Neuroanatomy of a hydrothermal vent shrimp provides insights into the evolution of crustacean integrative brain centers
Figures
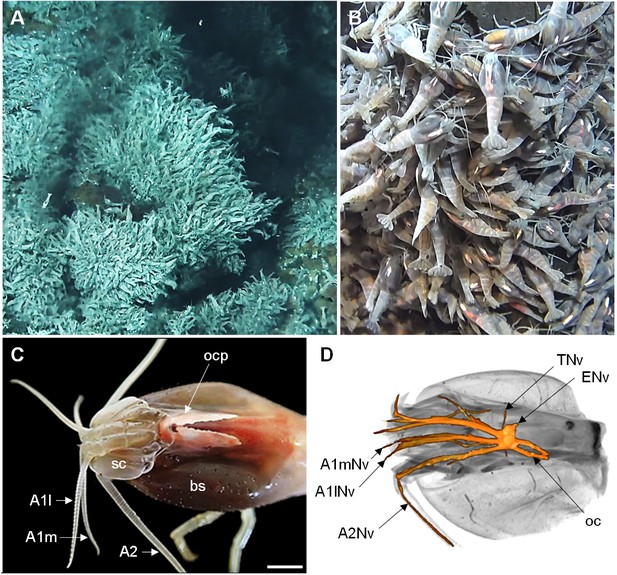
The Alvinocaridid vent shrimp Rimicaris exoculata.
(A,B) Swarms of thousands of R. exoculata individuals are crowded along the walls of black smoker hydrothermal vents at the TAG vent site (3600 m depth), Mid-Atlantic Ridge (IFREMER/Nautile6000, BICOSE 2018 cruise). (C) Dorsal view of the cephalothorax of R. exoculata, showing voluminous gill chambers covered by the branchiostegites, dorsal eyes (i.e. ocular plate) with two elongated retinae fused in the anterior region, and sensory appendages (antennae 1 and 2). Scale bar = 5 mm. (D) Black-white inverted image from an X-ray micro-CT scan showing a dorsal overview of the R. exoculata cephalothorax, with 3D reconstruction of the brain and associated nerves. Abbreviations: see text and appendix 1.
© 2018 IFREMER/Nautile6000, BICOSE 2018 cruise. Panels A and B reproduce images taken with the Nautile6000 submersible during the BICOSE 2018 cruise (https://doi.org/10.17600/18000004).
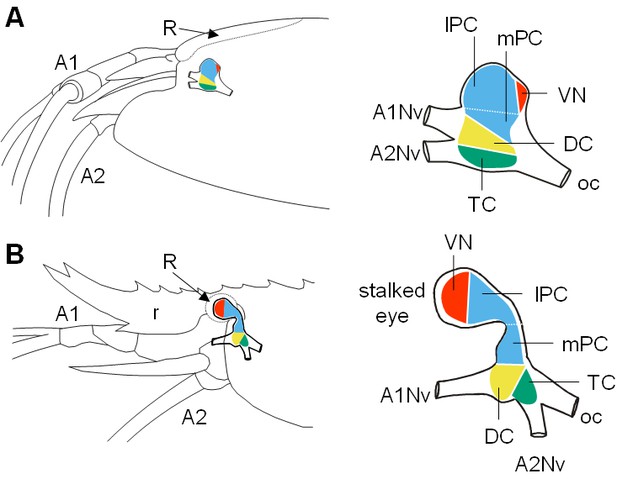
Comparative brain overview in Caridean vent and shallow-water species.
Lateral sketches of the brains of the vent shrimp Rimicaris exoculata (A) and the closely-related shallow-water shrimp Palaemon elegans (B), showing the brain position within the cephalothorax, the position of the main nerves and the subdivision of the brain into three neuromeres called proto-, deuto- and tritocerebrum, plus the visual neuropils. In contrast to P. elegans, R. exoculata does not possess eyestalks and the visual neuropils are fused to the median brain, in a dorsoposterior position behind the lateral protocerebrum. Abbreviations: see text and appendix 1.
