A protein secreted by the Salmonella type III secretion system controls needle filament assembly
Figures
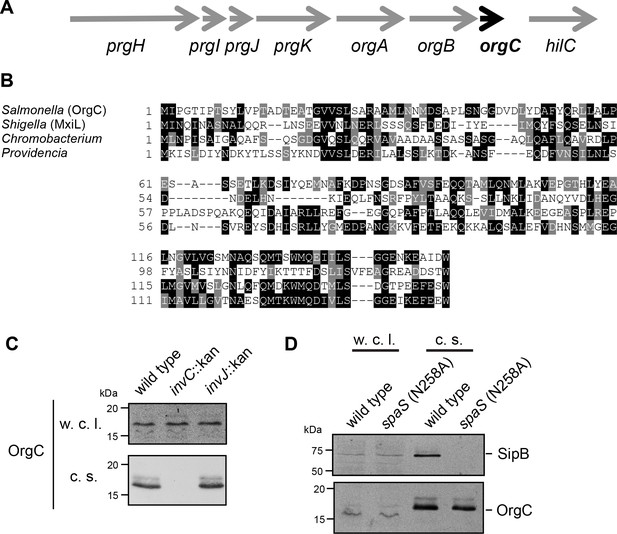
The T3SS-associated protein OrgC is secreted as an early substrate of the S. Typhimurium type III secretion system encoded within its pathogenicity island 1.
(A) Gene organization of the orgC locus within the S. Typhimurium pathogenicity island 1. (B) Amino acid sequence alignment of OrgC homologs. The sequences used in the alignment are: OrgC (S. Typhymurium), MxiL (Shigella flexneri), and hypothetical proteins from Chromobacterium violaceum and Providencia alcalifaciens. (C) Whole cell lysates (w. c. l.) or culture supernatants (c. s.) of wild-type S. Typhimurium, or the isogenic mutants ∆invC (T3SS-defective), ∆invJ, or spaSN258A (both mutant strains are able to secrete only early substrates), all expressing 3xFlag tagged OrgC, were analyzed by immunoblot with antibodies directed to the FLAG tag or the protein translocase SipB (as a control).
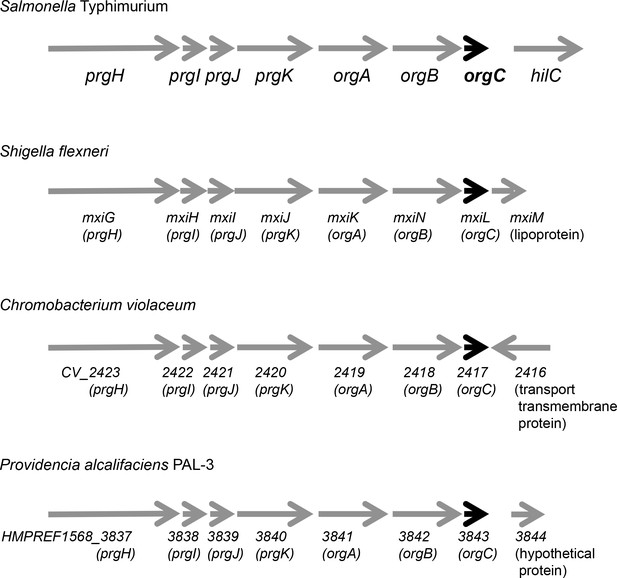
Genetic organization of different T3SS loci encoding OrgC homologs.
https://doi.org/10.7554/eLife.35886.003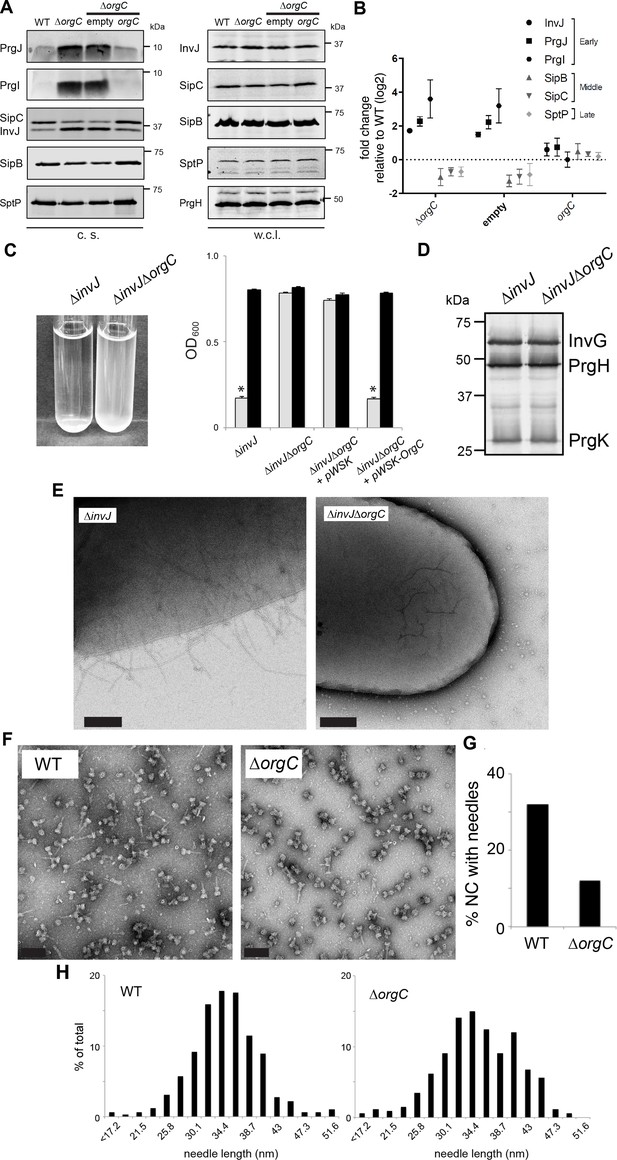
OrgC is necessary for efficient needle assembly but does not affect needle length.
