Clarinet (CLA-1), a novel active zone protein required for synaptic vesicle clustering and release
Figures
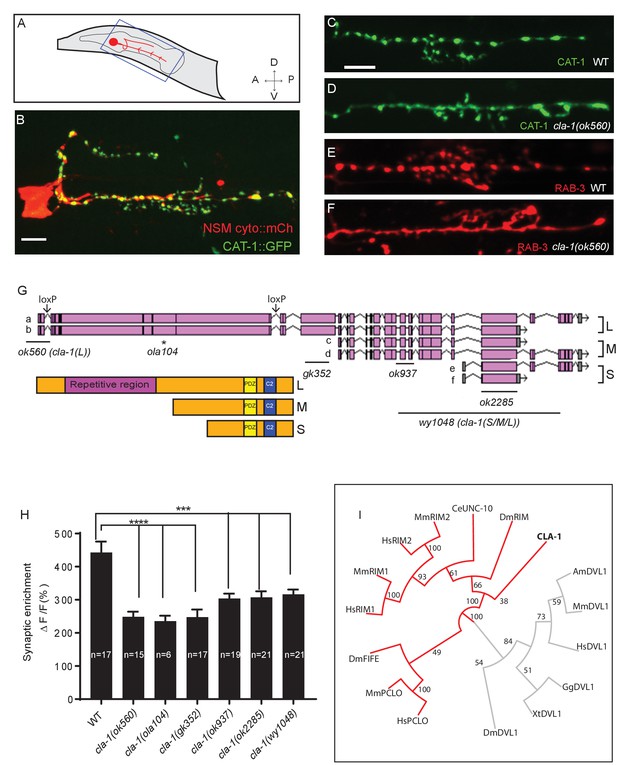
cla-1 mutants display disrupted synaptic vesicle clustering in NSM neurons.
(A) Schematic diagram of the nematode head and the NSM neuron (in red inside blue-boxed region). (B) Cytosolic mCherry (cyto::mCh) and the synaptic vesicle marker CAT-1::GFP expressed cell specifically in NSM. Scale bar = 5 μm. (C–F) Synaptic vesicle markers in NSM: CAT-1::GFP (C–D) or RAB-3::mCherry (E–F) in ventral neurite in wild type (WT; C and E) and cla-1(ok560) (D and F). Note how cla-1 mutants exhibit diffuse (D, F) rather than the wild type punctate (C, E) fluorescence patterns. Scale bar = 5 μm. (G) Schematics of the genomic region of cla-1 and the structure of three main isoforms of the CLA-1 protein. The locations of loxP sites and the genetic lesions of the cla-1 alleles examined in this study are indicated. In addition to the common C-terminus, CLA-1L contains a large N-terminal repetitive region (see Figure 1—figure supplement 1G). (H) Synaptic enrichment (ΔF/F) of CAT-1::GFP in NSM is greatly reduced in all cla-1 mutants compared to wild type (WT). n = number of animals. (I) The PDZ sequence of CLA-1 was aligned to RIM, Piccolo and Fife from C. elegans (CeUNC-10), Drosophila (DmRIM, DmFife), mouse (MmRIM1/2, MmPCLO) and human (HsRIM1/2, HsPCLO) by neighbor joining with 100 bootstrap replicates. PDZ domains of Dishevelled family proteins were used as an outgroup (grey).