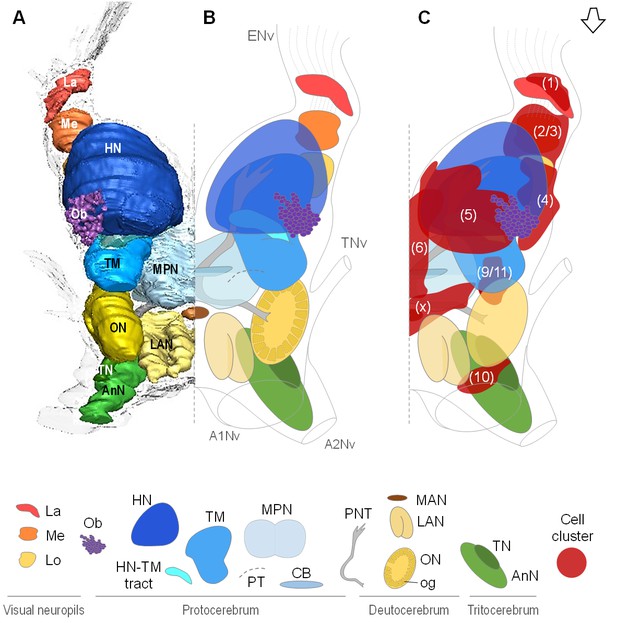
Overall organization of the brain of R.exoculata.
3D reconstruction (A) and schematic representations (B,C) of the brain and neuropils of R. exoculata viewed from a dorsal, slightly anterior direction. The open white arrow points towards anterior of the body axis. In (C), the clusters of cell somata associated with the neuropils are shown. The 3D reconstruction is based on an image stack obtained by serial sectioning of paraffin-embedded material; the sections were aligned manually by shifting and rotating each section using Amira. Abbreviations: see text and appendix 1.
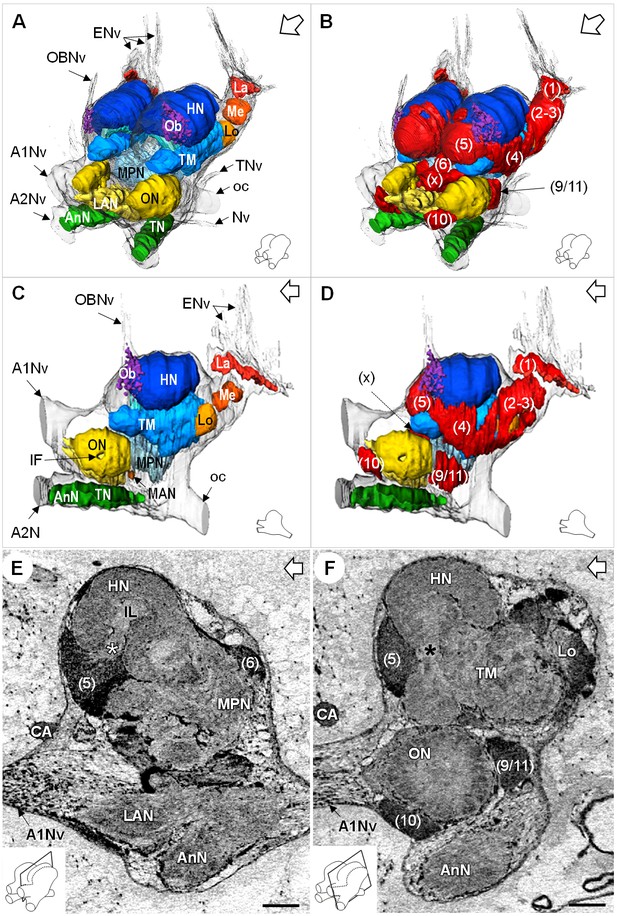
Additional views of the brain morphology in R.exoculata.
(A–D) 3D reconstruction of the brain of R. exoculata in anterior-left (A,B) and left (C, D) views, based on an image stack obtained by serial sectioning of paraffin-embedded material. (B and D) include the cell clusters. The brain orientation is sketched in the bottom right corners. (E,F) Lateral sections of the brain of R. exoculata from micro-CT scans (black-white inverted images). The section’s positions are depicted in the bottom left corners. White asterisk in (E) indicates the entrance of axons from the cell cluster (5) into the hemiellipsoid body. Black asterisk in (F) indicates the tract connecting the anterior region of the terminal medulla to the hemiellipsoid body. The open white arrows point towards anterior of the body axis. Scale bars = 100 µm. Abbreviations: see text and appendix 1.