(A) S. Typhimurium ΔorgC secretes elevated amount of early and reduced amount of middle/late substrates. Proteins in bacterial culture supernatants (c. s.) were concentrated by TCA precipitation and analyzed by SDS-PAGE, followed by immunoblot using specific antibodies for PrgI, PrgJ, InvJ (early substrates), SipB, SipC (middle substrates) and SptP (late substrate). The secretion phenotype of ΔorgC was complemented by introducing a plasmid expressing OrgC but not by introducing the empty plasmid vector alone. Expression levels of the indicated proteins in whole-cell lysates (w. c. l.) were also evaluated. (B) Western-blot band intensities from three independent experiments were quantified. Values were normalized to the wild-type parent strain and log2 transformed. The dotted horizontal line corresponds to the levels of the different proteins in wild type S. Typhimurium. (C) A bacterial cell-clumping assay implicates OrgC in needle assembly. Cultures of the S. Typhimurium ∆invJ mutant strain, which display long needle filaments, clump and precipitate to the bottom of the tube (left panel) resulting in drastically decreased OD600 (right panel, grey bars), which can be recovered by vortexing the samples (right panel, black bars). Bacterial cell clumping is abolished by introduction of a ∆orgC mutation (left and right panels), which can be complemented by introducing a plasmid encoding orgC (pWSK-orgC) but not by introducing the vector alone (pWSK) (right panel). Values represent OD600 before (grey bars) and after (black bars) vortexing and are the mean ± SEM (standard error of the mean) of three independent measurements. Asterisks indicate statistically significant differences from the values of the vortexed sample (p<0.001, Student t test). (D) Western blot analysis of the NC base components InvG, PrgH, and PrgK in whole cell lysates of the ∆invJ and ∆invJ ∆orgC S. Typhimurium mutant strains. (E) Electron micrographs of negatively stained S. Typhimurium showing the presence (∆invJ) or absence (∆invJ ∆orgC) of long T3SS needle filaments. Scale bar = 200 nm, (∆invJ); 100 nm (∆invJ ∆orgC). (F) Electron micrographs of negatively stained needle complexes isolated from wild type (WT) or ∆orgC S. Typhimurium. Scale bar = 100 nm. (G) Percentage of needle complexes exhibiting the needle filament in preparations obtained from wild type (WT) or ∆orgC S. Typhimurium (number of particles analyzed: w. t. = 1105; ∆orgC = 1273). (H) Needle length of needle complexes isolated from S. Typhimurium wild type or ∆orgC mutant strains. The percentage of needle complexes exhibiting the indicated length (in nm, x axis) is indicated (number of needle complexes analyzed: w. t. = 314; ∆orgC = 339.
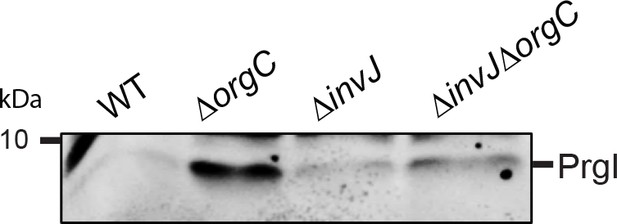
PrgI secretion profile of S.
Typhimurium wild-type, ΔorgC, ΔinvJ, and ΔorgC/ΔinvJ mutant strains. Culture supernatant from S. Typhimurium or its isogenic mutants were examined by western-blot for the presence of PrgI.
