Tuberculosis Vaccine: Dual delivery of antigens shows promise
The BCG vaccine was first used in 1921 and, remarkably, over 100 years later it is the only licensed vaccine for use against tuberculosis. While the BCG vaccine provides protection against tuberculosis that has spread beyond the lungs in young children, its efficacy against lung infections in adults – the main form of the disease – is variable (Trunz et al., 2006). Tuberculosis is caused by a bacterium called Mycobacterium tuberculosis (M. tb), and one reason for the lack of more effective vaccines is that we still do not know which M. tb antigens are able to induce protective immunity (Guinn and Rubin, 2017).
Almost 20 vaccine candidates are currently being evaluated in clinical trials. Most of these aim to induce a response from immune cells called type 1 helper T cells by exposing them to protein antigens from M. tb, although other approaches are also being explored. Now, in eLife, Evan Scott and Chyung-Ru Wang from Northwestern University and colleagues – including Eva Morgun as first author – report a new approach that involves using protein antigens and long-chain fatty acids called mycolic acids, found in the M. tb cell envelope, to induce an immune response (Morgun et al., 2023).
Previous studies have shown that the immune system generates a protective response when exposed to mycolic acids: this response is mediated by a subset of “unconventional” T cells, but the details of the response are not fully understood (Shang et al., 2018; Zhao et al., 2015). Moreover, there have been very few attempts to explore the use of mycolic acids as alternative antigens for a tuberculosis vaccine, partly because most vaccine delivery systems are designed for hydrophilic cargoes (such as proteins), whereas mycolic acids are hydrophobic. Morgun et al. have overcome this problem by developing a system that can deliver hydrophobic and hydrophilic cargoes at the same time.
Building upon evidence that vaccine formulations including M. tb lipids encapsulated in liposomes – hollow spherical structures made of lipid bilayers – provide protection (Dascher et al., 2003; Larrouy-Maumus et al., 2017), Morgun et al. developed structures called bicontinuous nanospheres. These self-assembled polymer-based structures can carry both hydrophobic and hydrophilic components because they possess an organized internal cubic structure including hydrophobic bilayers and hydrophilic aqueous channels (Figure 1). Furthermore, the nanospheres retain antigens more efficiently than other lipid nanostructures, and only release their contents when they reach the target compartment within antigen-presenting cells. These features increase the persistence of the antigens within cells and stimulate the immune system for longer periods of time, which leads to a more robust immune response (Correia-Pinto et al., 2013).
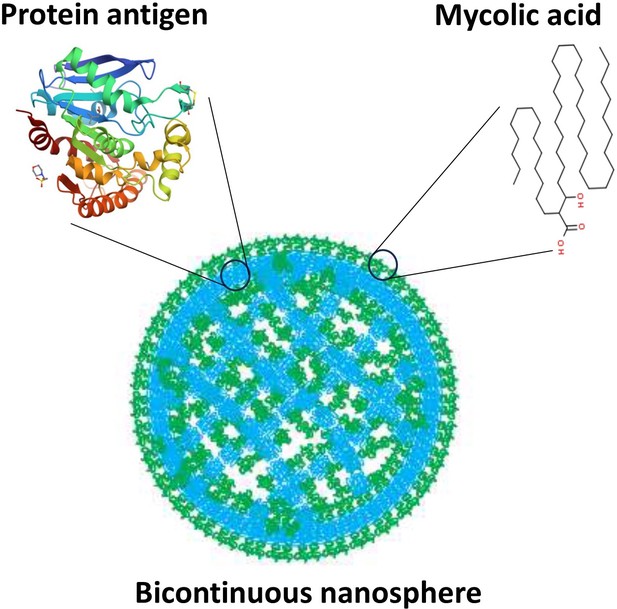
Bicontinuous nanospheres can be loaded with both protein and lipid antigens.
Structure of a bicontinuous nanosphere. Hydrophilic (blue) regions contain protein antigens and hydrophobic (green) regions contain mycolic acids, represented by tentative structures. Figure based on image from Allen et al., 2018.
Morgun et al. used a mouse model that allowed them to observe the immune response to dual vaccination with mycolic acids and proteins. This showed that T cells responded to both antigens: however, only mycolic acid remained detectable inside immune cells after vaccination. It remains unclear if this differential antigen persistence is due to the nanosphere delivery system, the protein itself, or a distinct mechanism of antigen capture and maintenance by immune cells.
