Sterol transfer by atypical cholesterol-binding NPC2 proteins in coral-algal symbiosis
Figures
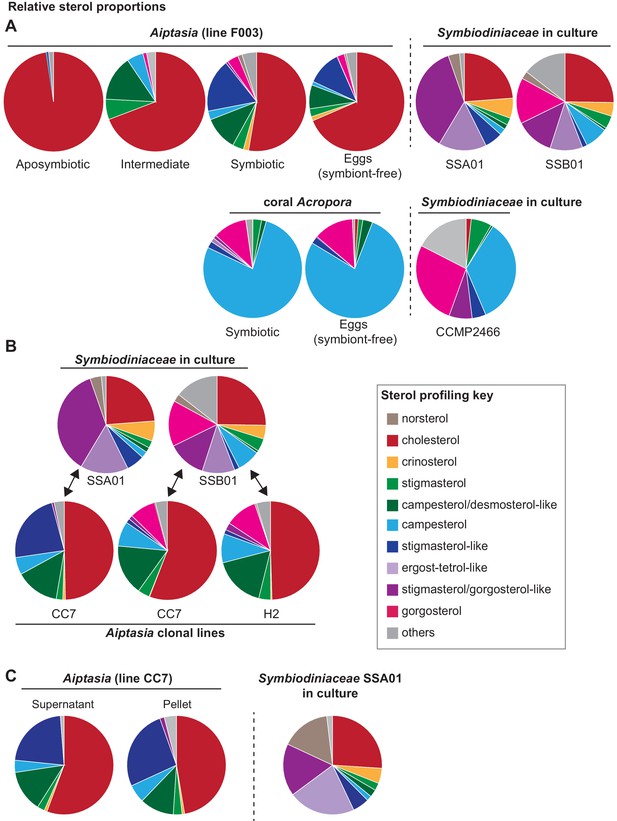
Transfer of symbiont-produced sterols reflects control by both host and symbiont.
(A) Gas chromatography/mass spectrometry (GC/MS)-generated sterol profiles of the given organisms, with relative composition (%) of each sterol in key. Values, Figure 1—source data 1. Symbiont-free animals (‘aposymbiotic’) were fed Artemia brine shrimp comprising nearly only cholesterol (Tolosa et al., 2011). ‘Intermediate’ were symbiotic Aiptasia more recently starved of brine shrimp diet than ‘symbiotic’ animals. Aiptasia strain F003 hosts Symbiodiniaceae strains SSA01 and SSB01 (Grawunder et al., 2015). Acropora digitifera endogenous Symbiodiniaceae are uncultured but closely related to the cultured strain CCMP2466 (see Materials and methods). (B) GC/MS-generated sterol profiles of Symbiodiniaceae strains in culture (upper row) and in symbiosis with adults of different Aiptasia host lines (Grawunder et al., 2015) (lower row). (C) Sterol profiles of Aiptasia CC7 homogenate separated by centrifugation into symbiont-enriched (Pellet) and symbiont-depleted (Supernatant) fractions with the corresponding Symbiodiniaceae strain SSA01 in culture.
-
Figure 1—source data 1
Relative sterol compositions of samples in pie graphs.
- https://doi.org/10.7554/eLife.43923.006
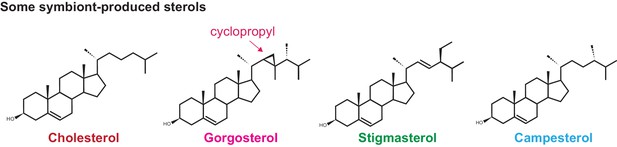
Some of the symbiont-produced sterols.
Symbiodiniaceae, like their dinoflagellate relatives, produce many sterols, including the unique gorgosterol with its unusual cyclopropyl group. Colors correspond to those in Figure 1.
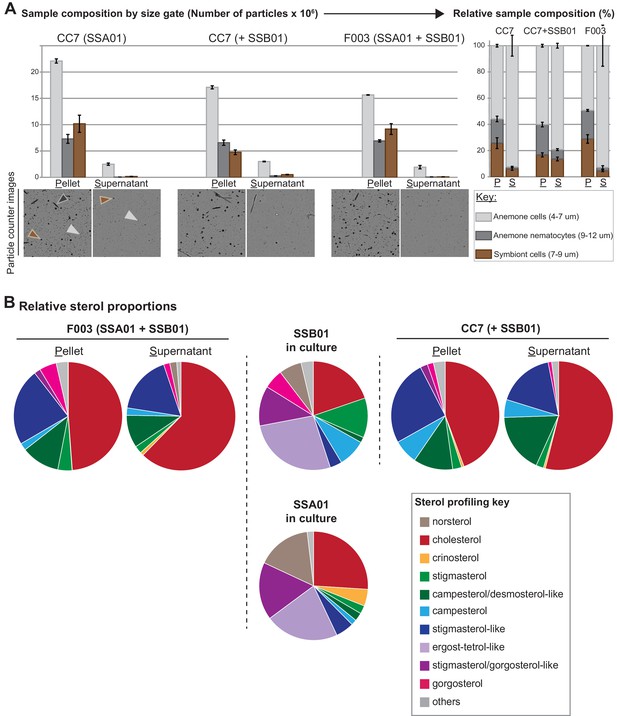
GC/MS-generated sterol profiling of symbionts in culture vs. in symbiosis.
