Expression dynamics of metabolites in diploid and triploid watermelon in response to flooding
- Published
- Accepted
- Received
- Academic Editor
- Atsushi Fukushima
- Subject Areas
- Agricultural Science, Molecular Biology, Plant Science
- Keywords
- Diploid and triploid watermelon, Flooding, Dynamic expressions, Antioxidants, Oxidants, Flooding, Dynamic expressions, Antioxidants, Oxidants
- Copyright
- © 2022 He et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Expression dynamics of metabolites in diploid and triploid watermelon in response to flooding. PeerJ 10:e13814 https://doi.org/10.7717/peerj.13814
Abstract
Watermelon (Citrullus lanatus) is an economically important cucurbitaceous crop worldwide. The productivity of watermelon is affected by both biotic and abiotic stresses. Flooding has significant impacts on the growth of watermelons by causing oxygen deficiency and a loss of agricultural productivity. Currently, we used the triploid and diploid watermelon Zhengzhou No.3 to study the dynamics of metabolites in response to flooding stress. Quantification of metabolites was performed by UPLC-ESI-MS/MS at different time intervals i.e., 0, 3, 5 and 7 days under flooding stress. We observed that the activities of oxidants were higher in the diploid watermelon, whereas the higher antioxidant activities in the triploid watermelon makes them more resistant to the flooding stress. We also observed that the root activity and the chlorophyll in the triploid watermelon plants were higher as compared to the diploid watermelon plants. Co-expression network analysis leads to the identification of twenty-four hub metabolites that might be the key metabolites linked to flooding tolerance. Resolving the underlying mechanisms for flooding tolerance and identification of key molecules serving as indicators for breeding criteria are necessary for developing flooding-resistant varieties.
Introduction
Cucurbit species, including watermelon, produce large edible fruits that serve as an important part of the diet of people around the world (Erickson et al., 2005). Watermelon, a cucurbitaceous crop, is an economically important crop. Watermelon production accounts for 2.56% of the world’s total vegetable production (http://faostat.fao.org). According to FAO data, China consumed more than 70 million tons of watermelon in 2020, and per capita, watermelon consumption exceeded 50 kilograms (kg) (http://faostat.fao.org).
Seedless watermelons are very popular around the world due to consumer preferences, and greater economic value to growers than traditional seed varieties (Liu, Offler & Ruan, 2014).
Watermelon productivity is affected by a variety of environmental stresses. Most crop hybrids (a genetic “cross” between different species, varieties, or cultivars) often exhibit increased yields, increased yield stability, and enhanced stress resistance ability against abiotic and biotic stresses (Schnable & Springer, 2013). Despite the importance of watermelon production, the functions of genes related to environmental stress in watermelon have not yet been reported. Stresses including biotic and abiotic stresses have a huge impact on the annual yield of watermelon. Hence, research on abiotic and biotic stresses on watermelon is needed.
Waterlogging significantly reduces crop yields and exacerbates the problem by decreasing crop production to feed a growing human population (Zhang et al., 2017). Flooding conditions are causing oxygen starvation, resulting from the slow diffusion of gases in the water and oxygen uptake by microorganisms as well as plant roots (Huang, Greenway & Colmer, 2003). Flooded soil rapidly becomes free of oxygen at depths under a few millimeters (Vartapetian et al., 2003). Floods are a major problem limiting crop growth by leading to hypoxia in the vicinity of the root and rhizomes, as well as consequently it could be fatal since aerobic respiration ceases thus levels of energy-rich adenylates decline sharply leading to a drastic decrease in ion uptake and transport (Huang, Greenway & Colmer, 2003; Vartapetian et al., 2003). Waterlogging mainly takes place due to heavy rains and poor soil drainage. The epistatic and dynamic quantitative trait loci (QTLs) for four watermelon traits, including the membership function value of waterlogging (MFVW), the wilting index (WI), the chlorosis score (Score), and the dead leaf ratio (DLR) to demonstrate waterlogging resistance based on genetic characteristics in a chrysanthemum population of 162 F1 lines were identified by (Su et al., 2018; Voesenek, Van & Sasidharan, 2014; Zhang et al., 2016) identified. Five putative QTLs in Oryza sativa L. were identified, as qAG-1-2, qAG-3-1, qAG-7-2, qAG-9-1, and qAG-9-2 on chromosomes 1, 3, 7, and 9, respectively. These were detected as tolerant QTLs and some QTLs were compatible with previously identified QTLs for tolerance-related symptoms (Angaji et al., 2010). The phenotypic responses of 277 genotypes of, Andean Diversity Paneled dry beans (Phaseolus vulgaris L.) against flooding at germination and seedling stages and genetic areas associated with flood tolerance have been identified, including the regions at Pv08/3.2 Mb by Soltani et al. (2018). Limited knowledge of watermelon is available in response to flooding stress. It was reported by Yetisir et al. (2006) that the impact of flooding on physiological parameters (fresh and dry weight, leaf number and main stem length. Leaf color, single leaf CO2 exchange rate (CER), stomatal conductance (SC) and transpiration rate (Ts)) in grafted and non-grafted watermelons and found that grafted watermelons performed well under flooding.
Metabolomics studies have identified crucial secondary metabolites of tolerant varieties for combating abiotic stress (Lothier et al., 2020). Metabolites are believed as signaling molecules because they are correlated with physiological practices and are exported from each organelle to the cytoplasm in the form of retrograde signals (Evans, 2003). The plant reaction to drought is affected not only by the drought-responsive transcriptome but also by interactions among genes, metabolites, and proteins. Hence, we can better recognize the mechanisms of fundamental plant resistance to drought stress by combining transcriptomics and metabolomics approaches. Plants have two types of metabolites i.e., primary and secondary metabolites which are linked to highly complex metabolic pathways. Modern techniques including GCMS, LCMS, and NMR are successful in the detection of different types of metabolites. Primary metabolites lead to the production of lipids, sugars, and amino acids thus, affecting plants’ growth as well as development. Secondary metabolites are formed as a result of the activities of primary metabolites and are produced in response to various stresses including flooding, high temperature, chilling, drought, salinity, and insect/pest attack. Moreover, secondary metabolites include reactive oxygen species (ROS), antioxidants, as well as coenzymes.