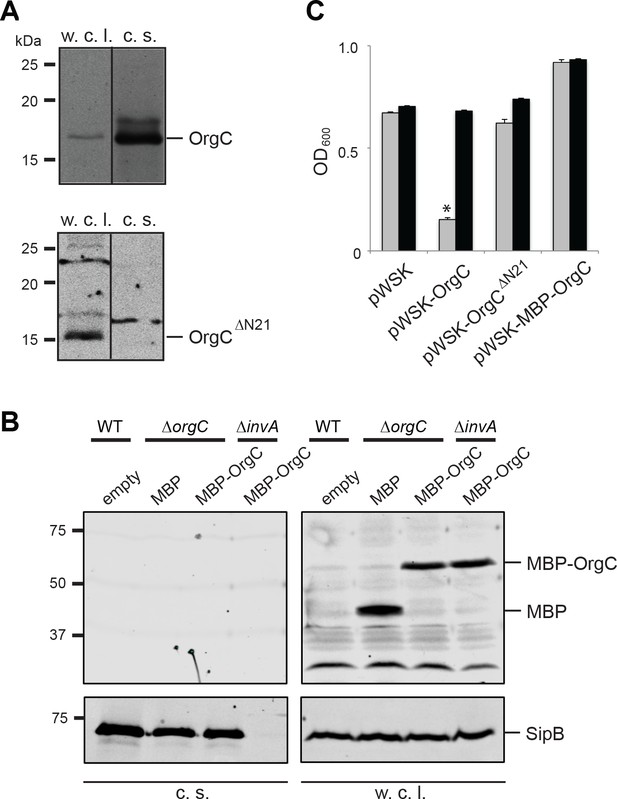
T3SS-mediated secretion of OrgC is required for its function.
(A and B) Removal of its first 21 amino acids (A) or N-terminal addition of MBP (B) prevents the secretion of OrgC. Whole cell lysates (w. c. l.) and culture supernatants (c. s.) of wild-type S. Typhimurium expressing, C-terminally FLAG-tagged full length OrgC or an equivalently tagged deletion mutant lacking its first 21 amino acids (N21) were analyzed by western immunoblot with a monoclonal antibody directed to the FLAG tag (A). Alternatively, whole cell lysates (w. c. l.) or culture supernatants of wild-type S. Typhimurium, or the indicated isogenic mutants carrying an empty pWSK129 plasmid or its derivatives expressing either maltose-binding protein (MBP) or MBP-OrgC fusion were analyzed by western-blot using specific antibodies directed to the MBP tag or the protein translocase SipB (as a secretion control) (B). (C) Non-secretable forms of OrgC are non-functional. The S. Typhimurium ∆invJ ∆orgC mutant strains carrying the indicated plasmids were analyzed by the clumping assay as indicated in Figure 2. Values represent OD600 before (grey bars) and after (black bars) vortexing and are the mean ± SEM of three independent measurements. Asterisks indicate statistically significant differences from the values of the vortexed sample (*p<0.001, Student t test).
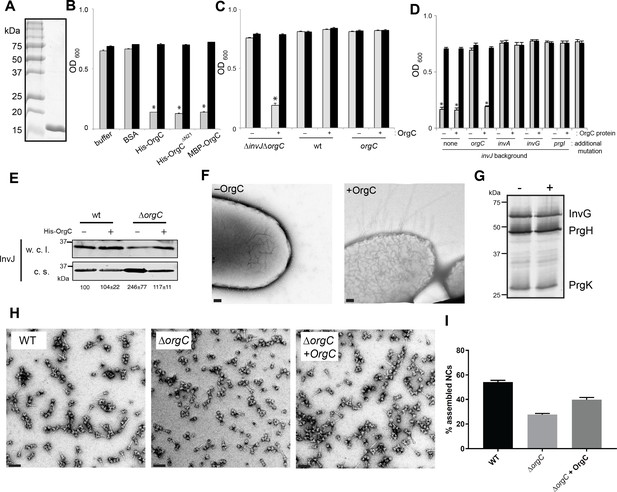
OrgC can exert its function when exogenously applied to bacterial cells.