(A) compositions of the centrifugally separated homogenates of the indicated Aiptasia clonal line (CC7, F003) and Symbiodiniaceae strain (SSA01, SSB01) pairings. Shown are total number of particles (left) and sample composition (right) per size gate. Average values ± SEM (error bars) of technical duplicates. (B) GC/MS-generated sterol profiles, as in Figure 1, of the samples shown in A and Symbiodiniaceae strains in culture. Values, Figure 1—source data 1.

Symbiotic anthozoans have expanded NPC2s with characteristics of differential adaptive evolution.
(A) Consensus Bayesian phylogeny of NPC2 homologues in metazoan genomes with anthozoan non-canonical (blue shading) and canonical (red shading) homologues. Also shown are alignments with intron/exon boundaries (green bars) and tandem duplication of NPC2 loci (where genome assemblies allow). Node values, posterior probabilities. Asterisks, new homologues from this study (Supplementary file 1). (B) Alignment of anemone and human NPC2 proteins, with shading by conservation. Shown are residues under positive (orange) or negative (purple) selection per NPC2 group as found in multiple tests of non-synonymous/synonymous substitution rates (dN/dS) in HyPhy (Pond et al., 2005); asterisks, significant in all tests. Indicated are also several functional regions in human NPC2 (Xu et al., 2007; Friedland et al., 2003; Ko et al., 2003; Wang et al., 2010; McCauliff et al., 2015).
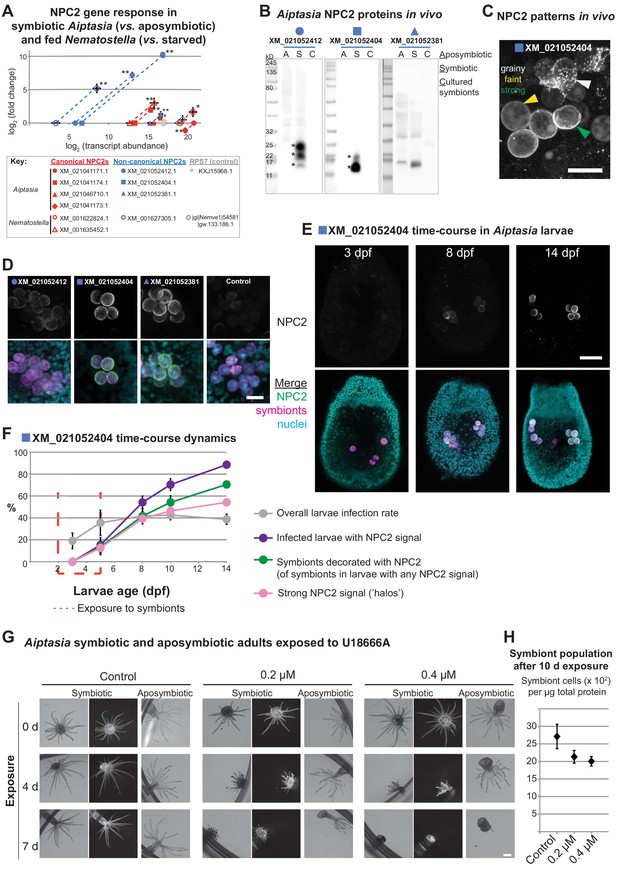
Non-canonical NPC2s respond to symbiosis and are spatiotemporally regulated to mature symbiosomes.
(A) Gene expression by RT-qPCR of canonical (red symbols) and non-canonical (blue symbols) NPC2s and 40S ribosomal subunit (RPS7, gray symbols). Filled symbols: Aiptasia. Open symbols: Nematostella. Average values ± SD (error bars). Statistical comparisons by Bayesian modeling (see Materials and methods), *p<0.05, **p<0.005. (B) Homogenates of aposymbiotic (A) or symbiotic (S) Aiptasia adults and cultured Symbiodiniaceae strain SSB01 (C) detected with affinity-purified antibodies to non-canonical Aiptasia NPC2 homologues. Asterisks, NPC2 glycoforms. (C) Immunofluorescence (IF) patterns of non-canonical NPC2 in 14 d post-fertilization (dpf) Aiptasia larvae containing intracellular symbionts of Symbiodiniaceae strain SSB01. Scale bar, 10 μm. (D) IF of several non-canonical Aiptasia NPC2s as in C. Merge channels: NPC2, secondary antibody Alexa488-anti-rabbit IgG; Nuclei, Hoechst; Symbionts, red autofluorescence of photosynthetic machinery. Control, secondary antibody only. Scale bar, 10 μm. (E) Time-course of immunofluorescence of non-canonical NPC2 in Aiptasia larvae infected with Symbiodiniaceae SSB01 from 2-5 dpf. Larvae oral opening facing up. Merge as in D. Scale bar, 25 μm. (F) Quantification of NPC2 IF time-course in E. Average value ± SEM (error bars). (G) Brightfield and fluorescence micrographs of symbiotic and aposymbiotic Aiptasia exposed to U18666A or DMSO negative control (vol. equiv. to 10 µM addition). Symbiont red autofluorescence as above. Scale bar, 1 mm. (H) Quantification of symbiont density in symbiotic anemones from G. Average values ± SEM (error bars).
