Abstract
To investigate the effects of incident molecular temperature on organic-thin-film growth by vacuum evaporation, quantitative analysis of molecular temperature is required. In this study, we propose a method of determining molecular temperature based on the heat exchange between a platinum filament and molecular vapor. Molecular temperature is estimated from filament temperature, which remains unchanged even under molecular vapor supply. The results indicate that our method has sufficient sensitivity to evaluate the molecular temperature under the typical growth rate used for fabrication of functional organic thin films.
Export citation and abstract BibTeX RIS
Vacuum evaporation (sometimes called thermal deposition) is a widely used technique for the preparation of metals, semiconductors, and dielectric thin films in electronics, optics, and other industrial fields. This method has also been used for the fabrication of active layers in various organic semiconductor devices, such as light-emitting diodes,1,2) thin-film transistors,3,4) and photovoltaics,5) owing to its applicability to the preparation of highly ordered molecular thin films. Since the molecular order in organic thin films significantly affects the performance of organic devices, precise control of a thin-film structure is required, and much effort has been made to fabricate thin films exhibiting high molecular order by vacuum evaporation.
To control the film structure precisely, it is necessary to understand the elementary processes in organic-thin-film formation. Many experimental and theoretical studies have been performed over the last thirty years.6–14) Ueda and Ashida6) and Matsuzaki et al.7) reported that the orientation of a linear-chain molecule can be controlled by substrate temperature and growth rate. Regarding theoretical studies, Kubono and Akiyama proposed theoretical models based on a kinetic or dynamic nucleation theory to explain the correlation between experimental conditions and molecular orientation.11,12) The difference in molecular orientation as a function of experimental conditions can also be observed not only in simple linear-chain molecules, but also in planar π-conjugated molecules such as pentacene.15,16) Thus, substrate temperature and growth rate are recognized as key parameters for controlling the thin-film structure.
However, since in most cases we change the temperature of the evaporation source to control the growth rate, it is necessary to consider that changing the temperature of the evaporation source changes not only the vapor flux but also the vapor temperature of the atoms or molecules impinging on the substrate. In the vacuum evaporation of metal or inorganic semiconductors, the temperature of vapor atoms impinging on the substrate does not affect thin-film growth since the thermal accommodation is perfect while the adsorption of atoms on the substrate surface is taking place,17,18) that is, atoms are in thermal equilibrium with the substrate. In contrast, perfect thermal accommodation is not always achieved in the growth of organic thin films. Our previous experiment, in which crucible temperature was changed but the molecular flux was maintained at a constant value using a metal mesh to suppress the vapor flow, suggests that molecular temperature affects the adsorption property of organic molecules on the substrate.19) The growth mode of thin films deposited by the supersonic molecular beam deposition technique, which can control the kinetic energy of impinging molecules, was also affected by the kinetic energy of the molecules.20,21) Thus, investigating the effects of incident molecular temperature is important for understanding the elementary processes in organic-thin-film formation.
The effect of vapor temperature or kinetic energy has been investigated by analyzing the thermal accommodation coefficient using quadrupole mass spectroscopy (QMS) or a torsion microbalance (TMB).17,18) However, QMS and a TMB measure the molecular flux and momentum change in the injecting and re-evaporating molecules, respectively, and neither of them can detect vapor temperature directly. In addition, both of them require complicated and sensitive equipment. In this work, we propose a simple method of directly measuring the temperature of incident molecules on the basis of the thermal exchange between the molecules and a platinum filament.
In thermodynamics, temperature is defined as the partial derivative of internal energy with respect to entropy.22) Since the definition of entropy has a physical meaning only in the thermodynamic equilibrium state, the temperature of a molecular flow, which corresponds to the nonequilibrium steady state, cannot be defined thermodynamically. However, we can estimate an alternative value that has the same dimension as thermodynamic temperature using a temperature sensor maintained at a constant temperature.