(A) His-OrgC was purified by Ni-affinity, ion exchange, and gel-filtration chromatography analyzed by SDS-PAGE and coomassie blue staining. (B – D) Administration of purified OrgC to bacterial cell culture can complement a ∆orgC mutation. S. Typhimurium ∆orgC ∆invJ (B) or the indicated S. Typhimurium strains (C and D) were grown in the presence of the indicated protein preparations and the clumping of the bacterial cells was measured as indicated in Figure 2. Values represent OD600 before (grey bars) and after (black bars) vortexing and are the mean ± SEM of three independent measurements. Asterisks indicate statistically significant differences from the values of the vortexed sample [*p<0.001 (B), p<0.005 (C and D), Student t test]. (E) The secretion profile abnormality of the ΔorgC mutant can be reversed by addition of purified OrgC. Secretion profile of ΔorgC S. Typhimurium grown in the presence or absence of purified OrgC. Proteins in the bacterial culture supernatant were concentrated by TCA precipitation and analyzed by Western blotting using specific antibodies directed to the early T3SS substreate InvJ. Numbers below the different lanes are the average ± SEM of the western-blot band intensities of InvJ in culture supernatants relative to wild-type (-)from three independent secretion assays. (F) Electron micrographs of negatively stained S. Typhimurium ∆orgC ∆invJ grown in the presence (+OrgC) or in the absence (−OrgC) of purified OrgC. Note the presence of long filaments when bacteria are grown in the presence of purified OrgC protein. Scale bar: 100 nm. (G) Western blot analysis of the NC base components InvG, PrgH, and PrgK in whole cell lysates of S. Typhimurium ∆invJ ∆orgC grown in the presence or absence of purified OrgC. (H) Electron micrographs of negatively stained needle complexes isolated from wild-type (WT), and ΔorgC S. Typhimurium mutant strains grown in the absence (−OrgC) or in the presence (+OrgC) of purified OrgC protein. Scale bar: 100 nm. (I) The proportion of needle complexes displaying the needle filament in the indicated strains was determined. Values are expressed as the mean percentage (±SEM) of needle complexes per micrograph exhibiting the needle filament. Number of particles analyzed from 28 micrographs: w. t. = 2979; ΔorgC (−OrgC) = 3334; ΔorgC (+OrgC) = 3957.
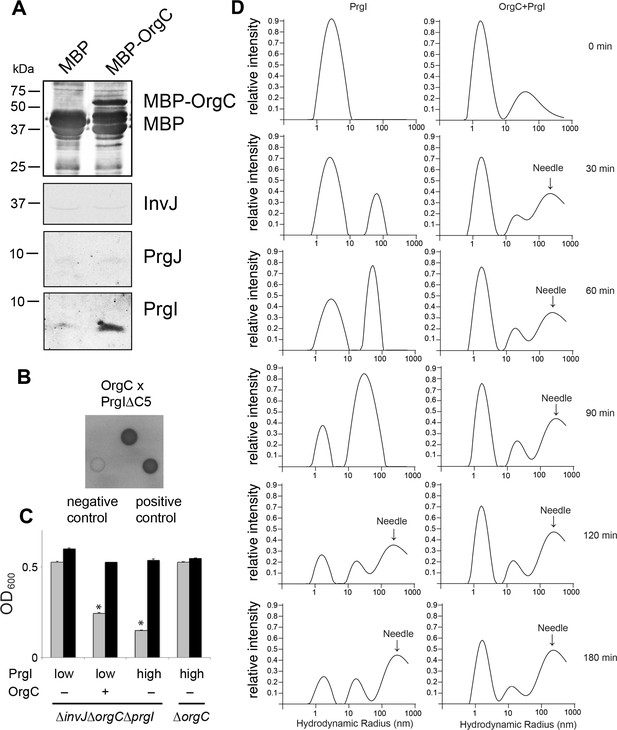
OrgC interacts with the needle filament protein PrgI and accelerates its in vitro polymerization.
(A) MBP-tagged OrgC or MBP alone were expressed in a ∆orgC S. Typhimurium strain and affinity-purified from whole cell lysates with amylose resin. Bound proteins were eluted with maltose and analyzed by Western blotting with the indicated antibodies. A specific PrgI signal was detected in the MBP-OrgC sample but not in the MBP control. Interaction of MBP-OrgC with other early T3SS substrates (PrgJ or InvJ) was not detected. (B) The interaction of OrgC with PrgI was examined in a bacterial adenylate cyclase-reconstitution two-hybrid assay. E. coli strains carrying plasmids encoding bait and target proteins fused to a PrgI deletion mutant lacking its five last residues (PrgI∆C5), and OrgC, respectively. Plasmids encoding the leucine zipper domain of the yeast transcription activator GCN4 (Zip) or strains carrying empty vectors served as positive and negative controls, respectively. All strains were plated on MacConkey agar to visualize interactions. Colonies of bacteria expressing interacting proteins appear green (dark in this figure). (C) Effect of PrgI overexpression on the ∆orgC phenotype. PrgI was expressed in a ∆invJ ∆orgC ∆prgI or ∆orgC S. Typhimurium strains from an arabinose-inducible promoter after growth in the presence of low (0.00075%) or high (0.015%) levels of arabinose, and bacterial cell clumping was assayed as indicated in Figure 2. Values represent OD600 before (grey bars) and after (black bars) vortexing and are the mean ± SEM of three independent measurements. Asterisks indicate statistically significant differences from the values of the vortexed sample (*p<0.001; Student t test). (D) OrgC stimulates PrgI polymerization in vitro. The effect of OrgC on the polymerization of recombinant PrgI into needles was monitored by dynamic light scattering (DLS) from 0 to 180 min. In the absence of OrgC, PrgI polymerized into needles at ~120 min. Addition of OrgC at 1:1 molar ratio accelerated the polymerization of PrgI into needles, which could be detected after 30 min. DLS peaks were assigned as monomeric PrgI (peak below 10 nm hydrodynamic radius), PrgI oligomers (10–100 nm), and PrgI polymers or needles (100–1000 nm). As control, OrgC did not show the needle DLS peak between 100–1000 nm, and remained well below 100 nm hydrodynamic radius.