Over the past decade, research on molecular-based mechanisms of flood tolerance has developed rapidly. Adaptations of waterlogging (root system induction) and sinking (root and aerial system flooding) were explained at the molecular level in rice (Fukao & Xiong, 2013; Mustroph et al., 2015) and low-O2 sensing knowledge was achieved, through Arabidopsis research and has been successfully translated into barley to improve tolerance (Mendiondo et al., 2015). Molecular regulators of flood-adaptive silencing (SUB1A) and escape (SNORKEL1/2) were found in rice (Xu et al., 2006; Hattori et al., 2009; Gibbs et al., 2012; Licausi et al., 2011; Sasidharan & Mustroph, 2011) exposed the elusive mechanism of O2 sensing with ethylene response transcription factors of subclass group VII (ERF-VII) and wild species intolerant to natural studies revealed unknown tolerance mechanisms and genes (Sasidharan et al., 2013; Veen et al., 2013). However, to our knowledge of reduced O2 sensing, signaling, and lower response networks, there are still many violations. Great challenges do exist in understanding and improving root aeration, regulation of low-O2 metabolism, and recovery after stress. Currently, we used a metabolomics-based approach to identify metabolites linked to flooding tolerance in watermelon. Here, we used diploid and triploid watermelon to check which ploidy level responds better to flooding stress. No such reports have been presented before in response to flooding stress in watermelon. Here in for the first time the quantification of metabolites was performed by UPLC-ESI-MS/MS at different time intervals under flooding stress in diploid and triploid watermelons. We tried to explain the dynamics of metabolites linked to flooding at different developmental stages.
Materials and Methods
The varieties Zhengzhou No.3 diploid (Z2x) and Zhengzhou No.3 triploid (Z3x) bred by the polyploid watermelon genetics and breeding research group of Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences. The fully mature, plump, and uniform seeds were broken, soaked in water for 3 h at room temperature, and then placed for germination at 35 °C for 36 h. The germinated seeds were sown in a plastic nutrition bowl containing the seedling substrate (mixed with carbendazim fungicide). The seedlings were cultured in a greenhouse located in Zhengzhou, Henan province, China, in March 2019 under 26 °C for 12 h light, and 23 °C for 12 h dark.
When the fourth true leaf was fully expanded, the whole plant together with the bowl was placed in a water tank. The plants were fully submerged in water. We added water every day to keep the same water level. Plant phenotypic observations and sample collections were carried out at 0, 3, 5, and 7 days after flooding treatment, and 0 days after flooding was considered as control. The samples from 6 plants were mixed as a replicate, three biological repeats were taken Table 1.
| Label | Tissue | Remarks |
|---|---|---|
| Z2xCK | Fresh root | Zhengzhou No.3 diploid, 0 days after flooding treatment |
| Z3x CK | Fresh root | Zhengzhou No.3 triploid, 0 days after flooding treatment |
| QZ2x-3 | Fresh root | Zhengzhou No.3 diploid, 3 days after flooding treatment |
| QZ3x-3 | Fresh root | Zhengzhou No.3 triploid, 3 days after flooding treatment |
| QZ2x-5 | Fresh root | Zhengzhou No.3 diploid, 5 days after flooding treatment |
| QZ3x-5 | Fresh root | Zhengzhou No.3 triploid, 5 days after flooding treatment |
| QZ2x-7 | Fresh root | Zhengzhou No.3 diploid, 7 days after flooding treatment |
| QZ3x-7 | Fresh root | Zhengzhou No.3 triploid, 7 days after flooding treatment |
Sample preparation and extraction for widely targeted metabolic analysis
The roots samples were collected at 0, 3, 5, and 7 days after flooding and stored at −80 °C. The freeze-dried samples were further used for metabolite extraction. Metabolites extraction protocol and machine conditions were used as described previously by Wu et al. (2020).
Principal component analysis (PCA) analysis
For the current research, an R-based statistical program prcomp (R Core Team, 2018) (3.50) has been used to execute principal component analysis Data was log2 transformed before doing the PCA.
Hierarchical cluster analysis and pearson correlation coefficients
The HCA (hierarchical cluster analysis) outcomes of samples, as well as metabolites, have been presented as heatmaps along with dendrograms, whereas Pearson’s correlation coefficients (PCC) among the samples were calculated in R by using the cor function and then presented as heatmaps. R package heatmap (1.0.12) was used for estimating the HCA and PCC. For HCA, the standardized signal strength of metabolites (unit variance scaling) is shown as a color spectrum.
Differential metabolites selection
Considerably regulated metabolites among the groups were defined by Variable influence on projection (VIP) >= 1 and the absolute Log2FC (fold change) >= 1. The VIP values were taken from orthogonal projections to latent structures discriminant analysis (OPLS-DA) results, which also include score plots as well as permutation plots have been generated using the R package MetaboAnalystR (1.0.1). The log2 transformation of data, as well as mean centering, was done before OPLS-DA. To prevent overfitting, a permutation test (200 permutations) was conducted.
Assay for antioxidant enzymes and osmoregulatory compounds
Leaf samples at 0, 3, 5, and 7 days were collected in replicates to perform the said assays. The peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and hydrogen peroxidase were estimated according to Wang et al. (2014), García-Triana et al. (2010), Aebi (1984) and Liu, Offler & Ruan (2014) respectively. The proline and malondialdehyde (MDA) contents were determined according to Vieira, Silva & Glória (2010); Castrejón & Yatsimirsky (1997) respectively.
Determination of chlorophyll contents and plasma membrane permeability
SPAD values of watermelon leaves were determined by using a SPAD-502 chlorophyll meter (Konica Minolta, Tokyo, Japan). Plasma membrane permeability was measured according to Aly-Salama & Al-Mutawa (2009).
Determination of root activity, relative water contents, and Lactate dehydrogenase (LDH)
The fresh diploid and triploid watermelon roots were used for the root activity measurements via the triphenyl tetrazolium chloride (TTC) method (Zhong et al., 2019). The relative water content (RWC) of the above-ground tissues was calculated by the following formula.
Lactate dehydrogenase (LDH) was assayed by the kit from Suzhou Comin Biotechnology Company (Li et al., 2020).
Coexpression network analysis
A coexpression regulation network of the metabolites linked to flooding stress was created using the Cytoscape software (version 3.7.2) (Shannon et al., 2003). The threshold for the co-expression network map was set as p > 0.99. The topological coefficient of each node with a degree > 20 was used to identify the hub metabolites.
Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA), and significant differences among the individual means were determined using Least Significant Difference (P < 0.05 and P < 0.01) at the 5% and 1% confidence levels, respectively. In addition, the graphs were drawn in MS excel.
Results
Phenotypic variation among diploid and triploid watermelon
At the 4th true leaf stage, watermelon plants were exposed to flooding stress. Triploid watermelon showed more resilient phenotypes to flooding stress as compared to the diploid watermelon plants at 3, 5, and 7 days of flooding (Fig. 1). Major differences were seen on the 5th day after flooding when diploid plants start to show wilting signs more clearly as compared to the triploid plants. Root density was recorded more for the triploid watermelon as compared to diploid watermelon. Both the roots and shoots of triploid watermelon seem to be healthier as compared to diploid watermelon plants.
Figure 1: Diploid and triploid watermelon plants in response to flooding stress at 0, 3, 5, and 7 days after flooding stress.