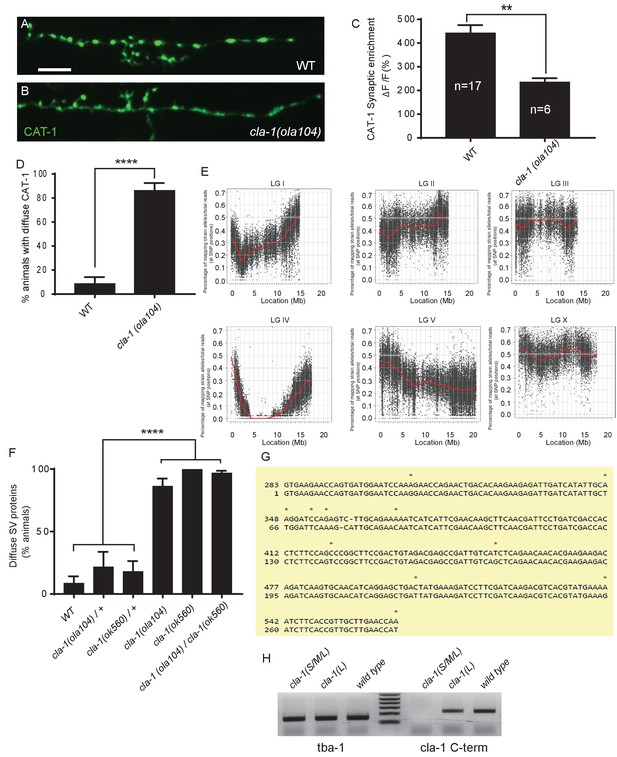
ola104 displays disrupted synaptic vesicle clustering in NSM neuron and was identified as a genetic lesion of cla-1.
(A–B) Synaptic vesicle marker CAT-1::GFP in the NSM ventral neurite in wild type (WT; A) and cla-1(ola104) (B). Note that cla-1(ola104) mutants exhibit diffuse (B and C) rather than the wild type punctate (A and C) fluorescence patterns. Scale bar = 5 μm. (C) Synaptic enrichment (ΔF/F) of CAT-1::GFP in NSM for WT and cla-1(ola104) animals. n = number of animals. (D) Percentage of animals displaying diffuse distribution of CAT-1::GFP in NSM of WT and cla-1(ola104) animals. n = 62 for WT; n = 63 for cla-1(ola104). (E) Graphic representation of the whole genome sequence (WGS)-SNP data. The positions of SNP loci are depicted as a XY scatter plot, where the ratio of Hawaiian/total number of reads for each SNP is represented. LGI to LGV and LGX are linkage groups/chromosomes of the worm. (F) cla-1(ok560) animals phenocopy cla-1(ola104) and do not complement cla-1(ola104) when assayed for CAT-1::GFP distribution in NSM. (G) Repeat unit (282 bp) of the repetitive region of cla-1L cDNA. Note the high similarity between two repeat units shown here. (H) RT-PCR shows that the common C-terminus is still expressed in a CLA-1L N-terminal deletion mutant cla-1(L), but not after deletion of the C-terminus itself, as in the null allele cla-1(S/M/L). tba-1 is used as a positive control for cDNA preparation.
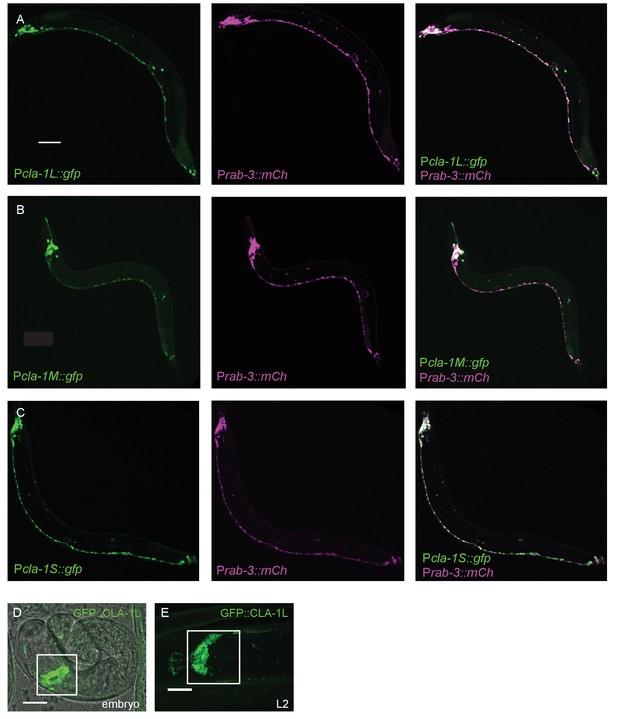
Expression pattern of CLA-1 isoforms.