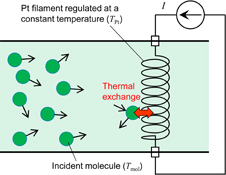
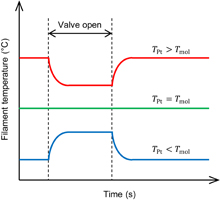
As shown in Fig. 1, a platinum filament is placed in the molecular flow and its temperature is maintained at a certain value by a constant current. When molecules impinge on the filament, the filament temperature TPt is varied by the thermal exchange between the molecules and the filament. If the impinging molecules take heat from (or provide heat to) the filament, TPt decreases (or increases) as shown in Fig. 2. At a certain initial filament temperature, thermal exchange does not occur and TPt will not vary. In this work, we consider this temperature as the temperature of the molecular flow. Here, note that the heat exchange efficiency between the filament and molecules does not influence the result. Since the heat exchange between most of the vapor molecules and a metal is imperfect,23) the filament temperature is different from the molecular temperature under the condition where the filament temperature is changed by supplying molecular vapor. However, the important point in this method is to find the filament temperature that remains unchanged following the supply of molecular vapor, that is, the temperature at which heat exchange does not occur. Thus, in our method, it is not necessary to take into account the heat exchange efficiency between the filament and molecules.
Fig. 1. Model of heat exchange between incident molecules and the platinum filament.
Download figure:
Standard image High-resolution imageFig. 2. Ideal time evolution curves of the temperature of the platinum filament.
Download figure:
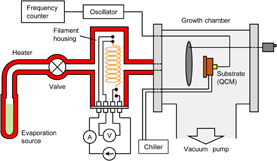
Standard image High-resolution imageFigure 3 shows the experimental setup. The system consists of four parts: the evaporation source, the inlet tubes, the filament housing, and the main chamber, and all the parts are made of stainless steel. The filament housing is a tube with a length of 100 mm and an inner diameter of 23 mm, and the molecules are supplied from the evaporation source into the main chamber through this housing. The platinum filament is placed in the filament housing and the resistance of the filament is measured to estimate the filament temperature. A helical filament is adopted to obtain an enlarged surface area. In the initial state, the filament temperature was maintained at a constant value by flowing a constant current from the laboratory-built current source with a resolution of 0.01 mA, and then molecular vapor is supplied by opening the valve. The time evolution of filament temperature is monitored every second as the change in the measured resistance of the filament.
Fig. 3. Schematic illustration of experimental system.
Download figure:
Standard image High-resolution imageAs the source material, stearic acid (C17H35COOH) was thermally evaporated at a system pressure of 5.0 × 10−4 Pa. The melting and sublimation points at this pressure are ca. 69 and 45 °C, respectively. The temperature of the evaporation source and the inlet tubes was maintained at 130 °C so that their surfaces did not adsorb stearic acid molecules, and the temperature of the filament housing was maintained at 60 °C. Under this condition, the molecular flux monitored using the quartz crystal microbalance placed at the main chamber was 0.015 × 10−7 mol/m2 s. This value is equal to a growth rate of ca. 0.02 Å/s if all the molecules were adsorbed on the substrate.
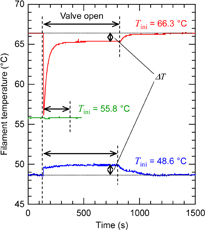
Figure 4 shows the typical time evolution curves of the filament temperature at various initial temperatures. When the initial temperature is 66.3 °C, the temperature of the platinum filament immediately responds upon the opening of the valve and reaches a constant value at 65.3 °C, which is lower than the initial temperature. After closing the valve, the filament temperature returns to the initial value. Thus, it is suggested that the molecular temperature is lower than 66.3 °C. When the initial temperature is 48.6 °C, the opposite behavior is observed, that is, the temperature of the Pt filament is higher than 48.6 °C. Finally, when the initial temperature is 55.8 °C, the filament temperature remains almost unchanged. Thus, the molecular temperature is expected to be approximately 55.8 °C. It is worth noting that this method has enough sensitivity to evaluate molecular temperature at low deposition rates as described above. We would also like to mention that a negative spike appears in the curve immediately after opening the valve at an initial temperature of 66.3 °C. This spike is commonly observed only in cases where the initial temperature is higher than the molecular temperature. We considered that the negative spike is probably due to the sudden pressure change caused by the opening of the valve; however, the detailed mechanism is still unclear.