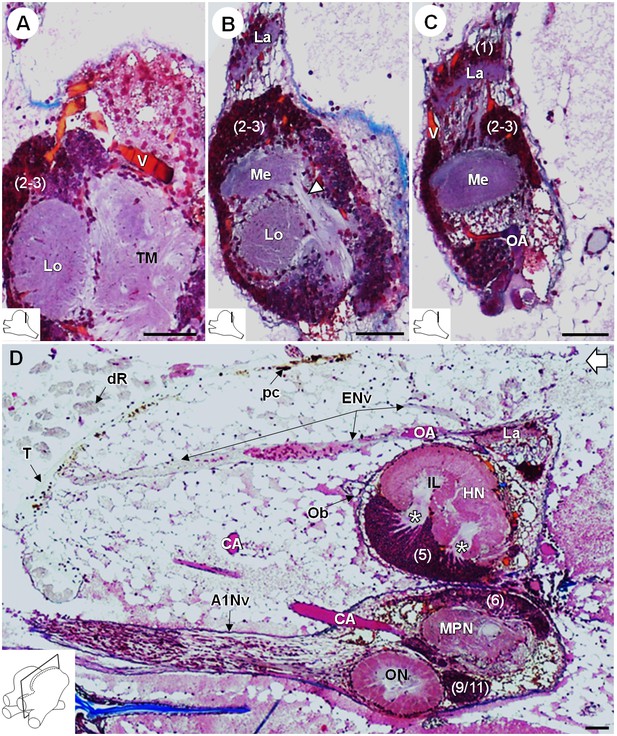
Lateral protocerebrum: the visual neuropils.
(A–C) Frontal histological sections in the posterior region of the brain, from anterior to posterior, showing the visual neuropils, associated cell clusters, and part of the vascular system. The white arrow head in (B) shows the fiber tract connecting the medulla to the terminal medulla. (D) Sagittal histological section of the brain, showing the eye nerve fibers projecting from the anterodorsal retina to the lamina. White asterisks indicate the entrance of axons from the cell cluster (5) into the hemiellipsoid body intermediate layer. The open white arrow points towards anterior of the body axis. The section’s positions are sketched in the bottom left corners. Scale bars = 100 µm. Abbreviations: see text and appendix 1.
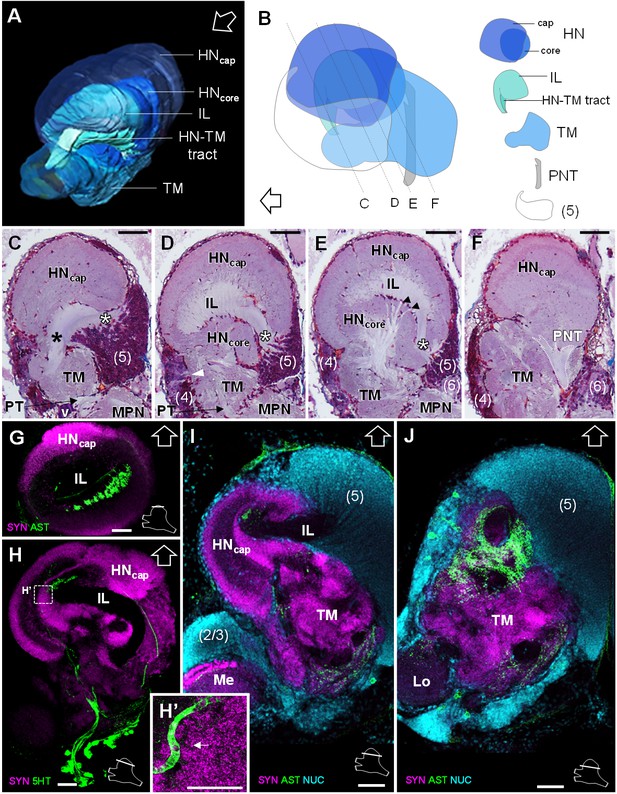
Lateral protocerebrum: the hemiellipsoid body and the terminal medulla.