(A–C) Expression pattern of the long (A), medium (B), and short (C) isoforms of CLA-1. Promoter reporters were generated by placing 2 kb of sequence upstream of the start codons of each isoform before gfp cDNA. The GFP reporters under all three promoters are expressed broadly in the nervous system, which is marked with an mCherry reporter under the rab-3 promoter (middle panels of A-C). Scale bar = 60 μm. (D–E) Endogenous expression of GFP::CLA-1L during development. GFP::CLA-1L is highly expressed in the nerve ring (the synapse-rich region in the nematode head considered the ‘brain’ of the worm; boxed region) during late embryogenesis (D) and throughout larval stages (E, L2 larval stage). Scale bar = 10 μm.
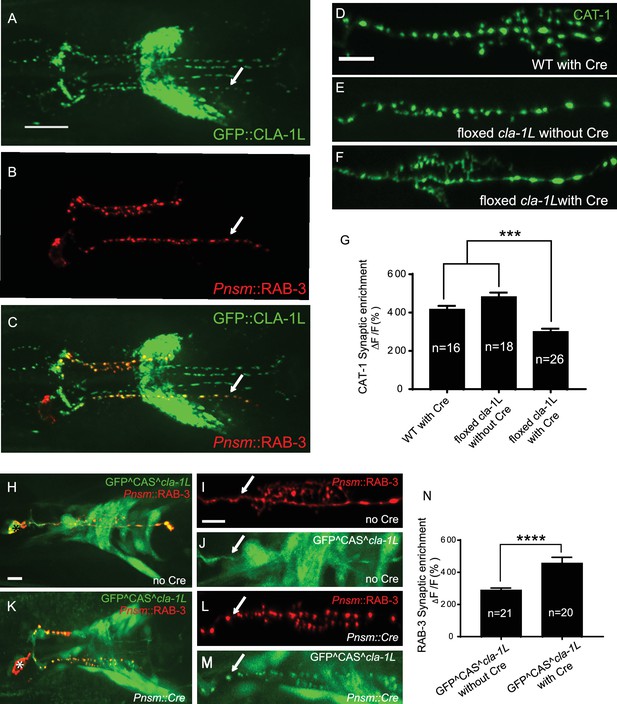
CLA-1 localizes to synapses and regulates synaptic vesicle clustering in a cell autonomous manner.
(A–C) Endogenous expression of GFP::CLA-1L (see Materials and methods) in the nerve ring of adult worms (A) along with NSM-specific expression of mCh::RAB-3 (B) CLA-1L colocalizes with RAB-3 in NSM (arrows) (A,C). Scale bar = 10 μm. (D–F) CAT-1::GFP distribution is normal in WT worms expressing Cre recombinase in NSM (D), and in floxed cla-1L worms without Cre (E), as expected. However, when Cre is expressed cell-specifically in NSM in the context of the floxed cla-1L allele, the synaptic vesicle pattern in NSM phenocopies that of cla-1 loss-of-function mutants (F). Scale bar = 5 μm. (G) Synaptic enrichment (ΔF/F) of CAT-1::GFP in NSM for control animals (‘WT with Cre’ and ‘floxed cla-1L without Cre’), and animals in which cla-1L was cell-specifically deleted in NSM (‘floxed cla-1L with Cre’). n = number of animals. (H–M) Cytosolic GFP driven by the endogenous cla-1L promoter in place of CLA-1L (GFP^CAS^cla-1L; H and J) overlaps with RAB-3 expressed under the NSM promoter (Pnsm::RAB-3::mCh; H and I). RAB-3 shows defective vesicle clustering before Cre excision of the translation termination sequence (GFP^CAS^cla-1L without Cre; I; arrow). Upon cell-specific Cre expression in NSM (K–M), a functional, translational fusion of GFP:CLA-1L results (see Materials and methods and Figure 2—figure supplement 1), rescuing the synaptic pattern in NSM (as determined by punctate distribution of both RAB-3 (L, arrow) and of GFP::CLA-1L (M, arrow)). Asterisk (H and K) corresponds to the location of the cell body of the NSM neurons. Scale bar = 5 μm. (N) Quantification of the synaptic enrichment (ΔF/F) of mCherry::RAB-3 in NSM for cla-1l null animals (‘GFP^CAS^cla-1L without Cre’) and animals expressing GFP::CLA-1L cell-specifically in NSM (‘GFP^CAS^cla-1L with Cre’). n = number of animals.