It is worth noting that the persistence of mycolic acids likely occurs during in vivo infection and, therefore, the dual vaccination strategy may mimic a previously overlooked feature of the infection process. Supporting this notion, Morgun et al. found that vaccination with an attenuated M. tb strain resulted in a persistence of mycolic acids similar to that observed with the dual approach. These results suggest that vaccines using live attenuated strains of M. tb likely provide protection via mycolic acid-specific T cells. This raises the question of whether the persistence of lipid antigens is a key aspect of the protective immune response: this is something that needs to be considered when developing tuberculosis vaccines in the future.
Although definitive proof of protective immunity induced by dual-loaded bicontinuous nanospheres remains elusive, this approach allowing combinations of antigens – both hydrophobic and hydrophilic – can be used as an alternative strategy to live attenuated vaccines. Moreover, the ability to test combinations of alternative antigens will aid the development of vaccines for tuberculosis more generally. Future work with this system could also investigate the role of lipid persistence in other overlooked aspects of tuberculosis immunity, such as antibody responses, which might have an important contribution to protective immunity.
References
-
Vaccine delivery carriers: insights and future perspectivesInternational Journal of Pharmaceutics 440:27–38.https://doi.org/10.1016/j.ijpharm.2012.04.047
Article and author information
Author details
Publication history
- Version of Record published: July 21, 2023 (version 1)
Copyright
© 2023, Luque-García and Prados-Rosales
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 540
- views
-
- 52
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Chromosomes and Gene Expression
- Immunology and Inflammation
Ikaros is a transcriptional factor required for conventional T cell development, differentiation, and anergy. While the related factors Helios and Eos have defined roles in regulatory T cells (Treg), a role for Ikaros has not been established. To determine the function of Ikaros in the Treg lineage, we generated mice with Treg-specific deletion of the Ikaros gene (Ikzf1). We find that Ikaros cooperates with Foxp3 to establish a major portion of the Treg epigenome and transcriptome. Ikaros-deficient Treg exhibit Th1-like gene expression with abnormal production of IL-2, IFNg, TNFa, and factors involved in Wnt and Notch signaling. While Ikzf1-Treg-cko mice do not develop spontaneous autoimmunity, Ikaros-deficient Treg are unable to control conventional T cell-mediated immune pathology in response to TCR and inflammatory stimuli in models of IBD and organ transplantation. These studies establish Ikaros as a core factor required in Treg for tolerance and the control of inflammatory immune responses.
-
- Evolutionary Biology
- Immunology and Inflammation
CD4+ T cell activation is driven by five-module receptor complexes. The T cell receptor (TCR) is the receptor module that binds composite surfaces of peptide antigens embedded within MHCII molecules (pMHCII). It associates with three signaling modules (CD3γε, CD3δε, and CD3ζζ) to form TCR-CD3 complexes. CD4 is the coreceptor module. It reciprocally associates with TCR-CD3-pMHCII assemblies on the outside of a CD4+ T cells and with the Src kinase, LCK, on the inside. Previously, we reported that the CD4 transmembrane GGXXG and cytoplasmic juxtamembrane (C/F)CV+C motifs found in eutherian (placental mammal) CD4 have constituent residues that evolved under purifying selection (Lee et al., 2022). Expressing mutants of these motifs together in T cell hybridomas increased CD4-LCK association but reduced CD3ζ, ZAP70, and PLCγ1 phosphorylation levels, as well as IL-2 production, in response to agonist pMHCII. Because these mutants preferentially localized CD4-LCK pairs to non-raft membrane fractions, one explanation for our results was that they impaired proximal signaling by sequestering LCK away from TCR-CD3. An alternative hypothesis is that the mutations directly impacted signaling because the motifs normally play an LCK-independent role in signaling. The goal of this study was to discriminate between these possibilities. Using T cell hybridomas, our results indicate that: intracellular CD4-LCK interactions are not necessary for pMHCII-specific signal initiation; the GGXXG and (C/F)CV+C motifs are key determinants of CD4-mediated pMHCII-specific signal amplification; the GGXXG and (C/F)CV+C motifs exert their functions independently of direct CD4-LCK association. These data provide a mechanistic explanation for why residues within these motifs are under purifying selection in jawed vertebrates. The results are also important to consider for biomimetic engineering of synthetic receptors.