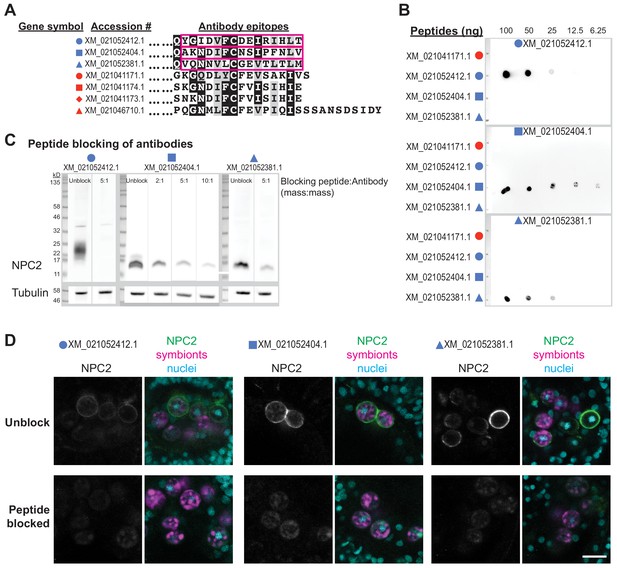
Validation of new antibodies raised against Aiptasia non-canonical NPC2s.
(A) N-terminal-truncated aligned Aiptasia NPC2s, with gene symbols as in Figure 2A. Epitopes used for immunogenic peptides in antibody production. (B) Dot blots of the peptides in A at the given concentrations probed with the antibodies indicated at the top of each blot. (C) Western blots of symbiotic Aiptasia extract probed with the indicated antibodies as is (Unblock) or incubated together with the peptides in A at the given ratios. (D) Immunofluorescence of 14 dpf Aiptasia larvae infected with Symbiodiniaceae strain SSB01 from 2-5 dpf, either with the indicated antibodies as is (Unblock) or incubated together with the peptides in A (Peptide blocked). Peptide:antibody (mass:mass) ratios were 5:1 (XM_021052412, XM_021052381) or 50:1 (XM_021052404). Scale bar, 10 μm.
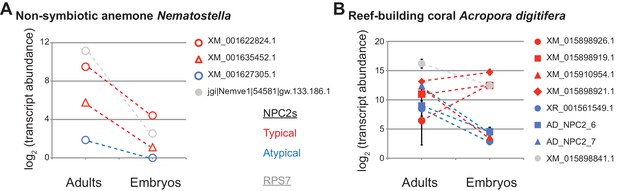
Differential maternal loading of canonical and non-canonical NPC2 transcripts in embryos of Acropora and Nematostella.
(A) Expression in Nematostella vectensis adults and in embryos. From RNAseq regeneration/development dataset on NvERTx server (Warner et al., 2018; Fischer et al., 2014). (B) Expression in both Acropora digitifera parent colonies and their offspring immediately after spawning. Average value ± SD (error bars). Difference between non-canonical NPC2s in adults and embryos significant, Student’s paired t-test, p=0.007 (canonical, p=0.18).
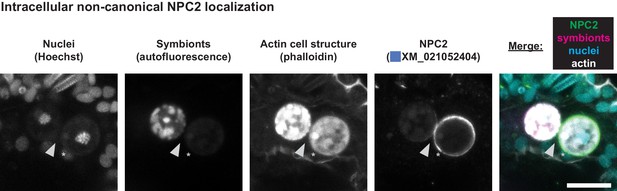
Intracellular symbiont surrounded by non-canonical NPC2.
NPC2 signal appears tightly localized around the symbiosome. Arrow, host cell cytoplasm within phalloidin-stained cell borders and near host cell nucleus (asterisk) showing absence of NPC2 signal. Scale bar, 10 μm.