QZ2 means diploid watermelon, whereas QZ3 means triploid watermelon.Measurement of chlorophyll content, root activity, plasma membrane permeability, intracellular CO2 concentration, and relative water content
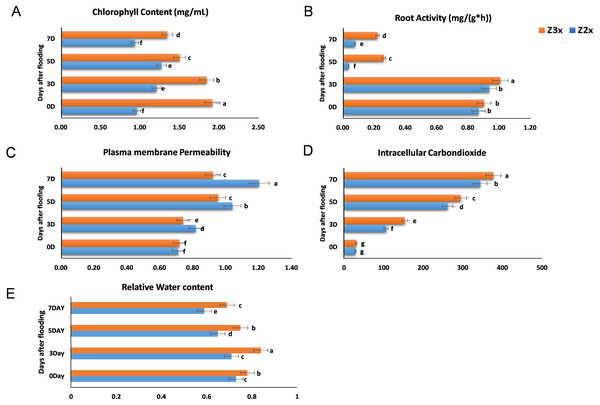
We measured the chlorophyll contents, plasma membrane permeability, root activity, and intracellular CO2 concentrations in the diploid and triploid watermelon plants after exposure to flooding stress at 0, 3, 5, and 7 days (Figs. 2A–2D). The contents of chlorophyll were observed more in the triploid watermelon at all the sampling stages. The highest chlorophyll contents were observed in triploid watermelon 3 days after flooding which is the most critical period. Higher root activities were recorded in triploid as compared to diploid watermelon. The highest root activity was observed 3 days after flooding stress. Plasma membrane permeability and intracellular CO2 concentrations were comparatively higher in diploid watermelon as compared to triploid watermelon. A continuous increase in the intracellular CO2 concentrations was observed in diploid and triploid watermelon plants. The highest intracellular CO2 concentrations were observed at 5 and 7 days after flooding stress. Relative water contents were recorded higher on the 3rd day after flooding (Fig. 2E). Overall, triploid watermelon plants have a higher relative water content as compared to diploid watermelon plants.
Figure 2: Measurement of (A) chlorophyll content, (B) root activity, (C) plasma membrane permeability, (D) intracellular CO2 concentration, and (E) relative water contents in triploid and diploid watermelon at 0, 3, 5, and 7 days after flooding stress.
Bars show standard error. Means followed by the same letter in the same graph are not significantly different at 5% probability level using least significant difference (LSD) test.Measurement of SOD in response to flooding stress in diploid and triploid watermelon
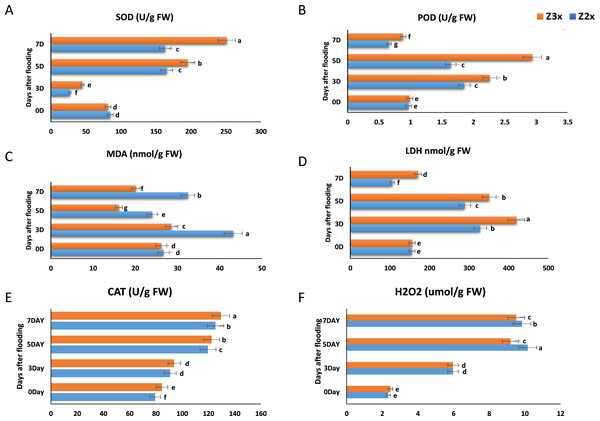
SOD is a vital ROS scavenger, which is catalyzing the superoxide dismutationand plays exceptional role in the biological oxidation structures (Fig. 3A). Results revealed that the SOD activity in Z2x (diploid) significantly decreased on the 3rd day as compared to 0 days and the highest SOD production was recorded on the 5th day, whereas in the case of Z3x (triploid) highest SOD activity was recorded on the 7th day and lowest was recorded on 3rd day. Higher SOD activities were recorded in the triploid watermelon at the later stages of flooding stress.
Figure 3: Measurement of (A) SOD, (B) POD, (C) MDA, (D) LDH, (E) CAT, and (F) H2O2 in response to flooding stress in diploid and triploid watermelon at 0, 3, 5, and 7 days.
Bars show standard error. Means followed by the same letter in the same graph are not significantly different at a 5% probability level using least significant difference (LSD) test.Measurement of POD in response to flooding stress in diploid and triploid watermelon
Both Z2x (diploid) and Z3x (triploid) showed the same trend in POD activity (Fig. 3B). POD started to increase gradually from day 0 and the highest POD activity was recorded on day 5 after flooding and a sharp decline was observed in POD activity on the 7th day. Comparatively higher POD activities were observed in triploid watermelon.
Measurement of MDA activity in response to flooding stress in diploid and triploid watermelon
Lipid decomposition also leads to the production of MDA, detecting the levels of MDA helps to estimate the lipids oxidation state. According to the results, the MDA activity in Z2x was seen to be highest on the third day of flooding, then it started to decrease on the 5th day and the highest MDA activity was observed on 7th day (Fig. 3C). In the case of Z3x, the highest MDA activity was observed on the 3rd day after flooding and the lowest was recorded on the 5th day. Overall, higher MDA activities were recorded in the diploid watermelon plants as compared to triploid watermelon plants.
Measurement of LDH in response to flooding stress in diploid and triploid watermelon
LDH showed a uniform trend of increasing and decreasing from day 0 to day 7 (Fig. 3D). In both varieties (Z2x (diploid) and Z3x (triploid)) a significant increase was recorded on day 3 and then it gradually started to decrease on day 5 and the lowest was recorded on day 7. Overall, the LDH activity was recorded higher in triploid watermelon at 3, 5, and 7 days after flooding stress.
Measurement of catalase in response to flooding stress in diploid and triploid watermelon
According to the results, the CAT activities in Z2x were lower as compared to the triploid watermelon plants (Fig. 3E). The highest CAT activity was observed on the 7th day of flooding in both triploid and diploid watermelon plants. The lowest CAT activities were recorded at the 0 and the 3rd days after flooding. Overall, we observed that the triploid watermelon plants had higher catalase activities than the diploid watermelon.
Measurement of H2O2 in response to flooding stress in diploid and triploid watermelon
In plants, H2O2 is considered a common reactive oxygen molecule, which can oxidize the nucleic acids, proteins, and other larger biological molecules and can damage the cell membranes, accelerating the process of aging and disintegration of cells, which can be catalyzed by the CAT and POD degradation. We observed that H2O2 was comparatively higher in the diploid watermelon as compared to the triploid watermelon (Fig. 3F). The highest levels of hydrogen peroxides were recorded on the 5th day after flooding in diploid and for triploid watermelon highest H2O2 was recorded on the 7th day after flooding.
