouration and pattern. Two members of the group Raorchestes keirasabinae sp. nov. and R. nerostagona have a distinct reddish-grey iris with faint or prominent horizontal brown band, iris periphery dark brown, and sclera scarlet blue; however, the iris colour and pattern of the provisionally placed member R. crustai is more closely related to that in Raorchestes graminirupes group, due to its light greyish-brown colour, iris periphery black with an inner scarlet blue ring, and sclera light silvery blue (Figs. 20E–20G; Table 1). Acoustic. All members of this group produce a single type of call, with similar call structure. The call of two of the studied species (R. nerostagona and R. crustai) consists of a single pulse with a short rise time followed by a relatively longer fall time (Figs. 20B and 20C; Table 2). Calling height. Members of this group can be found calling from high shrubs to the lower and higher tree canopy at heights of up to 40 m (Table 2). Geographical. This group is currently known only from the Western Ghats regions in the States of Karnataka, Kerala, and Tamil Nadu (Fig. 20H; Table 3). Habitat preference. Members of this group are predominantly canopy dwelling species found in forested areas.
Species level acoustic comparison. Within the R. nerostagona group, the call of R. nerostagona is longer (23.3 ms) compared to the call of R. crustai (13.3 ms). The dominant frequency of the calls of these two species are similar (2.0 kHz and 2.2 kHz, respectively) (Figs. 20B and 20C; Table 2).
Raorchestes signatus group
(Figs. 2, 21A–21C and 21K; Tables 1–3)
Figure 21: Raorchestes signatus species group and R. tinniens species group.
(A) Phylogenetic relationships and major morphological characters for the sole recognised member of the Raorchestes signatus species group: (1) adult size range (male SVL 24–29 mm, female SVL 34–42 mm); (2) presence of lingual papillae; (3) foot webbing small, not beyond the second subarticular tubercle on either side of toe IV. (B) Calling male, followed by a call oscillogram (above) and spectrogram (below) for R. signatus (0.1 s section showing a single call). (C) Eye colour and pattern in R. signatus. (D) Phylogenetic relationships and major morphological characters for members of the Raorchestes tinniens species group: (1) adult size range (male SVL 18–29 mm, female SVL 27–39 mm), and predominantly ground-dwelling species found in mountain grasslands or shola forests; (2) relatively short legs; (3) dorsum with a distinct metallic tinge in all colour forms, and often with mosaic pattern on lateral surfaces and groin; (4) foot webbing small, not beyond the second subarticular tubercle on either side of toe IV. (E) Calling male, followed by a call oscillogram (above) and spectrogram (below) for R. tinniens (0.1 s section showing a single call). (F and G) Adult males of R. montanus and R. primarrumpfi (calls not studied). (H–J) Eye colour and pattern in three members of the group. (K) Geographical distribution of members of the R. signatus group and R. tinniens group; species range colours on the map correspond to the square colours indicated alongside each species label in (B) and (E)–(G). Photo of front view of head (C) by Rajkumar K. P.Members included. Raorchestes signatus and R. thodai.
Group definition. Phylogenetic. The Raorchestes signatus group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. signatus and another closely related taxon R. thodai with doubtful taxonomic status, but none of the other currently recognised Raorchestes species (Fig. 2). This group is analogous to the Signatus clade of Vijayakumar et al. (2014). This group is likely to contain additional undescribed species (SG and SDB unpublished data). Morphological. This group can be diagnosed based on the combination of the following characters: small to large-sized frogs (male SVL 24.0–29.0 mm, female SVL 34.0–42.0 mm); dorsal colouration highly variable but usually with an ‘X’ mark; tongue with lingual papillae; and foot webbing small, not extending beyond the second subarticular tubercle on either side of toe IV (Fig. 21A). Eye colouration and pattern. Iris brown, dark brown or reddish-brown, with or without silver white or golden radiating lines and golden speckling; iris periphery without prominent ring; and sclera greyish-brown (Fig. 21C; Table 1). Acoustic. The sole studied member of the group produces a single type of non-pulsatile call and delivered at uniform intervals within call groups. Typically, the calls have a short rise time and a relatively longer fall time. The overall dominant frequency of the calls of the species is around 2.1 kHz (Fig. 21B; Table 2). Calling height. Members of the group calls from ground level to low bushes and shrubs, higher shrubs, lower and higher tree canopy up to 10 m high (Table 2). Geographical. This group is currently restricted to the Western Ghats regions in Kerala and Tamil Nadu States (Fig. 21K; Table 3).
Raorchestes tinniens group
(Figs. 2 and 21D–21K; Tables 1–3)
Members included. Raorchestes montanus, R. primarrumpfi, and R. tinniens.