(A) 3D reconstruction of the lateral protocerebrum (right hemisphere), viewed from an anterior-left perspective, based on an image stack obtained by X-ray micro-CT scan. A conspicuous arcuate tract connects the anterior region of the terminal medulla to the cap region of the hemiellipsoid body (see also in C). (B) Schematic representation of the lateral protocerebrum (right hemisphere), viewed from the left. Dotted lines indicate the section’s position in C-F. (C-F) Frontal histological sections of the lateral protocerebrum, from anterior to posterior. The hemiellipsoid body and the terminal medulla receive axons from the cell somata in the cell cluster (5) (white asterisks, (C–E) and (4) (white arrowhead, (D). An arcuate tract connects the terminal medulla to the cap region of the hemiellipsoid body in the anterior region (black asterisk, (C). The terminal medulla also connects to the hemiellipsoid body in the middle region, via arborizing fibers (black arrowheads, (E). The projection neuron tract enters the hemiellipsoid body in the posterior region (F). (G–J’) Horizontal sections of the lateral protocerebrum, from dorsal to ventral, double or triple-labeled for synapsin immunoreactivity (SYN, magenta), allatostatin-like immunoreactivity (AST) or serotonin immunoreactivity (5HT) (both showed in green), and a nuclear marker (NUC, cyan). The inset (H’) shows an enlargement of the hemiellipsoid body neuropil cap region, with microglomeruli (white arrow). Each section’s position is sketched in the bottom right corners. Black and white open arrows point towards anterior of the body axis. Scale bars = 100 µm (except in H’, scale bar = 50 µm). Abbreviations: see text and appendix 1.
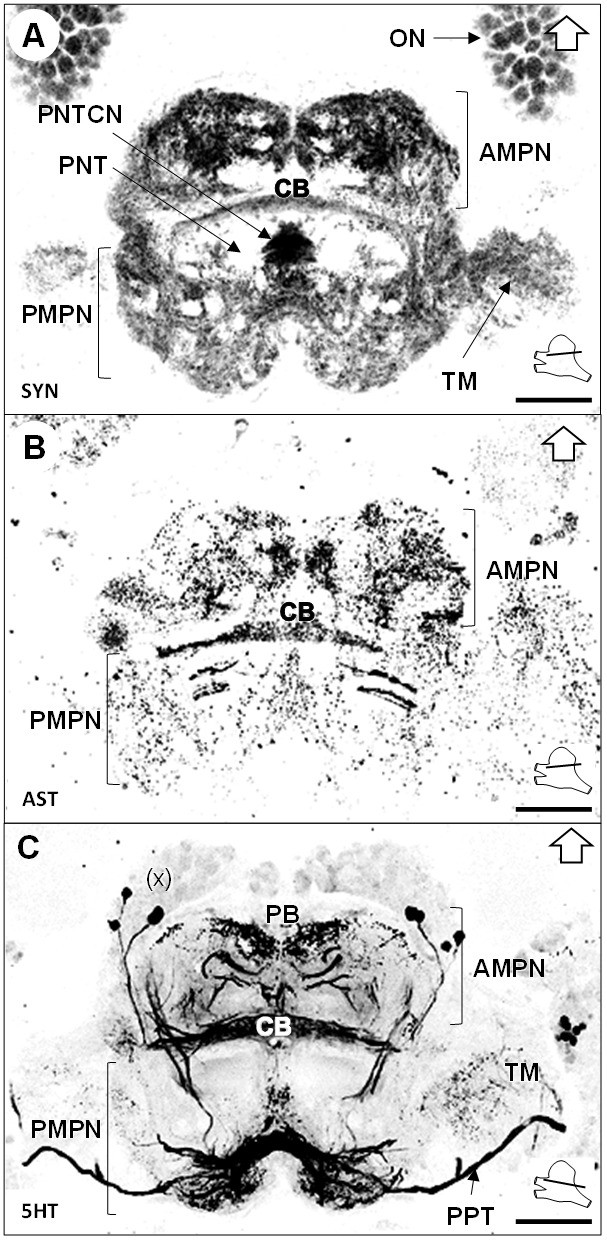
Median protocerebrum.