Identification of metabolites by UPLC-ESI-MS/MS and analysis of metabolites at different time points
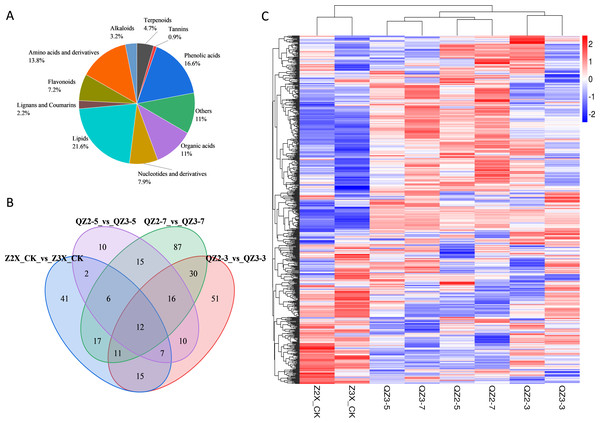
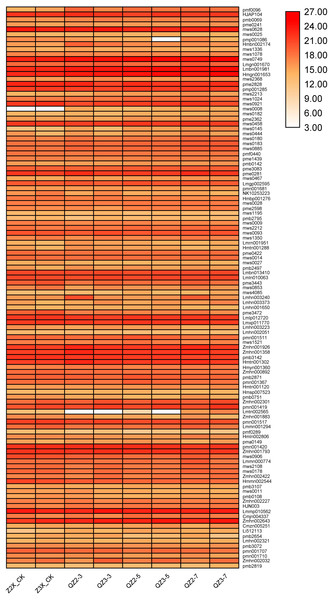
The root samples of diploid and triploid watermelon plants at 0, 3, 5, and 7 days after flooding were collected for metabolites identification. In total, 683 differentially expressed metabolites were annotated in both diploid and triploid watermelon roots. A heatmap of all the detected metabolites was drawn to show their contents in diploid and triploid watermelon at 0, 3, 5, and 7 days after flooding stress. PCA analysis shows the data variability among diploid and triploid at different sampling stages and thus increasing the confidence of our data (Figs. 4A, 4C and 4D). Kyoto Encyclopedia of Genes and Genomes (KEGG) showed that at 0 and 3 h of flooding stress fatty acid degradation, arginine biosynthesis, biosynthesis of amino acids, pentose phosphate pathway, and lysine biosynthesis pathways were highly enriched. At 5 and 7 h of flooding, the results suggested that arginine and proline metabolism, cysteine and methionine metabolism, starch and sucrose metabolism, flavonoid biosynthesis, and metabolic pathways were highly enriched (Fig. S1).
Figure 4: (A) Classification of metabolites in diploid and triploid watermelon under flooding stress. (B) Venn diagram presenting the common metabolites in diploid and triploid watermelon at 0, 3, 5, and 7 days after flooding stress. (C) Heatmap representing the contents of all the detected metabolites in diploid and triploid watermelon. (D) PCA plot showing the variability of data at different sampling stages in diploid and triploid watermelon.
Comparison of metabolites in diploid and triploid watermelon roots under flooding stress
Metabolic profiles of diploid and triploid watermelon roots were compared at four stages after applying flooding stress i.e., 0, 3, 5, and 7 days. Roots of diploid and triploid watermelon were used for the quantification of metabolites via UPLC-ESI-MS/MS high-throughput technology. In total, 683 metabolites were detected, of which 89% metabolites were grouped into different classes while 11% were clustered as others (Fig. 4A). The contents of metabolites belonging to different categories were different in both diploid and triploid watermelon at different time points. Overall lipid share was 21.6% and were the highest quantified metabolites in the current research, followed by phenolic acids (16.6%), amino acids and derivatives (13.8%), organic acids (11%), nucleotides and derivatives (7.9%), flavonoids (7.2%), terpenoids (4.7%), alkaloids (3.2%) and tannins (0.9%) respectively, Table 2.
| Group name | All | Down regulated | Up regulated |
|---|---|---|---|
| Z2X_0_vs_Z3X_0 | 111 | 60 | 51 |
| QZ2-3_vs_QZ3-3 | 152 | 99 | 53 |
| QZ2-5_vs_QZ3-5 | 78 | 41 | 37 |
| QZ2-7_vs_QZ3-7 | 194 | 112 | 82 |
Expression dynamics of different metabolites in diploid and triploid watermelon roots at different developmental stages
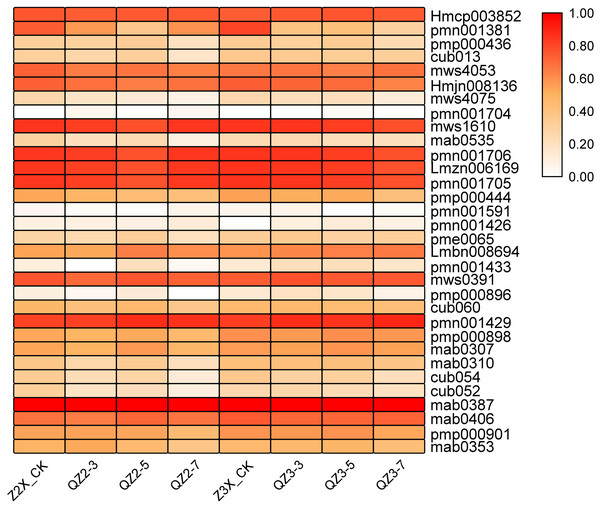
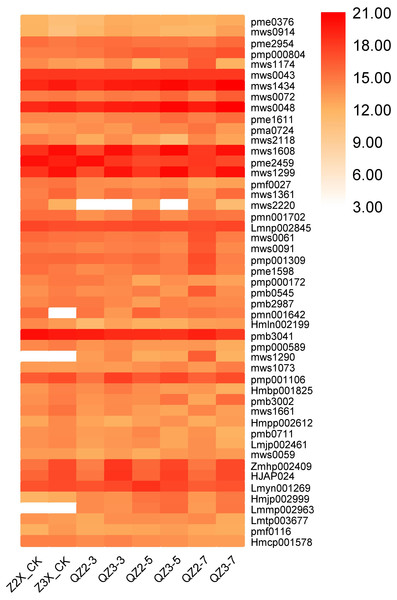
Terpenoids
Overall, in the current study, we quantified 32 terpenoids in response to waterlogging stress in diploid and triploid watermelon (Fig. 5). mab0387 (Cucurbitacin E-O-glucoside), Hmcp003852 (Elemol), pmn001706 (2-Hydroxyoleanolic acid), Lmzn006169 (Pomolic acid), pmn001705 (3,24-Dihydroxy-17,21-semiacetal-12(13)oleanolic fruit), pmn001429 (Cucurbitacin A) and mws1610 (Maslinic acid) showed a higher expression in both diploid and triploid watermelon. The content of these mentioned metabolites was more in triploid as compared to diploid watermelon.