Group definition. Phylogenetic. The Raorchestes tinniens group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. montanus, R. primarrumpfi, and R. tinniens, but none of the other currently recognised Raorchestes species. (Fig. 2). This group is analogous to the Tinniens clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to large-sized frogs (male SVL 18–29 mm, female SVL 27–39 mm); dorsum with a distinct metallic tinge in all colour forms and often with mosaic pattern on lateral surfaces and groin; relatively short legs; foot webbing small, not extending up to the second subarticular tubercle on either side of toe IV; and tongue with lingual papillae (Fig. 21D). Eye colouration and pattern. Members of the group have a dark brown, golden brown, or greyish-brown iris, occasionally with golden speckling; iris periphery black; sclera ash grey. Vijayakumar et al. (2014) noted the iris in R. primarrumpfi as having a dark maroon lower part and the upper half speckled with iridescent golden and silver. However, the same was not observed in any sample of this species from its type locality and vicinities in our study (Figs. 21H–21J; Table 1). Acoustic. A single member of the group for which calls are studied (R. tinniens) produces a single type of non-pulsatile call that is organised into call groups and separated by uniform intervals. Typically, the calls have a short rise time and a relatively longer fall time. The overall dominant frequency of the calls is around 2.6 kHz (Fig. 21E; Table 2). Calling height. Members of this group usually call from ground level to low bushes and shrubs up to 1.5 m high (Table 2). Geographical. Members of this group are currently restricted to the Western Ghats regions in Karnataka, Kerala, and Tamil Nadu States (Fig. 21K; Table 3). Habitat preference. Members of this group are predominantly ground-dwelling species inhabiting mountain grasslands or shola forests.
Raorchestes travancoricus group
Figure 22: Raorchestes travancoricus species group.
(A) Phylogenetic relationships and major morphological characters for members of the group: (1) adult size range (male SVL 16–30 mm, female SVL 21–39 mm); (2) snout sub-elliptical to pointed; (3) foot webbing rudimentary to small, not beyond the second subarticular tubercle on either side of toe IV. (B–E) Male calls in members of the group: (B, C, D and E) Calling individuals, followed by call oscillograms (above) and spectrograms (below) for R. kadalarensis (1 s section showing two Type 1 calls and two Type 2 calls, delivered in group), R. luteolus, R. travancoricus, and R. chlorosomma (1 s sections showing a single Type 1 call and a single Type 2 call for each of the latter three species, calls delivered in groups). (F) Adult male of R. agasthyaensis (call not studied). (G–K) Eye colour and pattern in five members of the group. (L) Geographical distribution of members of the group; species range colours on the map correspond to the square colours indicated alongside each species label in (B)–(F). Photos of calling males in (C) and (D) by Seshadri K. S. and Manoj P., respectively.Members included. Raorchestes agasthyaensis, R. chlorosomma, R. kadalarensis, R. luteolus, and R. travancoricus.
Group definition. Phylogenetic. The Raorchestes travancoricus group can be characterised as the most inclusive clade and a Western Ghats radiation that contains R. agasthyaensis, R. chlorosomma, R. kadalarensis, R. luteolus, and R. travancoricus, but none of the other currently recognised Raorchestes species (Fig. 2). This group is analogous to the Travancoricus clade of Vijayakumar et al. (2014). Morphological. Members of this group can be diagnosed based on the combination of the following characters: small to medium-sized adults (male SVL 16–30 mm, female SVL 21–39 mm); snout sub-elliptical to pointed; tympanum smaller than eyes; foot webbing rudimentary to small, not extending beyond the second subarticular on either side of toe IV (Fig. 22A). Eye colouration and pattern. Members of this group have variable iris colour and patterns: R. luteolus and R. travancoricus have golden yellow or light grey iris with brown speckling with a cobalt blue outsider ring, iris periphery black or bluish-black, and indistinct sclera; R. agasthyaensis and R. kadalarensis have dark brown iris with dense golden speckling, iris horizontally divided into light upper and dark lower halves, iris periphery dark brown, and sclera ash grey; or a distinctly metallic greyish-green or greenish-yellow iris with dark brown reticulations, iris periphery dark brown, and sclera scarlet blue in R. chlorosomma (Figs. 22G–22K; Table 1). Acoustic. Four members of the group for which calls were studied (R. chlorosomma, R. kadalarensis, R. luteolus, and R. travancoricus) produce two types of call. The Type 1 call in three species (R. chlorosomma, R. luteolus, and R. travancoricus) has a pulsatile temporal structure, whereas R. kadalarensis call comprises of a single pulse. On the other hand, the Type 2 call of two species (R. travancoricus and R. luteolus) consists of a single pulse, and that of R. chlorosomma and R. kadalarensis has a pulsatile temporal structure. The overall dominant frequency of Type 1 call of these species ranges from 2.2 to 3.4 kHz (Figs. 22B–22E; Table 2). Calling height. Members of this group usually call from heights of 0.5–4 m on low bushes and shrubs to high shrubs (Table 2). Geographical. Members of this group are widely distributed in the Western Ghats regions in Karnataka, Kerala, and Tamil Nadu States (Fig. 22L; Table 3).