(A–C) Black-white inverted images of horizontal sections of the median protocerebrum labeled for synapsin immunoreactivity (A), allatostatin-like immunoreactivity (B) or serotonin immunoreactivity (C). The section’s positions are sketched in the bottom right corners. Black arrows point towards anterior of the body axis. Scale bars = 100 µm. Abbreviations: see text and appendix 1.
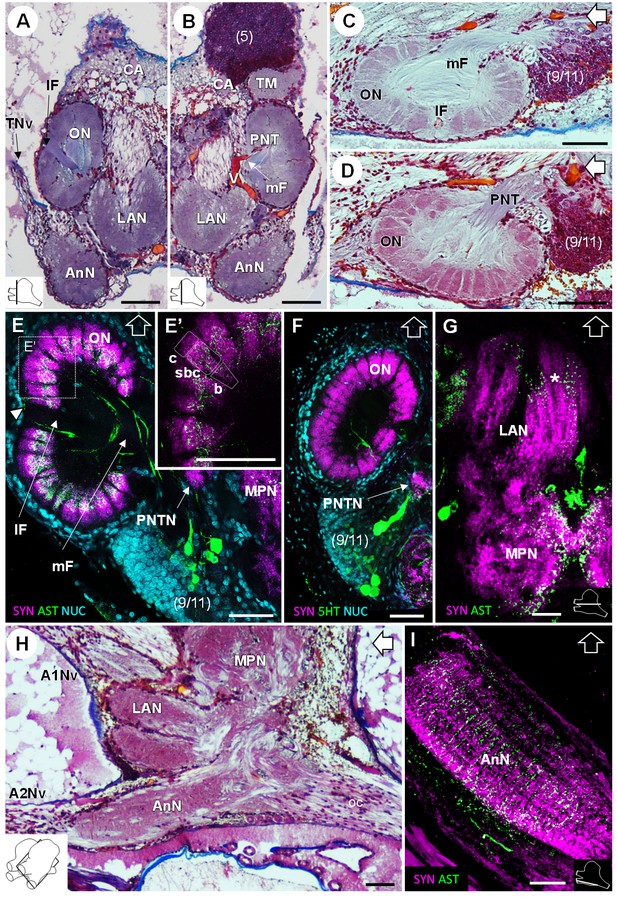
Deutocerebrum and tritocerebrum.
(A,B) Overview of the deutocerebrum and tritocerebrum (frontal histological sections). (A) is anterior to B. (C,D) Sagittal histological sections of the olfactory neuropils. (E,F) Horizontal sections of the olfactory neuropil, triple-labeled for synapsin immunoreactivity (SYN, magenta), allatostatin-like immunoreactivity (AST, (E,E’) or serotonin immunoreactivity (5HT, (F) (green), and a nuclear marker (NUC, cyan). (G) Horizontal section of the transversely stratified (white asterisk) lateral antenna one neuropil, double-labeled for synapsin immunoreactivity (SYN, magenta) and allatostatin-like immunoreactivity (AST, green). (H) Sagittal histological section of the tritocerebrum, and part of the deutocerebrum and median protocerebrum. (I) Horizontal section of the transversely stratified antenna two neuropil, double-labeled for synapsin immunoreactivity (SYN, magenta) and allatostatin-like immunoreactivity (AST, green). The section’s positions are sketched in the bottom corners. Black and white open arrows point towards anterior of the body axis. Scale bars = 100 µm. Abbreviations: see text and appendix 1.