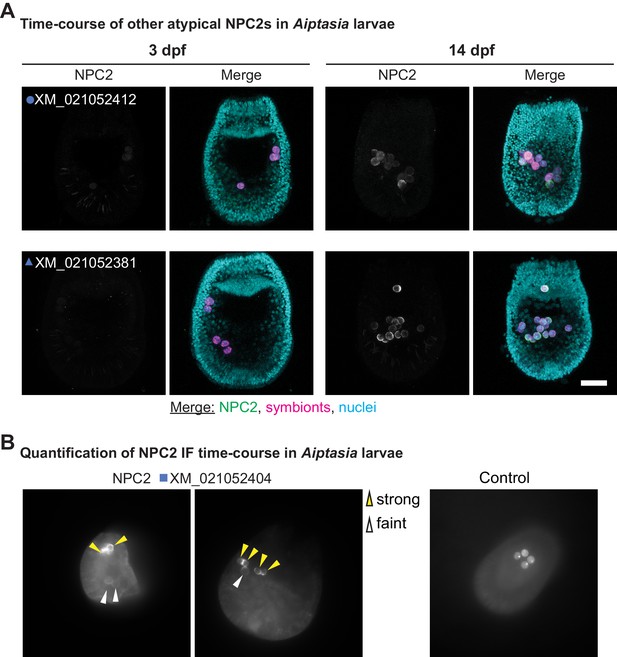
Dynamic recruitment of other non-canonical NPC2s to intracellular symbionts increases as symbiosis matures.
(A) Immunofluorescence (IF) of non-canonical NPC2s in Aiptasia larvae containing intracellular symbionts of Symbiodiniaceae strain SSB01 at 3 and 14 dpf. Merge channels as in Figure 3. Control, secondary antibody only. Scale bar, 25 μm. (B) Example images for quantification of NPC2 IF time-course in Figure 3E + F and Figure 3—figure supplement 7, with strong and weak NPC2 staining indicated by arrows. Control, secondary antibody only.
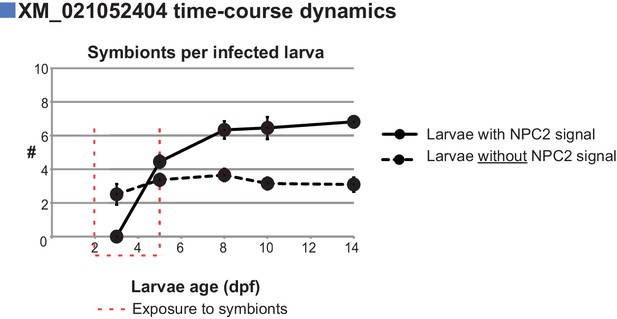
Quantification of symbiont load in Aiptasia larvae in a time-course of non-canonical NPC2 IF.
Quantification of NPC2 IF time-course in Figure 3E + F. n = triplicate samples of >50 larvae per time-point. Representative of two independent experiments. Average value ± SEM (error bars).
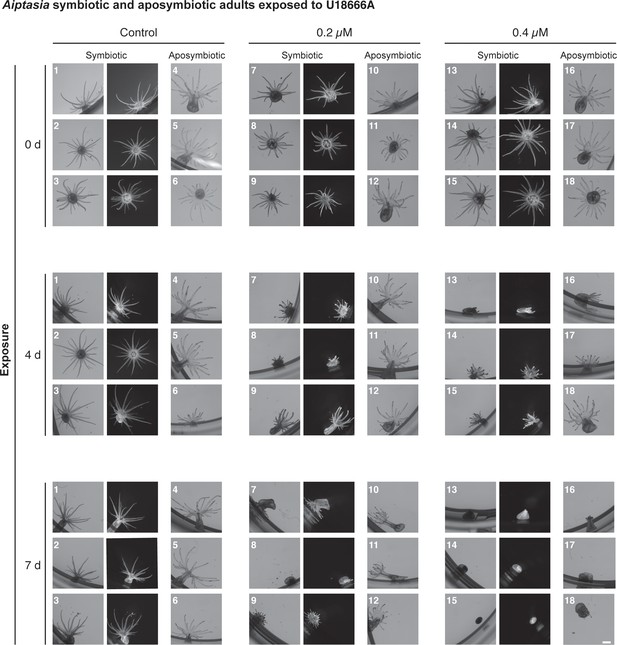
All symbiotic and aposymbiotic animals in the U18666A exposure experiment in Figure 3G + H.
Scale bar, 1 mm.
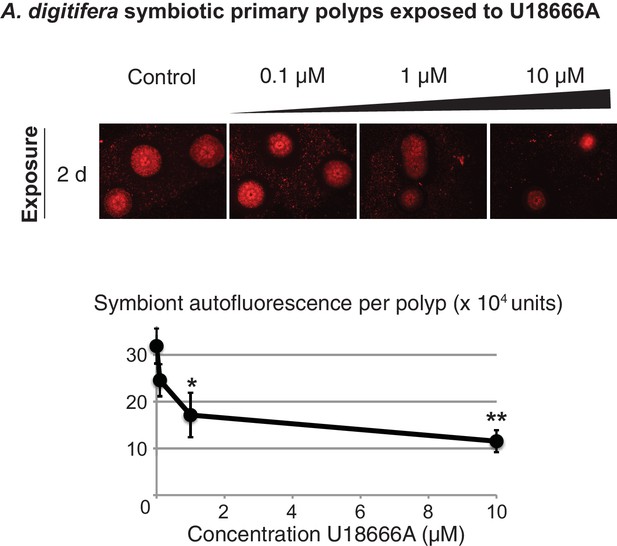
Acropora digitifera juvenile primary polyps hosting Symbiodiniaceae strain SSB01 exposed to U18666A or negative control.