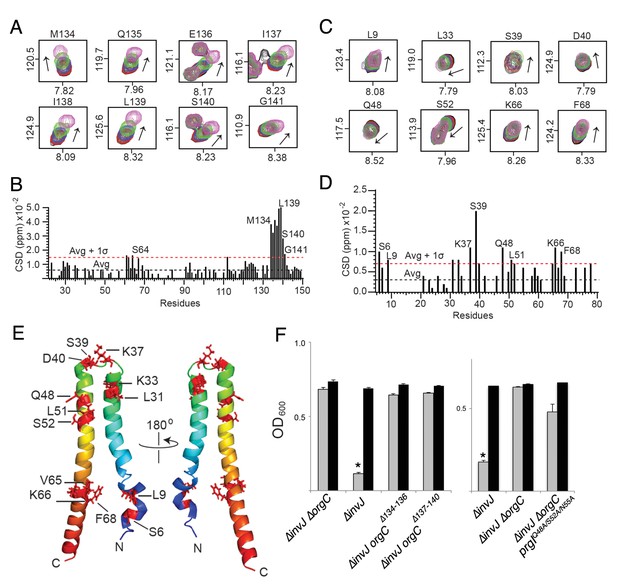
NMR titrations of OrgC and PrgI interaction.
(A) Selected 1H-15N peaks of 15N labeled OrgC that showed the largest changes upon titration with unlabeled PrgI. (B) Plot of the weighted chemical shift deviation of 15N OrgC titrated with PrgI. (C) Selected 1H-15N peaks of 15N labeled PrgI that showed the largest changes upon titration with unlabeled OrgC. (D) Plot of the weighted chemical shift deviation of 15N PrgI titrated with OrgC. In (A) and (C), peaks are colored according to the molar ratios of 15N protein: unlabeled binding partner as follows: black (1:0), red (1:0.5), blue (1:1), green (1:2), and pink (1:4). In (B) and (D), dashed lines show the average and average plus one standard deviation (σ) of the chemical shift deviation. (E) Location in the atomic structure of PrgI residues that showed the largest changes in the NMR peak positions of the backbone amides upon titration with OrgC. (F) Effect of mutations in the interacting residues in OrgC and PrgI. The indicated S. Typhimurium mutant strains were analyzed by the clumping assay as indicated in Figure 2. Values represent OD600 before (grey bars) and after (black bars) vortexing and are the mean ± SEM of three independent measurements. Asterisks indicate statistically significant differences from the values of the vortexed sample (*p<0.001, Student t test).
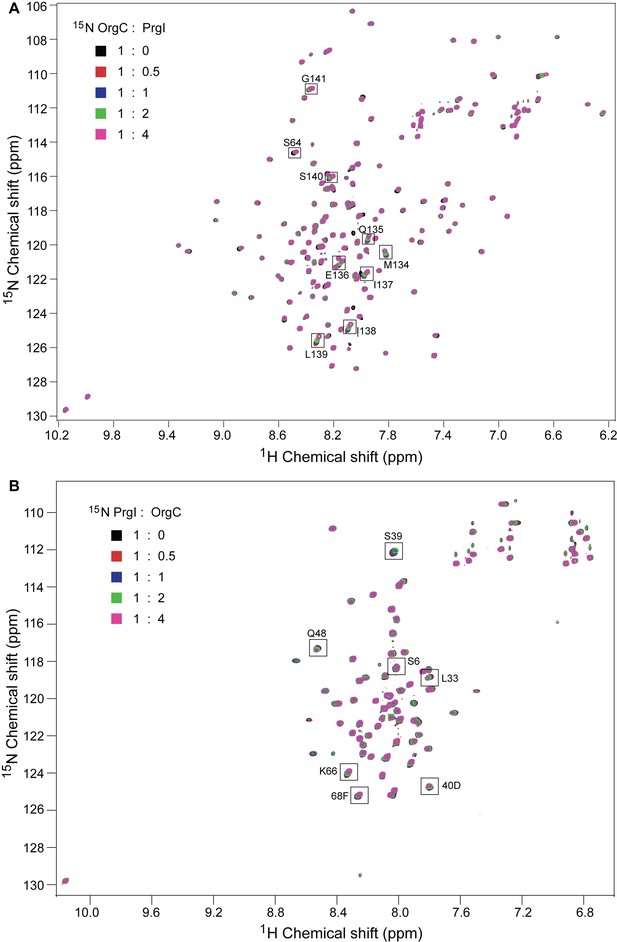
NMR titrations of OrgC and PrgI.