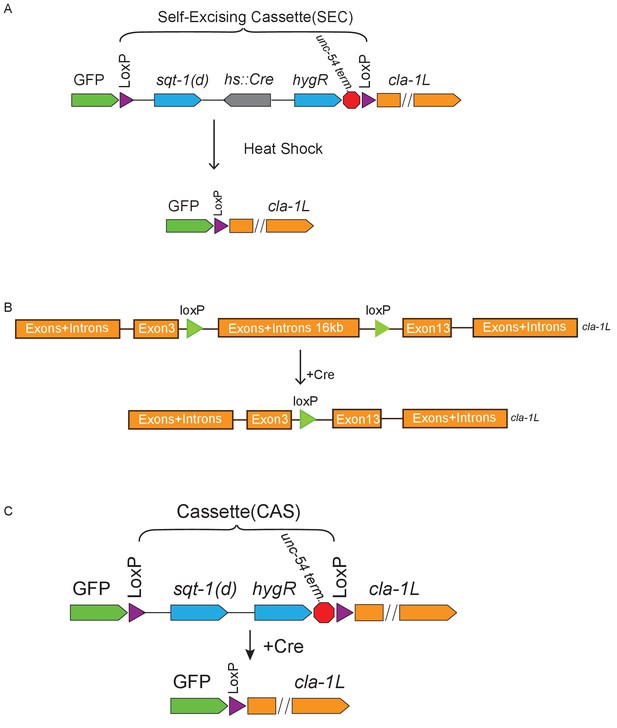
Schematics of CRISPR strategies to examine subcellular localization and cell autonomy of CLA-1L.
(A) CRISPR strategy for N-terminal GFP tagging of CLA-1L (described in Materials and methods). Briefly, a GFP followed by a self-excising cassette (SEC) was inserted in front of the cla-1L start codon, and then SEC was excised upon heat shock. (B) To determine cell autonomy, loxP sites were inserted into introns between exon 3 and 13 of cla-1L (Figure 1H) via CRISPR. Cre expression in NSM led to the removal of CLA-1L specifically in that neuron. (C) Cell-specific expression of CLA-1L. cla-1l null allele was generated by inserting GFP and a cassette (CAS) containing a transcriptional terminator before the start codon of cla-1l. Since the cassette was flanked by loxP sites, cell-specific expression of Cre resulted in cell-specific expression of GFP fused to the N-terminus of CLA-1L.
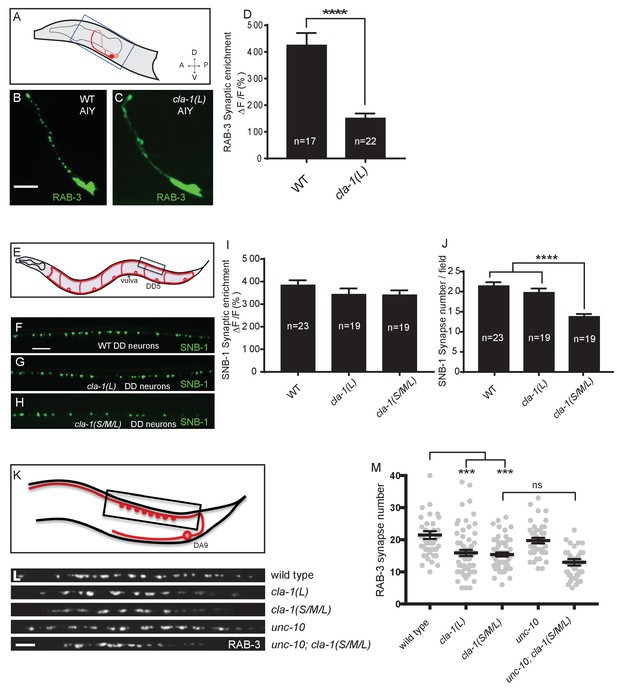
CLA-1 isoforms have discrete functions in several neuron types.