Average values ± SEM (error bars). Statistical comparisons to DMSO negative control (Student’s t-test: *p<0.05, **p<0.005).
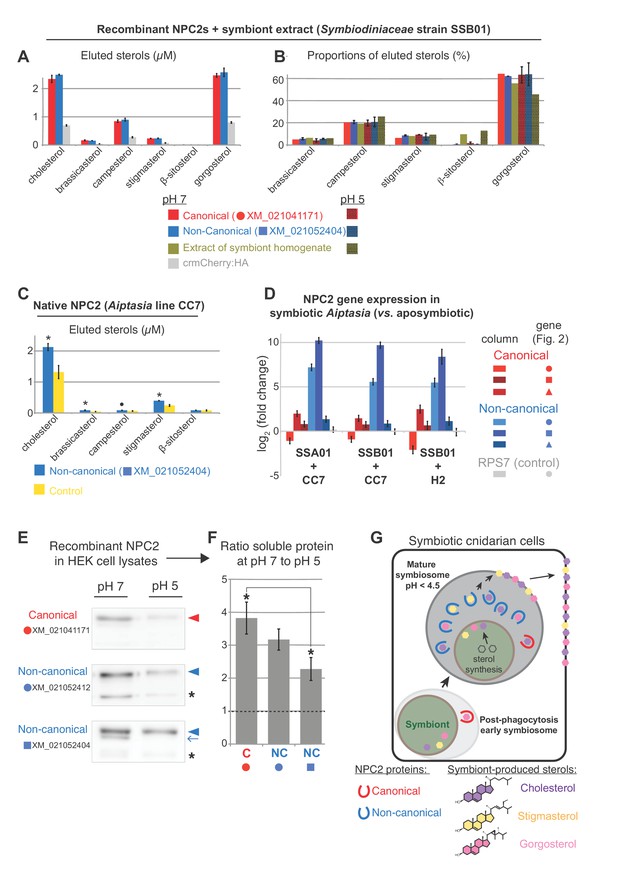
NPC2 binding to symbiont-produced sterols via immunoprecipitation-lipidomics, and differential stability of non-canonical NPC2 at varying pH.
(A) Quantification of bound lipids in the eluates following IP of recombinant canonical and non-canonical NPC2:crmCherry:HA and negative control crmCherry:HA at pH 7 (Figure 4—figure supplement 1). Sterols comprising <1.5% were omitted for clarity. crmCherry, lysosome-stable cleavage-resistant mCherry (Huang et al., 2014) . Average values ± SD (error bars). Except ß-sitosterol, each NPC2 was significantly different to crmCherry negative control (Student’s t-test, p<0.01). (B) Relative proportions of NPC2-bound sterols and the corresponding symbiont extract at pH 5 and 7. Average values ± SD (error bars) (C) Immunoprecipitation (IP) of native non-canonical NPC2 from Aiptasia and quantification of eluted bound sterols. Control, identical reaction omitting antibody. Average values ± SEM (error bars). Statistical comparisons to control (Student’s t-test: *p<0.05, •p<0.09). (D) NPC2 gene expression by qPCR in the various Aiptasia/Symbiodiniaceae host/symbiont combinations in Figure 1B. (E) Recombinant NPC2s detected by mCherry antibody in the soluble fractions of HEK cell lysates. Lysate preparations were identical except for buffer pH; equivalent volumes loaded per lane. (F) Quantification of protein abundances from Western blots in E. Average values ± SEM (error bars). Student’s t-test, *p<0.05. (G) We propose a model in which symbiotic anthozoans have evolved non-canonical NPC2 homologues that are spatiotemporally regulated to specifically respond to symbiosis, including through adaptation to the acidic environment of the symbiosome, the lysosomal-like organelle in which symbionts reside. NPC2 proteins bind and transport symbiont-produced sterols, and such trafficking is essential for cellular homeostasis of the sterol-auxotrophic hosts.