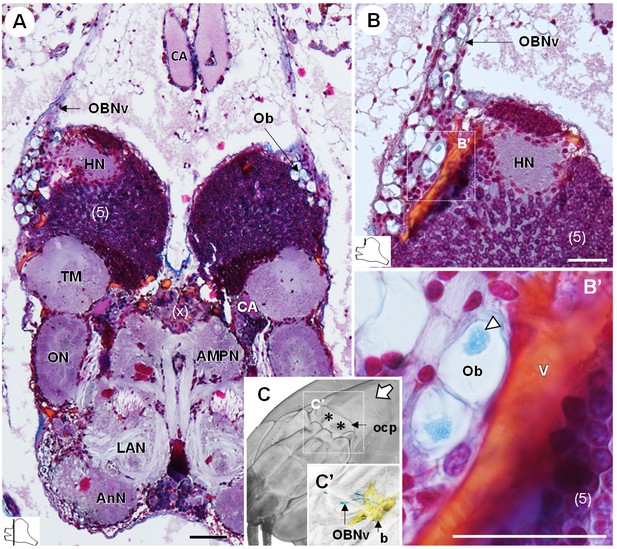
The organ of Bellonci.
(A–B’) Frontal histological sections of the anterior region of the brain, showing conspicuous onion body-structures from which a nerve tract emanates (A,B), and which are seemingly closely associated to the cerebral vascular system (B’) and contain elements of granular appearance (B’), white arrowhead). The section’s positions are sketched in the bottom left corners. (C) Anterodorsolateral overview of the cephalothorax from micro-CT scan. Asterisks indicate the position where the organ of Bellonci nerve connects to the cuticle beneath the anterior region of the ocular plate. (C’) shows a 3D reconstruction of the brain and the organ of Bellonci nerve in this region. White arrow points towards anterior of the body axis. Scale bars = 100 µm. Abbreviations: see text and appendix 1.
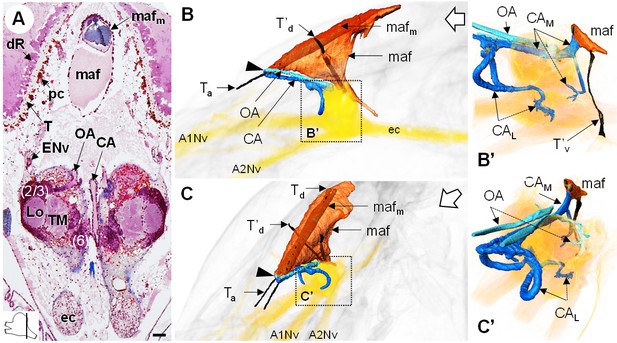
The myoarterial formation and cerebral vascular system.
(A) Frontal histological section of the myoarterial formation located between the bilateral retina and above the visual neuropils. The section’s position is sketched in the bottom left corner. Scale bar = 100 µm. (B,C) 3D reconstruction of the myoarterial formation (orange), part of the cerebral vascular system (blue and cyan) and the brain (yellowish), from lateral (B) and anterolateral (C) views, in the cephalothorax. (B’ and C’) show higher magnifications of the cerebral vascular system. Dotted arrows indicate structures inside the brain. Open white arrows point towards anterior of the body axis. Abbreviations: see text and appendix 1.
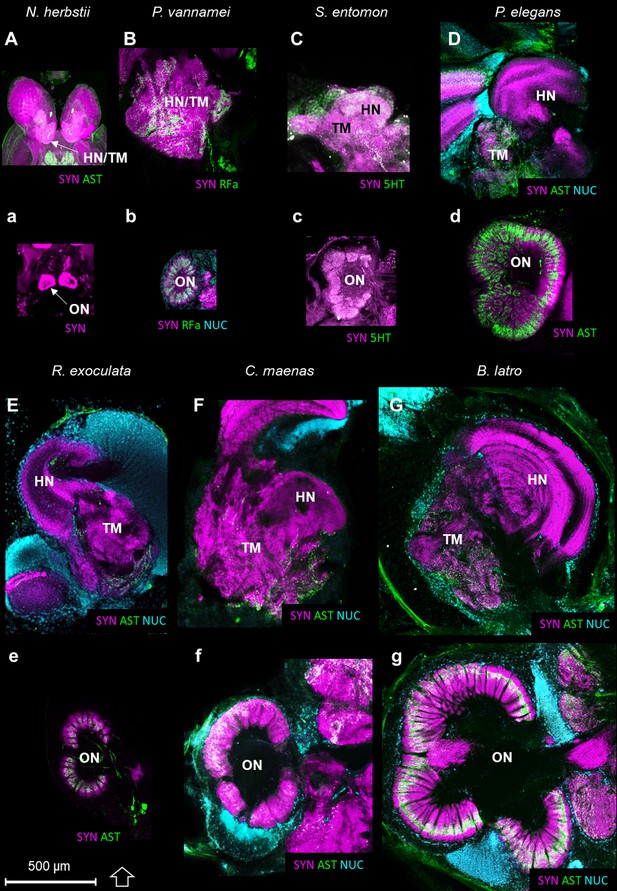
Comparison of the higher integrative centers and olfactory neuropils in several representatives of Malacostraca displayed at the same scale.