Figure 5: Expression patterns of quantified terpenoids in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
Tannins
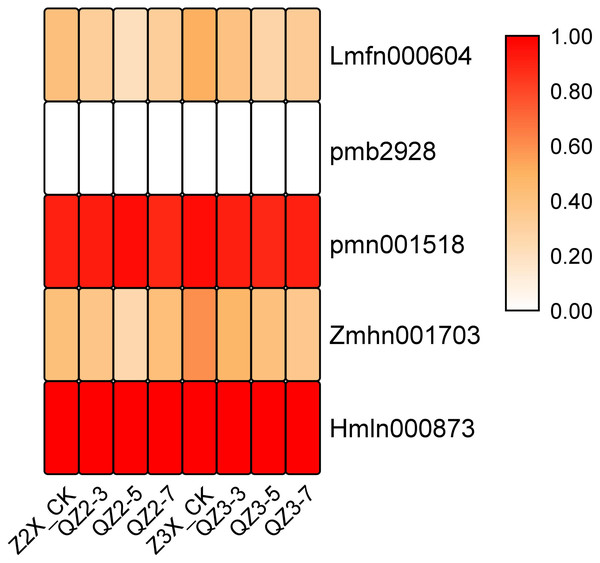
Five tannins have been quantified in the current experiment. Among the quantified tannins the expression of pmn001518 (1-O-Galloyl-D-glucose) and Hmln000873 (2-O-Galloyl-D-glucose*) were recorded highest at time points of sampling after waterlogging treatment (Fig. 6). The expression of Zmhn001703 (5-O-Galloyl-D-hamamelose) was recorded higher in triploid watermelon at all the time points after waterlogging treatment.
Figure 6: Expression patterns of quantified tannins in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
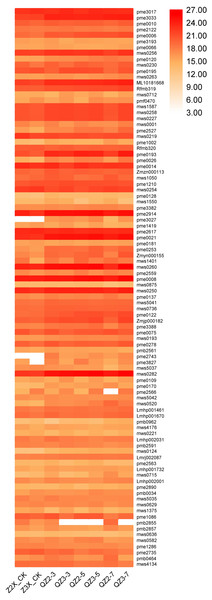
Phenolic acids
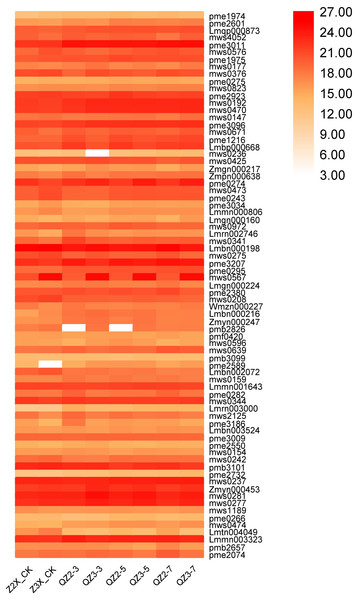
We have quantified 113 phenolic acids in response to waterlogging stress in diploid and triploid watermelon (Fig. 7). Among the phenolic acids HJAP104 (Benzaldehyde), mws0628 (4-Hydroxybenzaldehyde), mws0749 (4-Hydroxybenzoic acid), Lmbn001981 (2,5-Dihydroxybenzaldehyde), Hmgn001653 (Protocatechualdehyde), pme2828 (4-Nitrophenol), pmp001285 (Phthalic anhydride), mws0458 (Vanillin), pme0281 (Terephthalic acid), Lmlp012720 (Dibutyl phthalate), Lmxp011770 (Diisobutyl phthalate), Zmhn001926 (1-O-Salicyl-D-glucose*), Zmhn001358 (4-O-Glucosyl-4-hydroxybenzoic acid), pmb3142 (Salicylic acid-2-O-glucoside), Hmtn001302 (Glucosyloxybenzoic acid), Lmmp010562 (Diisooctyl Phthalate) and Zmhn002643 (4-O-(6′-O-Glucosyl-4″-hydroxybenzoyl)-4-hydroxybenzyl alcohol) have the higher expressions in response to waterlogging stress in both diploid and triploid watermelon at all the sampling stages. Zmhn002032 (4-O-(6′-O-Glucosylcaffeoylglucosyl)-4-hydroxybenzyl alcohol), pmn001707 (Quillaic acid), pmn001419 (1-O-[(E)-p-Cumaroyl]-D-glucose) contents were observed more in triploid watermelon as compared to diploid watermelon.
Figure 7: Expression patterns of quantified phenolic acids in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
Organic acids
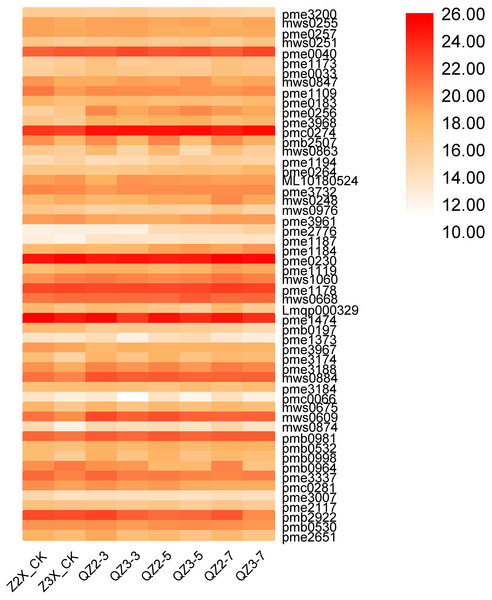
We have quantified 75 organic acids in response to waterlogging stress in diploid and triploid watermelon at 0, 3, 5, and 7 days after flooding stress (Fig. 8). We discovered that pme3011 (γ-Aminobutyric acid), Lmbn000198 (3-Dehydro-L-Threonic Acid), and pmb3101 (2-Isopropylmalic Acid), mws0237 (Azelaic acid), mws0277 (Quinic Acid) and Lmmn003323 (2-Hydroxyhexadecanoic acid*) had the higher contents at all the stages after flooding stress, whereas mws0192 (Succinic acid), mws0470 (Methylmalonic acid), pme3096 (Aminomalonic acid), and mws0567 (4-Guanidinobutyric acid) had the higher contents in triploid watermelon as compared to diploid watermelon at all the stages of sampling.