Species level acoustic comparison. Within the R. travancoricus group, R. travancoricus and R. luteolus produce calls with very similar structures, while the calls of R. chlorosomma and R. kadalarensis are different from other members of the group. Type 1 calls of R. travancoricus and R. luteolus have similar temporal properties with relatively longer call duration, 474.5 ms and 390.5 ms, respectively (vs. 246.2 ms in R. chlorosomma and 15.6 ms in R. kadalarensis), and much slower pulse rate, 33.1 pulse/s and 31.9 pulse/s, respectively (vs. 195.8 pulses/s in R. chlorosomma). The dominant frequency of Type 1 calls of R. travancoricus and R. kadalarensis are similar (3.3 kHz and 3.4 kHz, respectively) and both are higher in frequency than in the Type 1 calls of R. luteolus and R. chlorosomma, which are more similar to each other (2.5 kHz and 2.2 kHz, respectively). The non-pulsatile Type 2 calls of R. travancoricus and R. luteolus are relatively shorter, 17.2 ms and 27.2 ms, respectively, compared to the pulsatile Type 2 calls of R. chlorosomma (30.2 ms) and R. kadalarensis (52.6 ms). The dominant frequency of both Type 1 and Type 2 calls are similar in each species (Figs. 22B–22E; Table 2).
Ungrouped species: Raorchestes echinatus and R. indigo
Figure 23: Ungrouped species.
(A) Phylogenetic position of two ungrouped species, Raorchestes echinatus and R. indigo. (B–E) R. echinatus: (B) Dorsolateral view.(C) Lateral view. (D) Posterior view of thighs. (E) Eye colour and pattern. (F–I) R. indigo: (F) Dorsolateral view. (G) Lateral view. (H) Posterior view of thighs. (I) Eye colour and pattern. (J) Geographical distribution of R. echinatus and R. indigo.Two species, Raorchestes echinatus and R. indigo, cannot be unambiguously assigned to any of the defined species groups due to their unresolved phylogenetic relationship (Vijayakumar et al., 2014; Fig. 2, present study) and lack of clearly shared morphological characters with any other groups. We could also not study the acoustic characters of these species. Vijayakumar et al. (2014) discussed the superficial morphological similarities between R. echinatus and R. tuberohumerus largely based on skin colouration and texture, and certain body dimensions. We further note that R. echinatus could be more closely related to members of the R. bombayensis group due to comparable adult size, presence of mid-dorsal lines, spinular dorsum, and mottled ventral skin. On the other hand, R. indigo shows resemblance with R. beddomii, a member of the R. beddomii group, due to comparable adult size, green dorsal colour that extends on to the dorsal surfaces of limbs, loreal and tympanic regions, and occasional presence of blue colouration on groin, armpits, and thighs (Biju & Bossuyt, 2009). However, we currently refrain from assigning R. echinatus and R. indigo to any of the defined species groups (Fig. 23), with their grouping pending to be resolved based on further evidence (such as acoustic, ecological, reproductive, or behavioral aspects) or possible future discoveries of new and closely related taxa.
Discussion
The genus Raorchestes accounts for nearly one-fourth of the currently known frog diversity of the Western Ghats. Taxonomic studies on such a large radiation that lacks clear morphological synapomorphies among closely related species poses several challenges, particularly with a large number of new species accumulating within a short span of time. Despite already being one of the most actively researched groups of anurans of the region for nearly two decades (e.g., Biju & Bossuyt, 2005a, 2006, 2009; Biju et al., 2010; Zachariah et al., 2011, 2016; Vijayakumar et al., 2014, 2016), the known shrub frog diversity continues to rise. This is not surprising since groups where significant efforts have been made to resolve existing taxonomic confusions—other examples such as Nyctibatrachus (Biju et al., 2011; Van Bocxlaer et al., 2012; Garg et al., 2017), Indirana (Dahanukar et al., 2016; Garg & Biju, 2016), and Minervarya (Dubois, Ohler & Biju, 2001; Dinesh et al., 2015; Garg & Biju, 2017; Raj et al., 2018)—have also experienced renewed interest from researchers, consequently increasing the rate of new discoveries. On this backdrop, our discovery of another five new Raorchestes species indicates that much work remains to be done. Recent studies by Vijayakumar et al. (2014, 2016) have also shown the presence of undescribed diversity in this genus based on phylogenetic evidence that remains to be investigated further; however much of their available molecular data lacks the associated geographical or morphological information, with the corresponding species identity remaining largely unverified. Further studies are necessary to clarify the taxonomic status of all the currently known shrub frog populations and to better understand the patterns of diversification and distribution of this group in the Western Ghats.