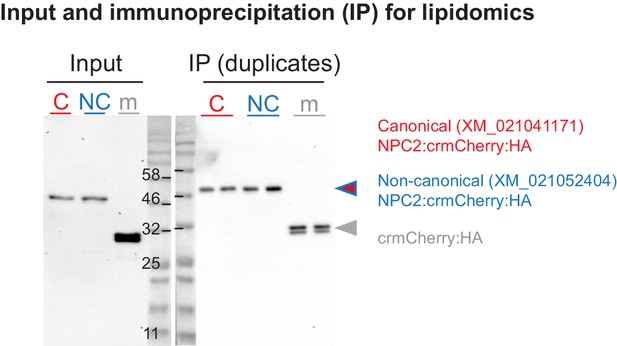
Immunoprecipitation-lipidomics: protein inputs and immunoprecipitation (IP) elutions.
HEK cell lysate input containing recombinant NPC2s or crmCherry control, and subsequent protein elutions of anti-HA IP reactions, in duplicate. After washing, lipids were eluted (Figure 4A + B) from IP reactions prior to protein elution shown here.

Soluble recombinant NPC2s in HEK 293T cell lysates.
Full Western blots from which the sections are shown in Figure 4E.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Exaiptasia pallida) | Aiptasia canonical NPC2 XM_021041171.1 | NA | XM_021041171.1 | |
| Gene (E. pallida) | Aiptasia canonical NPC2 XM_021041174.1 | NA | XM_021041174.1 | |
| Gene (E. pallida) | Aiptasia canonical NPC2 XM_021041173.1 | NA | XM_021041173.1 | |
| Gene (E. pallida) | Aiptasia canonical NPC2 XM_021046710.1 | NA | XM_021046710.1 | |
| Gene (E. pallida) | Aiptasia non-canonical NPC2 XM_021052412.1 | NA | XM_021052412.1 | |
| Gene (E. pallida) | Aiptasia non-canonical NPC2 XM_021052404.1 | NA | XM_021052404.1 | |
| Gene (E. pallida) | Aiptasia non-canonical NPC2 XM_021052381.1 | NA | XM_021052381.1 | |
| Gene (E. pallida) | Aiptasia RPS7 | NA | KXJ15968.1 | |
| Gene (Nematostella vectensis) | Nematostella canonical NPC2 XM_001622824.1 | NA | XM_001622824.1 | |
| Gene (N. vectensis) | Nematostella canonical NPC2 XM_001635452.1 | NA | XM_001635452.1 | |
| Gene (N. vectensis) | Nematostella non-canonical NPC2 XM_001627305.1 | NA | XM_001627305.1 | |
| Gene (N. vectensis) | Nematostella RPS7 | NA | jgi|Nemve1|54581|gw.133.186.1 | |
| Gene (Acropora digitifera) | Acropora canonical NPC2 XM_015898926.1 | NA | XM_015898926.1 | |
| Gene (A. digitifera) | Acropora canonical NPC2 XM_015898919.1 | NA | XM_015898919.1 | |
| Gene (A. digitifera) | Acropora canonical NPC2 XM_015910954.1 | NA | XM_015910954.1 | |
| Gene (A. digitifera) | Acropora canonical NPC2 XM_015898921.1 | NA | XM_015898921.1 | |
| Gene (A. digitifera) | Acropora non-canonical NPC2 XR_001561549.1 | NA | XR_001561549.1 | |
| Gene (A. digitifera) | Acropora non-canonical NPC2 AD_NPC2_6 | NA | AD_NPC2_6 | see Supplementary file 1 |
| Gene (A. digitifera) | Acropora non-canonical NPC2 AD_NPC2_7 | NA | AD_NPC2_7 | see Supplementary file 1 |
| Gene (A. digitifera) | Acropora RPS7 | NA | XM_015898841.1 | |
| Gene (Cladocopium goreaui) | Acropora digitifera resident Symbiodiniaceae symbionts, cp23S marker | NA | MK696595 | |
| Gene (C. goreaui) | CCMP2466 Symbiodiniaceae culture, cp23S marker | NA | MK696599 | |
| Strain, strain background (Exaiptasia pallida)(male) | Aiptasia line CC7 | DOI: 10.1038/srep15677 | in DOI: 10.1186/1471-2164-10-258 | |
| Strain, strain background (E. pallida)(female) | Aiptasia line F003 | DOI: 10.1038/srep15677 | ||
| Strain, strain background (E. pallida)(female) | Aiptasia line H2 | DOI: 10.1038/srep15677 | ||
| Strain, strain background (Nematostella vectensis)(male and female) | Nematostella | Prof. Dr. Thomas Holstein, Heidelberg University | ||
| Strain, strain background (Breviolum minutum) | Symbiodiniaceae strain SSB01 | DOI: 10.1111/jpy.12055 | GenBank:MK692539 | Accession number for rDNA 28S LSU marker (DOI: 10.1016/j.cub.2018.07.008) |
| Strain, strain background (Symbiodinium linuchae) | Symbiodiniaceae strain SSA01 | DOI: 10.1038/srep15677 | GenBank:MK692538 | Accession number for rDNA 28S LSU marker (DOI: 10.1016/j.cub.2018.07.008) |
| Strain, strain background (Symbiodinium necroappetens) | Symbiodiniaceae strain SSA02 | DOI: 10.1111/jpy.12055 | GenBank:MK692866 | Accession number for rDNA 28S LSU marker (DOI: 10.1016/j.cub.2018.07.008) |