Figure 8: Expression patterns of quantified organic acids in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
Nucleotides and derivatives
Fifty-four nucleotides and derivatives have been quantified in the current research in both diploid and triploid watermelon at 0, 3, 5, and 7 days after flooding stress exposure to the plants (Fig. 9). We have found that pmc0274 (6-Methylmercaptopurine), pme0230 (Adenosine), pme1474 (5′-Deoxy-5′-(methylthioadenosine), and pme1178 (Guanosine) have the highest contents in both diploid and triploid watermelon at all the stages of sampling. The contents of pme0040 (Adenine), and mws0884 (Cyclic 3′, 5′-Adenylic acid) were found to be higher in triploid watermelon as compared to diploid watermelon.
Figure 9: Expression patterns of quantified nucleotides and derivatives in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
Lipids
In total we have quantified 147 lipids in the roots of diploid and triploid watermelon at 0, 3, 5 and 7 days after flooding stress (Fig. 10). The contents of mws0752 (Undecylic Acid), Zmjn003398 (2-Dodecenedioic acid), mws0119 (Myristic Acid), pmp001264 (Hexadecylsphingosine), mws0366 (γ-Linolenic Acid, mws0367 (α-Linolenic Acid), pmb1650 (Octadeca-11E,13E,15Z-trienoic acid, mws1491 (Linoleic acid), mws0396 (Elaidic Acid), mws1489 (Stearic Acid) were observed highest in both triploid and diploid wateremlon. The contents of Lmyn006011 (Gingerglycolipid B), Lmyn005812 (Gingerglycolipid A), pmp001276 (1-linolenoyl-rac-glycerol-diglucoside), Lmhp007840 (LysoPC 19:2), Lmhp007598 (LysoPC 19:2(2n isomer)*), mws0126 (LysoPC 18:0), Lmhp009590 (LysoPC 17:1), Lmhp008718 (LysoPC 17:2), pmb0883 (LysoPE 18:0), Lmhp009129 (LysoPC 15:0(2n isomer)*), pmp001270 (LysoPC 16:1),were found to be higher in triploid as compared to diploid.
Figure 10: Expression patterns of quantified lipids in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
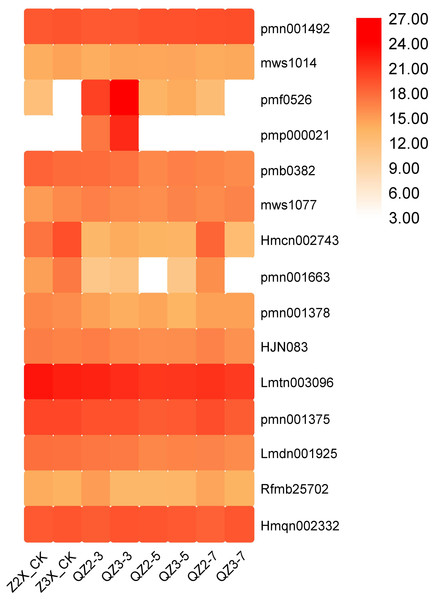
Lignans and coumarins
Fifteen lignans and coumarins have been quantified in the current study (Fig. 11). The contents of Lmtn003096 (secoisolariciresinol 4-O-glucoside), and Hmqn002332 (Pharsyringaresinol) were higher in both the triploid and diploid watermelon plants. We found out that the content of pmf0526 (Isoimperatorin) and pmp000021 (Isooxypeucedanine) was highest in the triploid on 3rd day of flooding stress.
Figure 11: Expression patterns of quantified lignans and coumarins in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
Flavonoids
In the current quantification, fourty-nine flavonoids have been quantified from the roots of diploid and triploid watermelon varieties (Fig. 12). Pme2954 (Quercetin), and pmp000804 (Isobavachalcone D) have the highest contents at all the stages, whereas the contents of mws1434 (Apigenin-6-C-glucoside (isovitexin)), mws0048 (apigenin-8-C-glucoside (vitexin)), Zmhp002409 (isoluteolin-6,8-di-C-glucoside), pmp001106 (vitexin- 2″-O-glucoside) and HJAP024 (kaempferol-6,8-di-C-glucoside) were higher in triploid as compared to diploid at all the stages of flooding stress.
Figure 12: Expression patterns of quantified flavonoids in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
Amino acids and derivatives
Among the amino acids and derivatives, we managed to quantify ninety-four of them from the roots of diploid and triploid watermelon plants in response to flooding stress (Fig. 13). Among the quantified amino acids and derivatives, we observed that 2-Aminoisobutyric acid, N,N-Dimethylglycine, L-methionine sulfoxide, and L-phenylalanine have higher expressions in both the diploid and triploid watermelon roots at all the sampling stages, whereas the contents of L-valine, cycloleucine, L-glutamine, L-glutamic acid, L-arginine, L-citrulline, L-tyrosine, and L-tryptophan were recorded higher in the triploid watermelon at all the stages of sampling as compared to diploid watermelon roots.
Figure 13: Expression patterns of quantified amino acids and derivatives in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
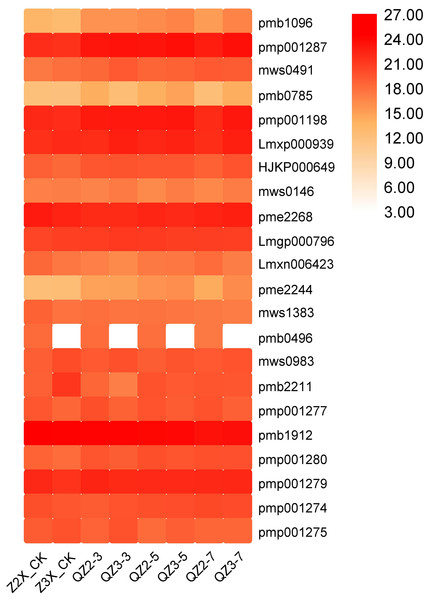
Alkaloids
In the current quantification, 22 alkaloids have been quantified from the roots of diploid and triploid watermelon varieties (Fig. 14). The contents of Pmp001287 (N-Benzylmethylene isomethylamine), pmp001198 (6-deoxyfagomine), pme2268 (trigonelline), and pmb1912 (10-formyltetrahydrofuran) were highest at all the stages, whereas the contents of HJKP000649 (N-benzylformamide), mws0491 (phenethylamine), pmb0785 (Isoquinoline), Lmxn006423 (2(3H)-benzothiazolone), pmp001274 (2-Hydroxy-5,8,11,14,17-icosapentaenoyloxy]propyl-2-(trimethylammonio)ethyl phosphate), and pmp001277 (2-hydroxypropyl palmitate) were higher in triploid watermelon as compared to diploid at all the stages of flooding stress.