Current knowledge about the distribution of Raorchestes frogs in the Western Ghats shows that half (28 species) of the known diversity (56 recognised species) is endemic to the southern Western Ghats, that is broadly considered as regions south of Palghat gap. In a phylogenetic perspective as well, the Western Ghats shrub frogs comprise two large radiations, (1) the Southern clade with ancestral range in the southern Western Ghats and (2) the Northern clade with ancestral range north of Palghat gap (Vijayakumar et al., 2016; Fig. 2 in present study). It is the northern clade that gives rise to radiations dispersed outside the Western Ghats, into Central India and the Eastern Ghats, that is the Raorchestes bombayensis species group (Figs. 2 and 12; Table 3), further into Northeast India and the remaining range of the genus in South, Southeast, and East Asia, that is the Raorchestes parvulus species group (Fig. 2). The geographical range of the Raorchestes charius group (northern clade) also extends from the Western Ghats to the Eastern Ghats. On the other hand, the southern clade represents ancient lineages restricted to the southern Western Ghats regions of Kerala and Tamil Nadu States, suggesting these hill ranges to be a reservoir of remnant and endemic anuran fauna (e.g., Biju & Bossuyt, 2003; Roelants, Jiang & Bossuyt, 2004). Our discoveries of the five new Raorchestes species described in this study were made from forested areas in the State of Kerala, encompassing the hill ranges of Agasthyamalai, Cardamom (south of Palghat gap), and Nilgiris, Siruvani, Wayand (North of Palghat gap). Amphibians in these regions are known to be facing increasing anthropogenic threats (Biju et al., 2008; Nair et al., 2011; Garg et al., 2017; Thorpe et al., 2018) and all the new species are found either outside protected areas or in fragmented primary forest patches and highly disturbed secondary forest areas. The new species will therefore require immediate assessment of threats to the known populations and habitats, and their conservation status.
One of the major aims of our study was to comprehensively infer the systematic relationships between Raorchestes species and define species groups using integrative approaches in order to facilitate a better working taxonomy for this large and morphologically challenging radiation of rhacophorid frogs. We define 16 species groups, primarily delimited based on phylogeny (Vijayakumar et al., 2014; present study), that are additionally diagnosable based on morphology, acoustics, and as well as geographical parameters. In doing so, we provide novel insights that will further enable proper laboratory as well as field-based identification and documentation of Raorchestes frogs in the Western Ghats, and thereby also assist in their conservation.
This is also the first time that bioacoustics studies have been carried out at a large-scale for this genus covering 45 of the now recognised 59 species from Peninsular India, thereby allowing a better understanding of the interspecific differences and possible group-level diagnostic call characters and their usefulness in taxonomy. The current work will pave the way for future acoustic studies on this group, which are required to describe the vocal repertoires of species in detail and understand intraspecific variations (Bee, Suyesh & Biju, 2013a, 2013b). An overall understanding of the basic call structures of members of the various species groups will also promote non-invasive documentation and monitoring of shrub frogs in the Western Ghats. The calling height of frogs also plays an important role in niche segregation (Hödl, 1977; Martins, Almedia & Jorge, 2006; Wells, 2007), hence future studies can investigate the evolutionary processes that are likely to have driven premating and postmating isolation mechanisms in this unique group of direct-developing tree frogs. Future research is also necessary on various understudied aspects of the group members such as reproductive behavior, ecology, and natural history (barring a few studies such as Bossuyt et al., 2001; Krishnamurthy, Gururaja & Manjunatha Reddy, 2002; Biju, 2003; Biju & Bossuyt, 2005a, 2005b, 2009; Biju et al., 2010; Seshadri, Gururaja & Bickford, 2015; Princy & Kannan, 2018), which can further enhance the diagnosability of various species and species groups. Our study also highlights a larger set of morphological characters, including various traits such as eye colour and patterns for group-level diagnosis of Raorchestes frogs. This will not only assist future taxonomic research within the Western Ghats but also of the extended radiations of Raorchestes frogs in South, Southeast and East Asia, for which no such comparable studies are currently available.