(A) Schematic diagram of the bilaterally symmetric AIY interneuron (in red, within blue-boxed region) in the worm head. (B–C) RAB-3::GFP forms discrete presynaptic clusters in AIY of wild type animals (WT; B), but is diffuse in cla-1(L) mutants (C). Scale bar = 5 μm. (D) Synaptic enrichment (ΔF/F) of RAB-3::GFP in AIY for WT animals and cla-1(L) mutants. n = number of animals. (E) Schematic diagram of DD motor neurons. Synaptic vesicle clustering in DD neurons was assessed by examining the localization of SNB-1::GFP in the boxed area. (F–H). SNB-1::GFP forms discrete presynaptic clusters in DD axons of cla-1(L) or cla-1(S/M/L) mutants (G–H), similar to the wild type animals (WT; F). Scale bar = 10 μm. (I) Synaptic enrichment (ΔF/F) of SNB-1::GFP in the DD axons for WT animals and cla-1(L) or cla-1(S/M/L) mutants. n = number of animals. (J) SNB-1::GFP puncta number in DD axons of cla-1(S/M/L) and cla-1(L) mutants, compared to WT animals. n = number of animals. (K) Schematic of the DA9 cholinergic motor neuron. Synapses (boxed region) labeled by RAB-3::GFP were examined. (L) Straightened synaptic domain (boxed region in K) showing the localization of RAB-3::GFP in WT animals and various mutants. Scale bar = 5 μm. (M) Synapse number was reduced in cla-1(S/M/L) as well as cla-1(L) mutants compared to WT animals (although with greater variability in cla-1(L) mutants), but was not significantly different between cla-1(S/M/L) single mutants and cla-1(S/M/L);unc-10 double mutants.
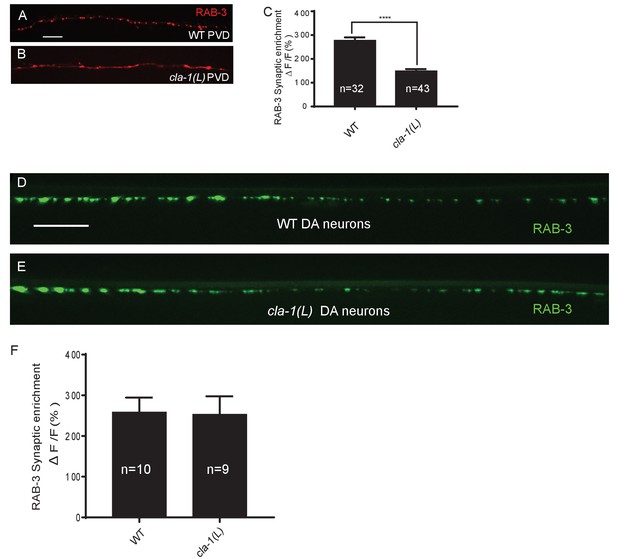
Synaptic vesicle clustering assessed in various neuronal types.
(A–B) RAB-3::GFP forms discrete presynaptic clusters in the mechanosensory neuron PVD of wild type animals (WT; A), but is diffuse in cla-1(L) mutants (B). Scale bar = 10 μm. (C) Synaptic enrichment (ΔF/F) of RAB-3::mCh in the PVD axon for WT animals and cla-1(L) mutants. n = number of animals. (D–E) RAB-3::GFP in DA motor neurons of WT (D) or cla-1(L) (E) animals was indistinguishable. Scale bar = 10 μm. (F) Synaptic enrichment (ΔF/F) of RAB-3::GFP in the cholinergic DA motor neurons for WT animals and cla-1(L) mutants. All images were taken of the dorsal nerve cord posterior to the vulva. n = number of animals.
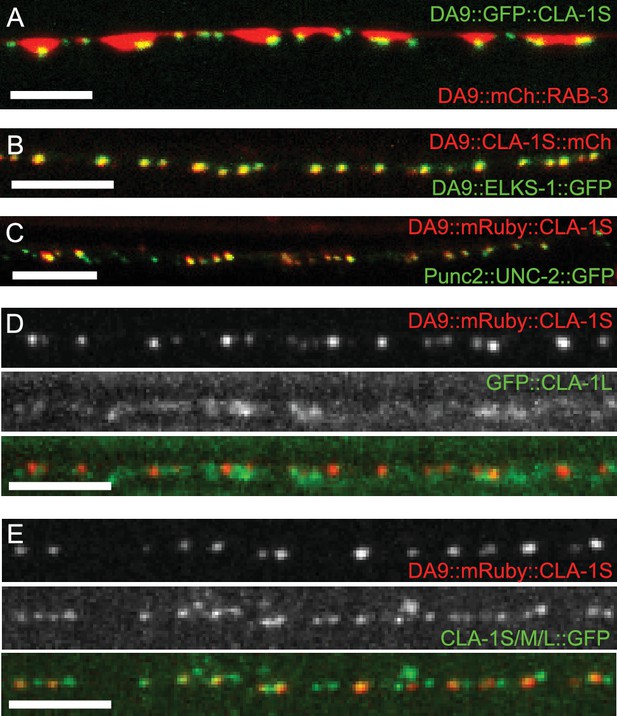
Subcellular localization of CLA-1 proteins.