Sections of the higher integrative centers (i.e. hemiellipsoid body and terminal medulla) (A–G) and horizontal sections of the olfactory neuropil (a–g) labeled with different sets of antibodies (see below), in several malacostracan species: Nebalia herbstii (Aa), Leptostraca, from Kenning and Harzsch (2013); Kenning et al. (2013), Penaeus vannamei (Bb), Dendrobranchiata, from Meth et al. (2017), Saduria entomon (Cc), Isopoda, from Kenning and Harzsch (2013), Palaemon elegans (Dd) and Rimicaris exoculata (Ee) (Caridea, this study), Carcinus maenas (Ff), Brachyura, from Krieger et al. (2012b) and Birgus latro (Gg), Anomala, from Krieger et al. (2010). Markers: a, SYNir; B, SYNir +RFair; b, SYNir +RFair + NUC; Cc), SYNir +5 HTir; A,d,e, SYNir +ASTir; D,E,F-g, SYNir +ASTir + NUC. ASTir, allatostatin-like immunoreactivity (green); NUC, nuclear marker (cyan); RFair, RFamide-like immunoreactivity (green); SYNir, synapsin immunoreactivity (magenta); 5HTir, serotonin immunoreactivity (green). Abbreviations: see text and appendix 1.
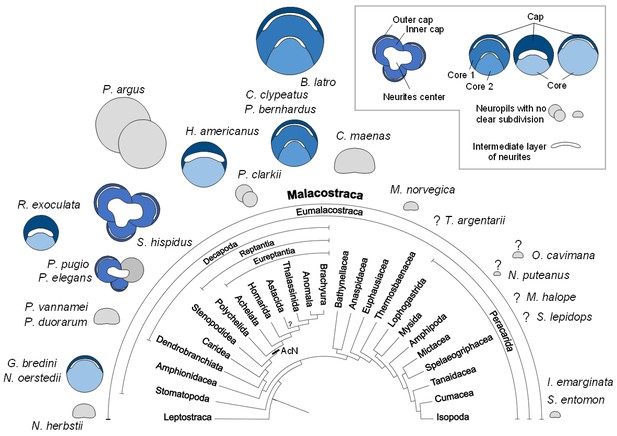
Structure of the hemiellipsoid body in several representatives of Malacostraca.