Figure 14: Expression patterns of quantified alkaloids in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
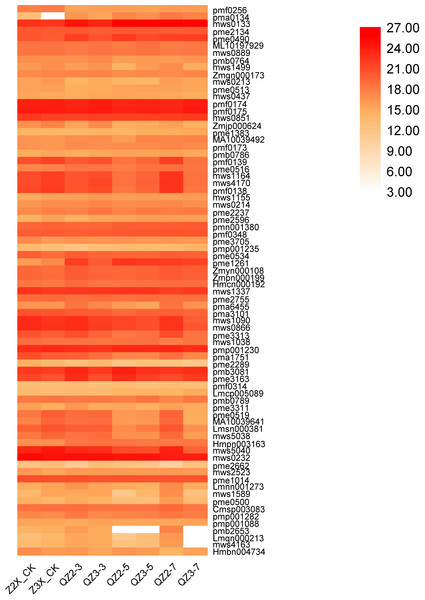
Others
In the current quantification, 77 metabolites were categorized in others in diploid and triploid watermelon varieties (Fig. 15). Contents of mws0133 (nicotinamide), pmf0174 (1-Decanol), pmf0176 (2-Decanol*) and mws0232 (riboflavin (vitamin B2)) were highest at all the stages, whereas the contents of Pme0490 (nicotinic acid (vitamin B3)), were higher in triploid watermelon as compared to diploid at all the stages of flooding stress.
Figure 15: Expression patterns of other quantified metabolites in diploid and triploid watermelon roots after 0, 3, 5, and 7 days of waterlogging treatment.
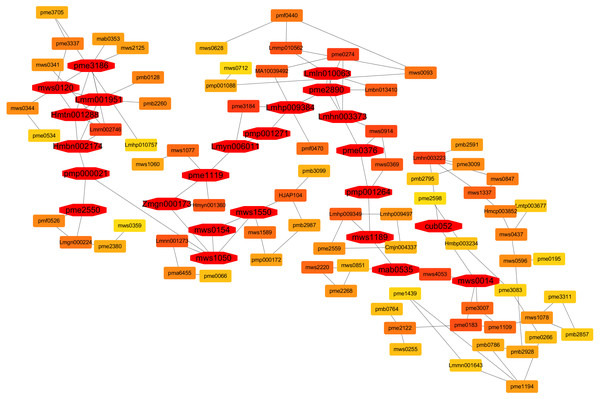
Co-expression network analysis of metabolites in diploid and triploid watermelon in response to flooding stress for the identification of hub metabolites
Contents of metabolites at each sampling time point i.e., 0. 3, 5, and 7 days after flooding stress were used for the co-expression network analysis. In the current research, twenty-four hub metabolites were identified that are involved in the flooding tolerance and their expressions in the triploid watermelon roots were higher as compared to the diploid watermelon roots (Fig. 16). Out of twenty-four, six were phenolic acids including Lmrn001951 ((S)-2-Hydroxy-3-(4-Hydroxyphenyl) propanoic acid), Lmln010063 (2,6-Di-tert-butylphenol) mws0014 (ferulic acid) Lmhn003373 (p-hydroxybenzoylmalic acid, Hmtn001288 (Methyl 2,4-dihydroxyphenylacetate), Hmbn002174 (4-hydroxyacetophenone). Four organic acids were also found to be involved in the flooding tolerance including, Pme3186 (DL-glyceraldehyde-3-phosphate), pme2550 (Cis-aconitic acid), mws0154 (shikimic acid), mws1189 (D-galacturonic acid). Among lipids, five were found to be the key lipids including, mws0120 (choline alfoscerate), Lmyn006011 (gingerglycolipid B), pmp001271 (1-Linoleoyl-sn-glycerol-diglucoside, Lmhp009384 (1-Linoleoylglycerol-2,3-di-O-glucoside), pmp001264 (hexadecylsphingosine). One metabolite belonging to Lignans and Coumarins “Pmp000021 (Isooxypeucedanine)”, was also included in the list of key metabolites. Three Amino acids and derivatives including mws1050 (O-Acetylserine) mws1550 (S-Allyl-L-cysteine), and pme2890 (L-homocystine), were also found to be involved in the flooding stress tolerance. Zmgn000173 (D-ribose), pme1119 (Inosine), pme0376 (naringenin (5,7, 4′-trihydroxyflavanone)) which belongs to others, nucleotides, derivatives, flavonoids respectively are also found as a result of metabolite correlation. Two terpenoids “mab0535 (11-carbonyl-2β, 20β-dihydroxycucurbitadienol), cub052 (cucurbitacin B O-rhamnoside)” were also selected as key metabolites in response to flooding stress.
Figure 16: A co-expression network analysis of all the identified metabolites at 0-, 3-, 5- and 7-days post flooding in diploid and triploid watermelon roots.
The red color represents the hub metabolites.Discussion
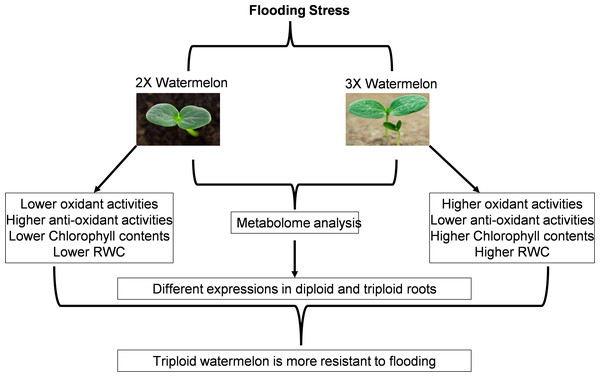
Flooding stress is among the major abiotic stresses agonized by plants. Limited aerobic respiration during flooding decreases energy metabolism thus restricting limits the growth and other developmental processes starting from the germination of the seed to vegetative and reproductive growth processes. Plants usually respond to flooding by maintaining their morphological stature, balancing energy metabolisms, hormonal levels, and other signaling processes (Fig. 17). We have tried to highlight the dynamic metabolic changes, morphological changes, damage caused by reactive oxygen species plus signaling cascades after exposing plants to flooding. Similar studies have been reported on cucumber and summer squash.
Figure 17: Schematic representation of current research work.