(A–C) CLA-1S localizes to the active zone. GFP::CLA-1S and mCherry::RAB-3 expressed in DA9 (A) show overlapping expression patterns, with CLA-1S fluorescence limited to a subregion of the RAB-3 domain. CLA-1S::mCherry or mRuby::CLA-1S expressed in DA9 colocalize well with ELKS-1::GFP (B) and the N-type calcium channel UNC-2::GFP (C), respectively. Scale bars = 5 μm. (D) mRuby3::CLA-1S expressed in DA9 along with endogenous expression of N-terminally tagged GFP::CLA-1L. Scale bar = 5 μm. (E) mRuby3::CLA-1S expressed in DA9 along with endogenous expression of C-terminally tagged CLA-1S/M/L::GFP. Scale bar = 5 μm.
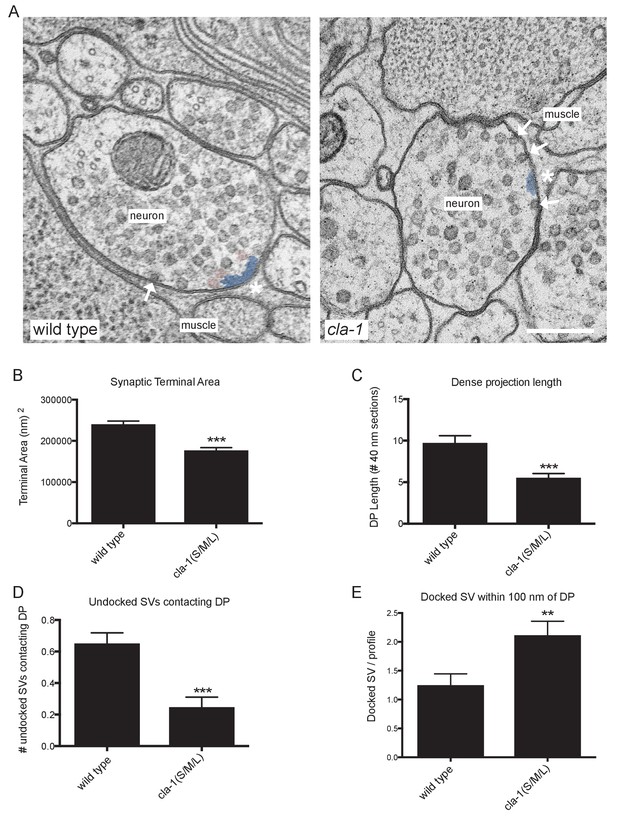
Ultrastructural analysis of cla-1 mutants reveals changes in synaptic vesicle localization.
(A) Representative micrographs of wild type and cla-1(S/M/L) mutant synaptic profiles. Arrows indicate docked vesicles; asterisk indicates the dense projection (DP), which is also colored blue; undocked vesicles touching the dense projection are colored pink. Scale bar = 200 nm. (B) Synaptic terminal area (measured in nm2) is decreased in cla-1(S/M/L) mutants. (C) The length of the dense projection, measured in the number of 40 nm profiles in which it is observed, is decreased in cla-1(S/M/L) mutants. (D) The number of undocked synaptic vesicles directly in contact with the DP is reduced in cla-1(S/M/L) mutants. (E) The number of synaptic vesicles docked at the plasma membrane within 100 nm of the dense projection is increased in cla-1(S/M/L) mutants.