The sketches of the hemiellipsoid body structure are displayed in relative size and include representatives of Leptostraca (Nebalia herbstii, Kenning et al., 2013), Stomatopoda (Neogonodactylus oerstedii, Wolff et al., 2017; Gonodactylus bredini, Sullivan and Beltz, 2004), Dendrobranchiata (Penaeus vannamei, Meth et al., 2017; Penaeus duorarum, Sullivan and Beltz, 2004), Caridea (Rimicaris exoculata and Palaemon elegans, this study; Palaemonetes pugio, Sullivan and Beltz, 2004), Stenopodidea (Stenopus hispidus, Sullivan and Beltz, 2004 and Krieger et al. unpublished), Achelata (Panulirus argus, Blaustein et al., 1988), Homarida (Homarus americanus, Sullivan and Beltz, 2001), Astacida (Procambarus clarkii, Sullivan and Beltz, 2001), Anomala (Birgus latro, Krieger et al., 2010; Coenobita clypeatus, Wolff et al., 2012); Pagurus bernhardus, Krieger et al., 2012a), Brachyura (Carcinus maenas, Krieger et al., 2012a), Euphausiacea (Meganyctiphanes norvegica, unpublished), Thermosbaenacea (Tethysbaena argentarii, Stegner et al., 2015), Amphipoda (Orchestia cavimana and Niphargus puteanus, Ramm and Scholtz, 2017), Mictacea (Mictocaris halope, Stegner et al., 2015), Spelaeogriphacea (Spelaeogriphus lepidops, Stegner et al., 2015) and Isopoda (Saduria entomon, Kenning and Harzsch, 2013; Idotea emarginata, Stemme et al., 2014). Sketches were made from sections stained using antibody raised against synapsin, except N. puteanus (antibody raised against tubulin), M. norvegica, P. argus and O. cavimana (histological sections), and N. herbstii (optical section). The symbol ‘?” indicates that the presence of a hemiellipsoid body is uncertain. The phylogram showing phylogenetic relationships of malacostracan crustaceans is modified from Harzsch and Krieger (2018) (therein modified from Sandeman et al., 2014, as compiled after Richter and Scholtz, 2001; Scholtz and Richter, 1995; Wirkner and Richter, 2010). Abbreviations: see text and appendix 1.
Tables
Comparative table summarizing characteristics of aesthetascs and olfactory neuropils in several malacostracan species.
https://doi.org/10.7554/eLife.47550.013| Aesthetascs | Olfactory neuropils (ON) | References | ||||
|---|---|---|---|---|---|---|
| Species (body length) | Total number | Length (µm) | Neuropil total volume (x10^6 µm3) | Mean glomerular volume (x10^3 µm3) | Glomerular number | |
| Leptostraca | ||||||
| Nebalia herbstii (1.4 cm) | - | - | 0.1 | 2 | 60 | Kenning et al., 2013 |
| Stomatopoda | ||||||
| Neogonodactylus oerstedii (4 cm) | 80 | 400 | - | 110 | 70 | Derby et al., 2003 |
| Isopoda | ||||||
| Saduria entomon (8 cm) | 40–60 | 240 | 3 | 34 | 80 | Kenning and Harzsch, 2013; Pynnönen, 1985 |
| Dendrobranchiata | ||||||
| Penaeus vannamei (7 cm) | 280 | - | - | - | <100 | Wittfoth and Harzsch, 2018; Zeng et al., 2002 |
| Caridea | ||||||
| Palaemon elegans (7 cm) | 280 | 230 | 120 | 225 | 530 | Zbinden et al., 2017; this study* |
| Rimicaris exoculata (6 cm) | 206 | 170 | 56 | 155 | 370 | Zbinden et al., 2017; this study |
| Achelata | ||||||
| Panulirus argus (20–60 cm) | 3000 | 1000 | 154 | 118 | 1332 | Beltz et al., 2003; Grünert and Ache, 1988 |
| Homarida | ||||||
| Homarus americanus (20–60 cm) | 2000 | 600 | 141 | 592 | 249 | Beltz et al., 2003; Guenther and Atema, 1998 |
| Astacida | ||||||
| Procambarus clarkii (9 cm) | 133 | - | 10 | 20 | 503 | Beltz et al., 2003 |
| Anomura | ||||||
| Birgus latro (20 cm) | 1700 | - | 375 | 280 | 1338 | Krieger et al., 2010 |
| Coenobita clypeatus (6 cm) | 519 | - | 120 | 154 | 799 | Beltz et al., 2003 |
| Pagurus bernhardus (3 cm) | 673 | - | - | 171 | 536 | Tuchina et al., 2015 |
| Brachyura | ||||||
| Carcinus maenas (9 cm) | 200 | 750 | - | 247 | - | Fontaine et al., 1982; Hallberg and Skog, 2011 |
-
Estimates of the animal’s body lengths are given for comparison. Carapace width is given for B. latro and C. maenas, and total length is given for all other species.
* The palaemonid shrimp Palaemon elegans was investigated in the present study for comparison, as a species closely-related to R. exoculata among the Caridea family.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47550.016