| Strain, strain background (Effrenium voratum) | Symbiodiniaceae strain SSE01 | DOI: 10.1111/jpy.12055 | GenBank:MK696597 | Accession number for rDNA 28S LSU marker (DOI: 10.1016/j.cub.2018.07.008) |
| Strain, strain background (Cladocopium goreaui) | Symbiodiniaceae strain CCMP2466 | National Center for Marine Algae and Microbiota (NCMA), Bigelow Laboratory for Ocean Sciences, Maine, USA | GenBank:MK696600 | Accession number for rDNA 28S LSU marker (DOI: 10.1016/j.cub.2018.07.008) |
| Strain, strain background (Durusdinium trenchii) | Symbiodiniaceae strain CCMP2556 | National Center for Marine Algae and Microbiota (NCMA), Bigelow Laboratory for Ocean Sciences, Maine, USA | GenBank: MK692915 | Accession number for rDNA 28S LSU marker (DOI: 10.1016/j.cub.2018.07.008) |
| Cell line (Homo sapiens) | HEK 293T | Deutsche Sammlung von Mikroorganismen und Zellkulturen (DMSZ) from Dr. C. Niehrs and Dr. S. Acebrón | ACC 305 | authenticated by DMSZ, confirmed mycoplasma-free |
| Biological sample (Acropora digitifera) | Acropora digitifera | this paper | Collected off Sesoko Island, Okinawa Prefecture, Japan (26°37'41"N, 127°51'38"E) | |
| Antibody | anti-Aiptasia NPC2 XM_021052412 | this paper | Rabbit polyclonal, 0.3 mg/ml. (Westerns 1:500-1:1000, Dot Blot 1:1000, IF 1:200) BioScience GmbH. | |
| Antibody | anti-Aiptasia NPC2 XM_021052404 | this paper | Rabbit polyclonal, 0.45 mg/ml. (Westerns 1:2000-1:4000, Dot Blot 1:5000, IF 1:100-1:750) BioScience GmbH. | |
| Antibody | anti-Aiptasia NPC2 XM_021052381 | this paper | Rabbit polyclonal, 0.4 mg/ml (Westerns 1:500, Dot Blot 1:500, IF 1:200) BioScience GmbH. | |
| Antibody | HRP-coupled anti-rabbit (goat polyclonal) | Jackson ImmunoResearch | Jackson ImmunoResearch:111-035-144 | (Western blot 1:10,000) |
| Antibody | anti-alpha-tubulin (mouse monoclonal) | Sigma-Aldrich | Sigma-Aldrich:T9026 | (Western blot 1:1000) |
| Antibody | HRP-coupled anti-mouse (goat polyclonal) | Jackson ImmunoResearch | Jackson ImmunoResearch:115-035-044 | (Western blot 1:10,000) |
| Antibody | anti-rabbit IgG-Alexa488 (goat polyclonal) | Abcam | Abcam:ab150089 | (IF 1:500) |
| Antibody | anti-mCherry (rabbit polyclonal) | Thermo Fisher Scientific | Thermo Fisher Scientific:PA5-34974 | (Western blot 1:3000) |
| Antibody | conformation-specific HRP-coupled anti-rabbit IgG (mouse monoclonal) | Cell Signaling Technology | CST:5127S | (Western blot 1:2000) |
| Recombinant DNA reagent | NPC2 XM_021052412 for recombinant expression in HEK293T cells (plasmid) | this paper | NPC2-5P-crmCherry (DOI: 10.1371/journal.pone.0088893)−3xHA (YPYDVPDYA). Progenitors: PCR (cDNA), vector pCEP | |
| Recombinant DNA reagent | NPC2 XM_021052404 for recombinant expression in HEK293T cells (plasmid) | this paper | NPC2-5P-crmCherry (DOI: 10.1371/journal.pone.0088893)−3xHA (YPYDVPDYA). Progenitors: PCR (cDNA), vector pCEP | |
| Recombinant DNA reagent | NPC2 XM_021041171 for recombinant expression in HEK293T cells (plasmid) | this paper | NPC2-5P-crmCherry (DOI: 10.1371/journal.pone.0088893)−3xHA (YPYDVPDYA). Progenitors: PCR (cDNA), vector pCEP | |
| Recombinant DNA reagent | crmCherry:3xHA control for recombinant expression in HEK293T cells (plasmid) | this paper | crmCherry (DOI: 10.1371/journal.pone.0088893)−3xHA (YPYDVPDYA). Progenitors: PCR (cDNA), vector pCEP | |
| Sequence-based reagent | Primers for qPCR of Aiptasia, Acropora, Nematostella NPC2s | this paper | see Supplementary file 3 for all primer sequences | |
| Peptide, recombinant protein | K-YGIDVFCDEIRIHLT | Custom peptide, INTAVIS Bioanalytical Instruments AG | Epitope for antibody against Aiptasia NPC2 XM_021052412 | |