(A) 15N labeled OrgC titrated with unlabeled PrgI. (B) 15N PrgI titrated with unlabeled OrgC. The titrations were monitored by acquiring 2D 1H-15N HSQC spectra.
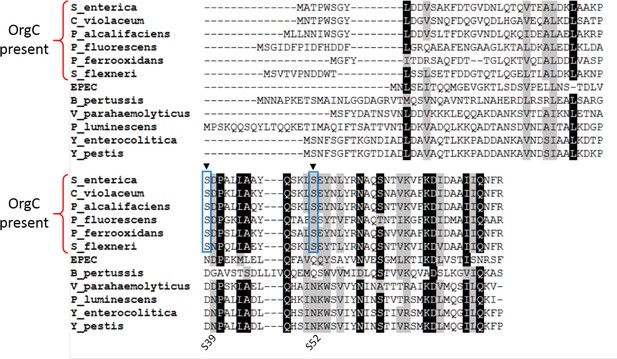
PrgI residues (Ser39 and Ser52) directly involved in OrgC binding are exclusively conserved in PrgI homologs that have an OrgC partner.
Multiple sequence alignment of different needle proteins from several bacteria carrying T3SSs. Ser39 and Ser52 are marked with filled black arrowheads. NCBI accession numbers for the needle proteins: Salmonella enterica (PrgI, CBW18951), Chromobacterium violaceum (OQS28935), Providencia alcalifaciens (EUC99172), Pseudomonas fluorescens (MxiH, AKV07676), Pseudogulbenkiania ferrooxidans (ERD99717), enteropathogenic Escherichia coli (EscF, CAS11480), Bordetella pertussis (BscF, CAC79556), Vibrio parahaemolyticus (PscF, KIT21774), Photorhabdus luminescens (LscF, AAO18031), Yersinia enterocolitica (YscF, AAD16839), Yersinia pestis (YscF, NP_395189). The alignment was carried out using Muscle and prepared for visualization using BioEdit. Residues identical are indicated in a black background whereas similar residues are marked gray if they are present in ≥80% of the sequences.
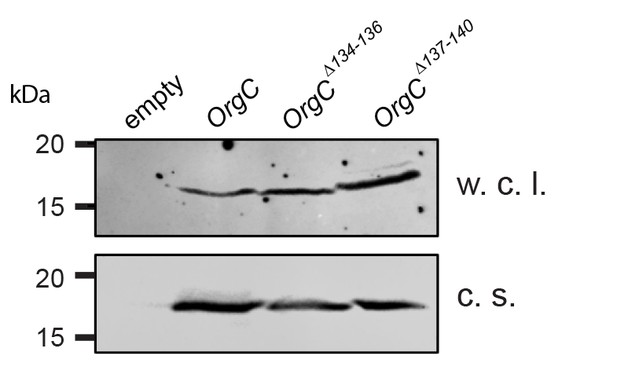
Stability of the OrgC protein deletion mutants.
Whole cell lysates (w. c. l.) or culture supernatants (c. s.) of S. Typhimurium ΔinvJ ΔorgC mutant strains carrying the empty vector pWSK129 (empty) or expressing the indicated carboxy-terminal FLAG-epitope-tagged OrgC deletion mutants were analyzed by immunodetection using antibodies directed to the FLAG tag. All variants were produced and secreted at equivalent levels.
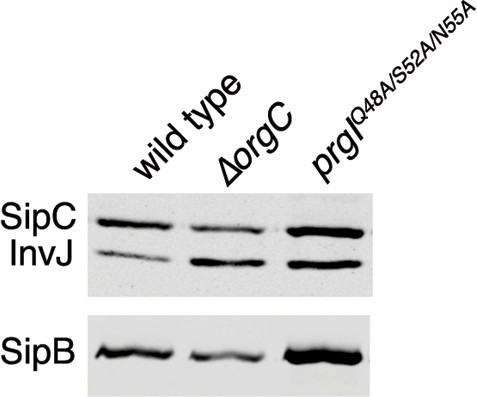
Mutations in the PrgI residues important for its interaction with OrgC do not affect type III secretion.
Proteins in the bacterial culture supernatant were concentrated by TCA precipitation and analyzed by SDS-PAGE, followed by immunoblot using specific antibodies to the indicated proteins.