Root development in diploid and triploid watermelon in response to flooding
In the current research work, we aimed the identification of metabolites that are linked to flooding tolerance in the roots. Thus, we evaluated variations in response to flooding stress at the metabolite levels in diploid and triploid watermelon roots at different stages after applying flooding stress. Triploid watermelon plants grew well as compared to diploid watermelon plants at all the sampling stages. Obvious differences were found on the 5th day of flooding. Diploid watermelon plants start to wilt (Fig. 1). The root density of triploid watermelon plants was more as compared to diploid watermelon plants. In general, the growth of the shoots is depending on the nutrients being supplied from the roots. On the other hand, roots rely on photosynthetic products provided by shoots. So, the performance of shoots and roots is vital for the conservation of materials being produced under stress. In the current experiment, it was found that, under flooding stress, triploid watermelon plants tend to minimize the above-ground losses by efficiently maintaining the development of the roots thus upholding their ability to receive photosynthetic end-products for good development of their roots (Fig. 1). It has been reported that the accumulation of sucrose is crucial for maintaining many regulatory functions in the plants that are under stress (Casartelli et al., 2018; Gowda et al., 2011; Kojima et al., 2005; Lynch, 2007; Matsunami et al., 2012; Rodziewicz et al., 2014; Slama et al., 2015; Suzuki et al., 2012; Toyofuku, Matsunami & Ogawa, 2015; Uga et al., 2009; Yamakawa & Hakata, 2010). It has been reported that sucrose is involved in the development of lateral roots that occupy most of the root system (Lucob-Agustin et al., 2020; Ogawa, Kawashima & Yamauchi, 2005). Higher contents of sucrose in the roots under flooding stress in triploid watermelon plants advocate that sucrose serves as the source of energy and plays a critical role in the osmoregulation for maintaining the development of roots in triploid watermelon plants (Matsunami et al., 2020; Sanders & Arndt, 2012; Sonobe et al., 2010).
Biochemical changes in response to flooding stress
In the current research work, we measured the chlorophyll content, plasma membrane permeability, root activity, intracellular CO2 concentration, and relative water content along with the oxidants and antioxidant enzyme activities in response to flooding stress. We observed an overall decrease in the chlorophyll content, however high chlorophyll content was recorded in triploid watermelon as compared to diploid watermelon. Similar research has already proved a reduction in chlorophyll level in response to flooding (Kuai et al., 2014). A decrease in the CO2 was observed in the current research work in response to flooding stress, however triploid possesses a higher CO2 content as compared to diploid watermelons. This reduction in CO2 level has already been reported by Drew, He & Morgan (2000), Evans (2003), Hattori et al. (2009), Pedersen, Rich & Colmer (2009), Qi et al. (2019), Yamauchi et al. (2013) and Yamauchi et al. (2017). Plasma membrane permeability, relative water content, and root activities were also found to be decreased, triploid watermelon has comparatively higher values as compared to diploid watermelon in response to flooding but as reported previously in kiwi by Li et al. (2020).
Reactive oxygen species (ROS) are overproduced as a result of flooding. ROS are detoxified by various cellular enzymatic and non-enzymatic mechanisms (Jia et al., 2019; Paradiso et al., 2016). The altered activities of SOD, POD, CAT, H2O2, MDA, and LDH specified their roles of them in ROS scavenging. Lactate dehydrogenase also participates in the flooding stress response. In the current research, we observed higher LDH activities in triploid watermelon as compared to diploid watermelon. It was reported previously that the overexpression of LDH meaningfully improved the PDC activity and flooding resistance in Arabidopsis, while knockdown of LDH resulted in a differing phenotype (Dolferus et al., 2008).
Generally, under flooding stress, the activities of SOD, POD, and CAT showed a decrease as the stress was prolonged but the decrease is more prominent in the sensitive genotypes as compared to the resistant genotypes. We also observed that the activities of SOD, POD, and CAT were higher in the triploid watermelon as compared to the diploid watermelon plants. Previously maize genotypes were exposed to flooding and it was observed that resistant genotypes possess higher SOD, POD, and CAT activities (Li et al., 2018). Similarly, flooding-resistant Sorghum bicolor lines “JN01” and “JZ31” have higher activities of SOD and CAT (Zhang et al., 2019). Flooding stress was applied for 21 days to the resistant and susceptible barley genotypes and an increase in the activities of SOD, CAT, and POD was observed (Luan et al., 2018). In plants, the damage caused by the oxidation as a result of flooding can be minimized by the antioxidants (Zhang et al., 2007; Bin et al., 2010; Doupis et al., 2017; Hasanuzzaman et al., 2020). Previously it was reported that higher activities of CAT and SOD under flooding stress were observed and the activities were more prominent in the resistant lines as compared to the resistant lines (R Core Team, 2018). Li (2007) found higher activities of antioxidants in response to flooding in cucumber along with higher chlorophyll contents in the resistant variety.
Metabolic changes in response to flooding in watermelon
In the current research work, a total of 683 metabolites were identified with either high or low abundance between diploid and triploid watermelon roots under flooding stress. Different classes of metabolites detected here have different expressions at different developmental stages under flooding stress. Here we found out that among the sugars sucrose has a higher content in the roots of triploid watermelon plants as compared to the diploid watermelon plants and it has been proved already that sucrose is involved in the lateral root formation suggesting that sucrose in serving as an energy source to keep up the osmoregulation process (Matsunami et al., 2020; Ogawa, Kawashima & Yamauchi, 2005; Slama et al., 2015; Toyofuku, Matsunami & Ogawa, 2015).
We observed an increase in alanine and GABA suggesting the pyruvate to 2-oxoglutarate recycling under flooding conditions when there is limited oxygen and catabolism is stopped. Flooding inhibits the Krebs cycle as there is a limited supply of oxygen and leads to the increased production of NO causing the inhibition of aconitase (Gupta et al., 2012). An increase in the NO levels results in the aconitate to isocitrate conversion (Castro, Rodriguez & Radi, 1994; Lloyd et al., 1999; Tórtora et al., 2007).
We found that the increase in nitrogen-containing compounds was more abundant such as β-alanine, putrescine, arginine, and ornithine thus suggesting that there is an upsurge in the polyamine biosynthesis linked to the urea cycle. Polyamines are important players in inducing tolerance to the abiotic stress conditions including flooding (Cui et al., 2020; Reggiani, Hochkoeppler & Bertani, 1989; Verma & Mishra, 2005). Moreover, the aspartate cycle was also found to be involved in response to flooding stress due to the presence of polyamines and amino acids (Cui et al., 2019).
Conclusions
Currently, we tried to compare the expression dynamics of metabolites in response to flooding in triploid and diploid watermelon roots. We managed to examine the flooding stress effects on a diverse compound. Comparative analysis revealed larger differences between diploid and triploid watermelon thus paving new insights into the flooding stress response in watermelon. In the current study, we found that, in the roots of triploid watermelon, the contents of amino acids (L-valine, cycloleucine, L-323 glutamine, L-glutamic acid, L-arginine, L-citrulline, L-tyrosine, and L-tryptophan) increased and metabolites in TCA cycle intermediates (succinic acid, succinic anhydride, L-Malic acid, α-Ketoglutaric acid, citric acid) were maintained under flooding stress. Furthermore, the oxidants and antioxidant enzyme activities also suggest that triploid watermelon is more resistant to flooding as compared to diploid watermelon. In the future, deep knowledge about how these metabolites were regulated at transcriptional and post-transcriptional levels is required for enhancing the root functions under flooding stress. Resolving the underlying mechanisms for flooding tolerance and identification of key molecules serving as indicators for breeding criteria are necessary for developing flooding-resistant varieties.