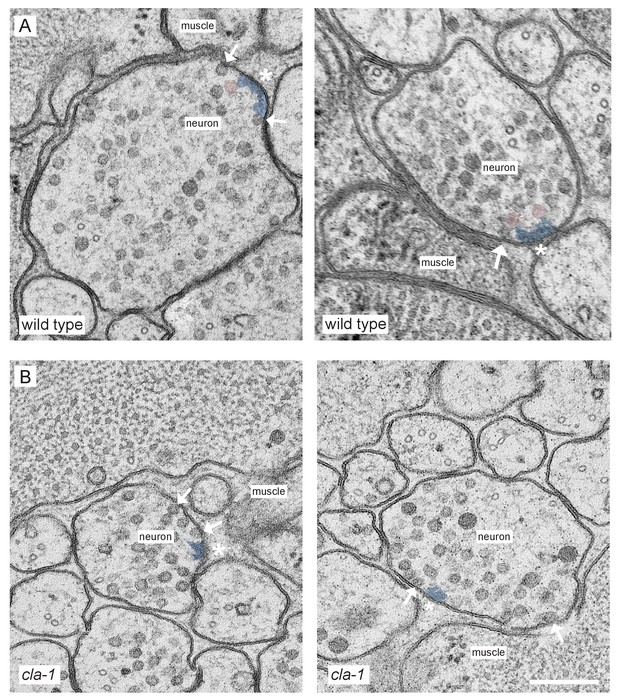
Ultrastructural analysis of cla-1 mutants reveals changes in synaptic vesicle localization.
(A–B) Additional representative EM images of wild type (A) and cla-1(S/M/L) (B) mutant synaptic terminals. Arrows indicate docked vesicles; asterisk indicates the dense projection (DP), which is also colored blue; undocked vesicles touching the dense projection are colored pink. Scale bar = 200 nm.
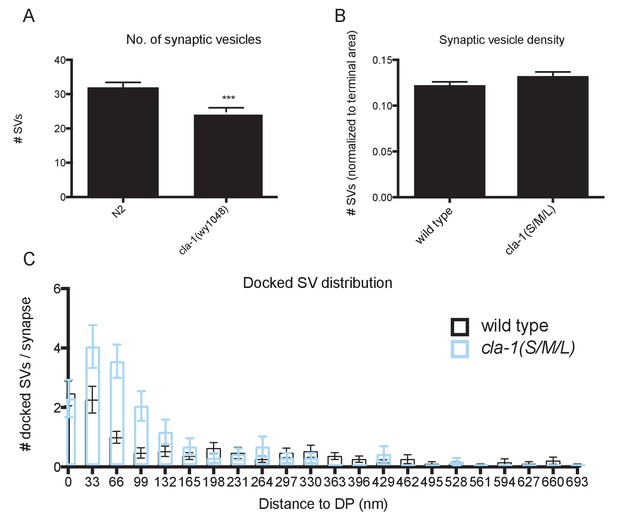
Ultrastructural analysis of cla-1 mutants reveals changes in synaptic vesicle localization.
(A) Total number of synaptic vesicles is reduced in cla-1(S/M/L) mutants. (B) Number of synaptic vesicles normalized to terminal area is unchanged in cla-1(S/M/L) mutants. (C) Distribution of synaptic vesicles docked at the plasma membrane plotted against distance from the dense projection.