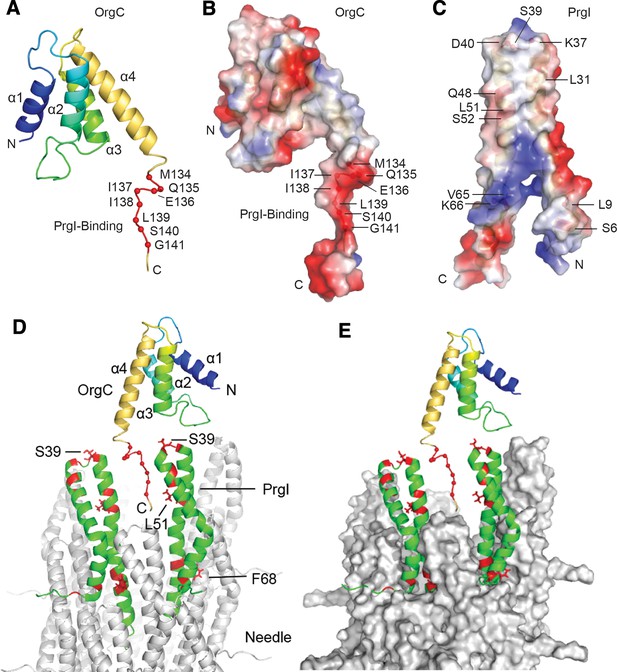
NMR structure of OrgC and structural model for its binding to PrgI in the assembled needle filament.
(A and B) The structured domain of OrgC consists of a 4-helix bundle and the PrgI-binding region is located at the C-terminus of the OrgC domain (A). The surface of the PrgI-binding region of OrgC is electronegative (B), which complements the electropositive surface on its binding partner, PrgI (C). (D and E). A model of OrgC with the assembled PrgI needle filament. The tip of the assembled PrgI needle is shown as a ribbon (D), and as a surface representation (E). The structure of the needle filament was from Loquet et al (Loquet et al., 2012). Two adjacent PrgI monomers at the needle tip are shown in green ribbon and the residues identified to be involved in binding OrgC are shown in red. There are spaces between the PrgI monomers at the needle tip that expose some of the PrgI residues (i.e. S39 and L51) involved in the interaction with OrgC. Residues near F68 are involved in PrgI-PrgI contacts in the assembled needle.
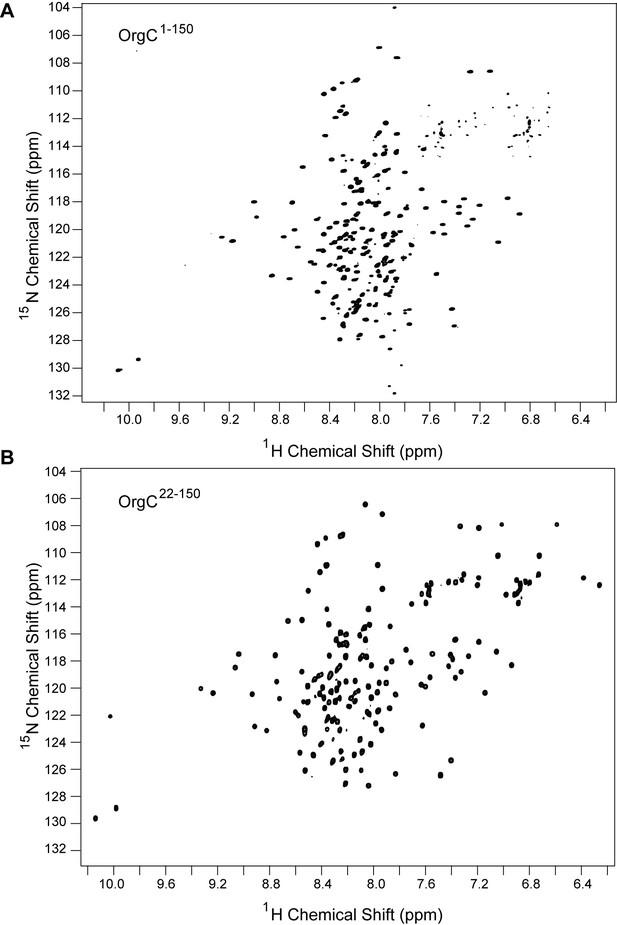
Comparison between the 2D proton-nitrogen correlation NMR spectra of (A) full length OrgC and (B) N-terminal truncation construct of OrgC.
https://doi.org/10.7554/eLife.35886.015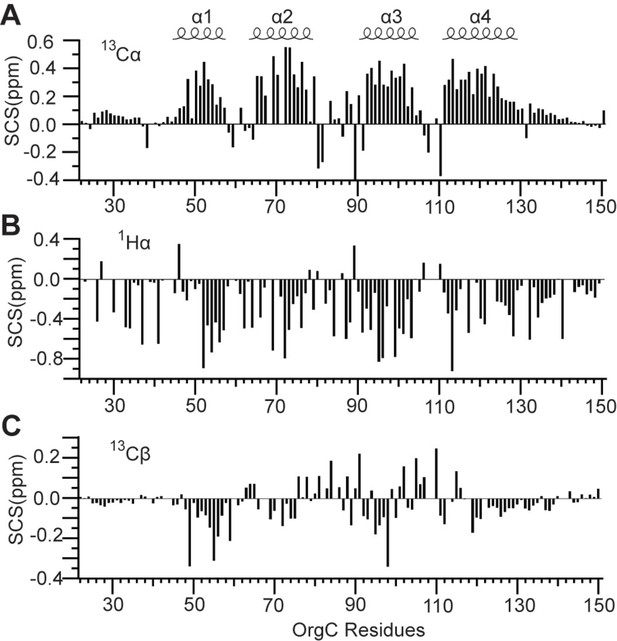
Secondary (A) 13Cα, (B) 1Hα, and (C) 13Cβ chemical shifts of OrgC identified four helices (α1, α2, α3, and α4) in the structure of OrgC.