| Peptide, recombinant protein | K-AKNDIFCNSIPFNLV | Custom peptide, INTAVIS Bioanalytical Instruments AG | Epitope for antibody against Aiptasia NPC2 XM_021052404 | |
| Peptide, recombinant protein | K-VQNNVLCGEVTLTLM | Custom peptide, INTAVIS Bioanalytical Instruments AG | Epitope for antibody against Aiptasia NPC2 XM_021052381 | |
| Commercial assay or kit | RNeasy kit | Qiagen | Qiagen:74104 | |
| Commercial assay or kit | SYBR Hi-ROX qPCR master mix | Bioline | BIO-92005 | |
| Commercial assay or kit | NHS-activated Sepharose Fast Flow 4 | GE Health Care Life Sciences | GE Healthcare Sciences:17090601 | |
| Commercial assay or kit | anti-HA magnetic beads | Miltenyi Biotech | Miltenyi Biotech:130-091-122 | |
| Commercial assay or kit | Dynabeads Antibody Coupling Kit | Thermo Fisher Scientific | Thermo Fisher Scientific:14311 | |
| Commercial assay or kit | Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | Thermo Fisher Scientific:23227 | |
| Chemical compound, drug | Trizol | Life Technologies | Life Technologies:15596026 | |
| Chemical compound, drug | MSTFA (N-Methyl-N-(trimethylsilyl)trifluoroacetamide | Sigma-Aldrich | Sigma-Aldrich:69479 | |
| Chemical compound, drug | Lipofectamine2000 | Thermo Fisher Scientific | Thermo Fisher Scientific:11668019 | |
| Chemical compound, drug | Cholesterol-D6 | Cambridge Isotope Laboratories | Cambridge Isotope Laboratories:DLM2607 | |
| Chemical compound, drug | Acetyl chloride in methylene chloride | Sigma-Aldrich | Sigma-Aldrich:708496 | |
| Chemical compound, drug | U18666A | Sigma-Aldrich | Sigma-Aldrich:U3633 | |
| Chemical compound, drug | RNAlater | Thermo Fisher Scientific | Thermo Fisher Scientific:AM7020 | |
| Software, algorithm | Geneious | DOI: 10.1093/bioinformatics/bts199 | v. 9 | |
| Software, algorithm | SignalP 4.0 | DOI: 10.1038/nmeth.1701 | v. 4.0 | |
| Software, algorithm | MEGA | DOI: 10.1093/molbev/msw054 | v. 7.10.8 | |
| Software, algorithm | MrBayes | DOI: 10.1093/bioinformatics/17.8.754 | v. 3.2.6; plugin for Geneious | |
| Software, algorithm | DataMonkey server | DOI: 10.1093/bioinformatics/bti320 | Datamonkey classic server | |
| Software, algorithm | HyPhy program suite | DOI: 10.1093/bioinformatics/bti079 | accessed via DataMonkey classic server | |
| Software, algorithm | single-likelihood ancestor counting (SLAC) | DOI: 10.1093/molbev/msi105 | accessed via DataMonkey classic server | |
| Software, algorithm | mixed effects models of evolution (MEME) | DOI: 10.1371/journal.pgen.1002764 | accessed via DataMonkey classic server | |
| Software, algorithm | MCMC.qPCR | DOI: 10.1371/journal.pone.0071448 | R library | |
| Software, algorithm | GCMS Postrun Analysis software | Shimadzu | ||
| Software, algorithm | Analyst | SCIEX | v. 1.6.3. Control and analysis software for QTRAP 5500 MS | |
| Software, algorithm | LipidView | SCIEX | v. 1.2 | |
| Software, algorithm | Fiji | DOI: 10.1038/nmeth.2019 | v. 2.0.0-rc-67/1.52d | |
| Other | Phalloidin; Phalloidin-Atto 565 | Sigma-Aldrich | Sigma-Aldrich:94072 | |
| Other | Hoechst; Hoechst 33258 | Sigma-Aldrich | Sigma-Aldrich:B2883 |
Additional files
-
Supplementary file 1
Newly annotated or corrected NPC2 homologues.
- https://doi.org/10.7554/eLife.43923.019
-
Supplementary file 2
Genome, transcriptome, and proteome accession information.
- https://doi.org/10.7554/eLife.43923.020
-
Supplementary file 3
Primers for qPCR in Aiptasia, Nematostella, and Acropora digitifera.
- https://doi.org/10.7554/eLife.43923.021
-
Transparent reporting form
- https://doi.org/10.7554/eLife.43923.022