Fig. 4. Time evolution curves of the temperature of the Pt filament at initial temperatures of 48.8, 55.8, and 66.4 °C.
Download figure:
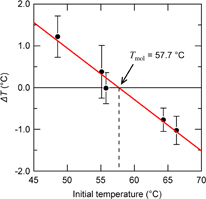
Standard image High-resolution imageThe sensitivity of the measurement within a temperature range near the molecular temperature is quite low because the resistance of the Pt filament is almost the same before and after opening the valve. For more accurate determination of molecular temperature, we plotted the temperature variations from the initial temperature (ΔT) for various initial temperatures. As shown in Fig. 5, ΔT shows a linear correlation with the initial temperature. This linear correlation means that the platinum filament exchanges heat only with the molecules in this temperature range. Fitting the plot with a straight line, we can more accurately determine the molecular temperature to be 57.7 °C from the x-intercept of the fitted line. Although the melting point of stearic acid is 69 °C, it is not surprising that the estimated molecular temperature is lower than the melting point. Indeed, the bulk of stearic acid exhibits the solid phase at 55.7 °C; nevertheless, our previous study suggests that the vapor molecules cannot be adsorbed on a substrate surface even at temperatures below the melting point.24) Hence, it is not necessary to consider the effects of the heat of fusion and sublimation.
Fig. 5. Temperature change from the initial filament temperature (ΔT) as a function of initial temperature. Plots and error bars indicate the average value and standard deviation of the ΔT at each initial temperature, respectively.
Download figure:
Standard image High-resolution imageNote that the estimated molecular temperature was much lower than the temperature of the evaporation source (130 °C), and was almost the same as the temperature of the filament housing (60 °C). To confirm that the housing temperature affects the molecular temperature, another set of experiments was performed at a filament housing temperature of 90 °C. The determined molecular temperature was 85.6 °C, which is almost the same as the temperature of the filament housing. These results indicate that the molecular temperature is dominated by the housing temperature. This is probably due to the size of the experimental system. Since we used thin tubes with a diameter of 1/4 in. to connect the filament housing located between the evaporation source and the main chamber because of space limitation, the molecular vapor is expected to stagnate in the filament housing and be close to thermal equilibrium before injection into the main chamber. From the above, the estimated temperature in this work is the temperature of low-pressure thermal-equilibrium molecular vapor rather than that of the molecular flow. To investigate the effect of incident molecular temperature on organic-thin-film growth more directly, the platinum filament should be placed close to the substrate in the main chamber, and we are currently updating the experimental system.
In summary, we have proposed a method of measuring incident molecular temperature during vacuum evaporation based on the heat exchange between molecular vapor and the platinum filament. It has been demonstrated that the temperature of stearic acid vapor can be quantitatively measured with sufficient sensitivity by applying this method. The determined molecular temperature was almost the same as the temperature of the filament housing rather than that of the evaporation source. This finding implies that the heat exchange between the molecular vapor and the filament housing exerts the most dominant effect on the temperature of the incident molecules in our experimental system. Although further improvement is still required, we expect our method to be a useful tool for investigating the effect of incident molecular temperature on organic-thin-film growth.
Acknowledgments
This work was partially supported by JSPS KAKENHI Grant Number 17K14106. The authors would like to thank Professor Masakazu Nakamura (NAIST) and Dr. Ichiro Hirosawa (JASRI) for helpful discussion. We would also like to thank Professor Daniel Moraru (Shizuoka University) for his support in the preparation of the manuscript.