https://doi.org/10.7554/eLife.35886.016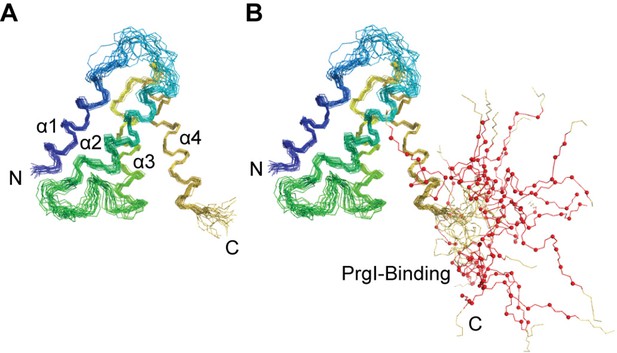
Superposition of the lowest energy 20 NMR structures of OrgC, (A) showing only the structured 4-helix domain of OrgC from residues 44–130, and (B) shown with the flexible PrgI-binding region, residues 134–141 (red lines and spheres).
https://doi.org/10.7554/eLife.35886.017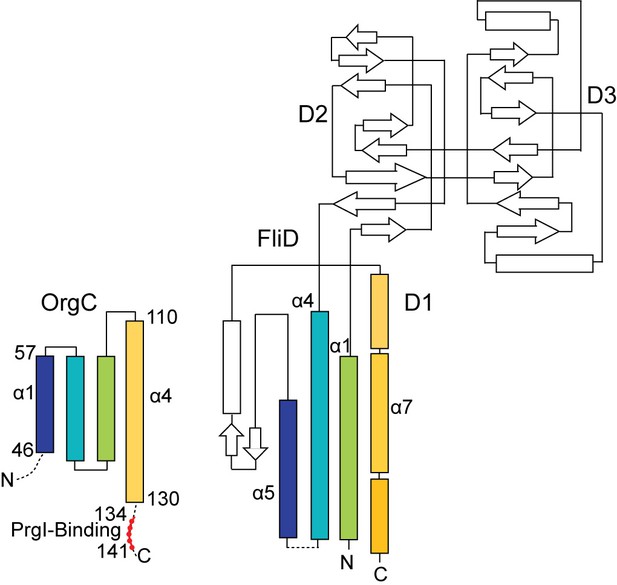
The topology of the OrgC fold shares similarity only partly with the topology of the FliD D1 domain.
The OrgC fold is depicted similar to the topology fold of FliD as reported by Song et al (Song et al., 2017). Shown in colors are the similarity in the folding topology of OrgC and FliD.
Tables
| Reagent type (species) | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB300 | Nature, 291:238 | wild type | Mouse isolate of SL1344 |
| Strain, strain background strain background (Salmonella enterica serovar Typhimurium) | SB2942 | This study | orgC 3xFlag invC::kan | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2943 | This study | orgC 3xFlag invJ::kan | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2946 | This study | orgC 3xFlag spaS-3xFlag | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2947 | This study | orgC 3xFlag spaSN258A-3xFlag | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2326 | This study | ∆invJ flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2939 | This study | ∆invJ∆orgC flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB3079 | This study | mbp-prgH flhD::tet | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB3275 | This study | mbp-prgH flhD::tet ∆orgC | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB762 | Infect. Immun. 68:2335 | flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB1679 | This study | ∆orgC | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2639 | This study | ∆invJ flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB2944 | This study | ∆orgC flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB3272 | This study | ∆invJ invA::kan flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB3273 | This study | ∆invJ invG::kan flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB3274 | This study | ∆invJ ∆prgI flhD::Tn10 | |
| Strain, strain background (Salmonella enterica serovar Typhimurium) | SB3289 | This study | ∆invJ ∆orgC ∆prgI flhD::Tn10 | |
| Antibody | M2 | Sigma | Mouse monoclonal antibody to the FLAG epitope |
Additional files
-
Supplementary file 1
Restraints and structural statistics for 20 NMR structures of OrgC.
- https://doi.org/10.7554/eLife.35886.019
-
Supplementary file 2
Bacterial strains used in this study.
- https://doi.org/10.7554/eLife.35886.020
-
Supplementary file 3
Plasmids used in this study.
- https://doi.org/10.7554/eLife.35886.021
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35886.022