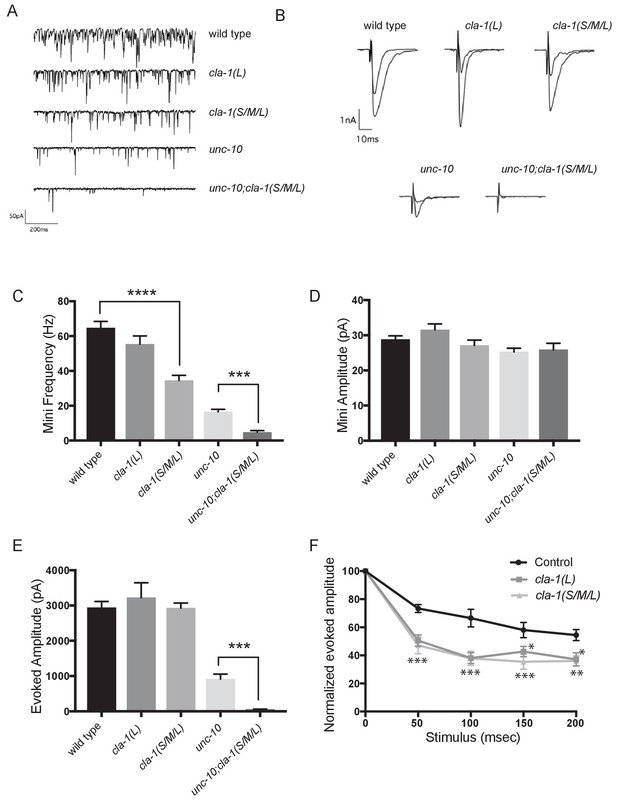
cla-1 mutant animals show defects in synaptic transmission.
(A) Representative traces of endogenous post-synaptic current events. (B) Representative traces of evoked EPSCs, including the first and last recording from a five-stimulus train given at 20 Hz. (C) Frequency of endogenous miniature postsynaptic currents is reduced in cla-1(S/M/L) but not cla-1(L) mutants, compared to wild type. It is also further reduced in cla-1(S/M/L);unc-10 double mutants compared to unc-10 single mutants. (D) Amplitude of endogenous miniature postsynaptic currents is unchanged in cla-1 and unc-10 single and double mutants. (E) The amplitude of electrode-evoked responses to a single stimulus is unchanged in cla-1 mutants compared to wild type, but is reduced in cla-1(S/M/L);unc-10 double mutants when compared to unc-10 single mutants. (F) Normalized amplitude of currents evoked by repeated electrode stimulation (interpulse interval = 50 msec) reveals increased depression in both cla-1(S/M/L) and cla-1(L) mutants.
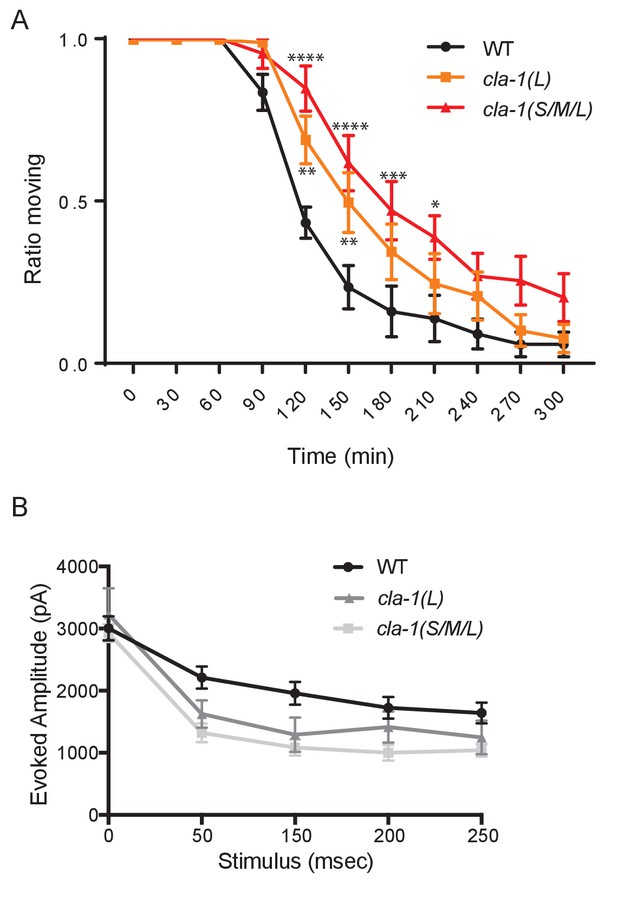
cla-1 mutant animals show defects in synaptic transmission.
(A) Quantification of the ratio of moving worms from each genotype on 1 mM aldicarb at the indicated exposure time reveals aldicarb resistence in both cla-1(S/M/L) and cla-1(L) mutants. Data are from five separate blinded experiments with ~25 animals per experiment (see Materials and methods). (B) Current amplitude (non-normalized) evoked by repeated electrode stimulation (interpulse interval = 50 msec).
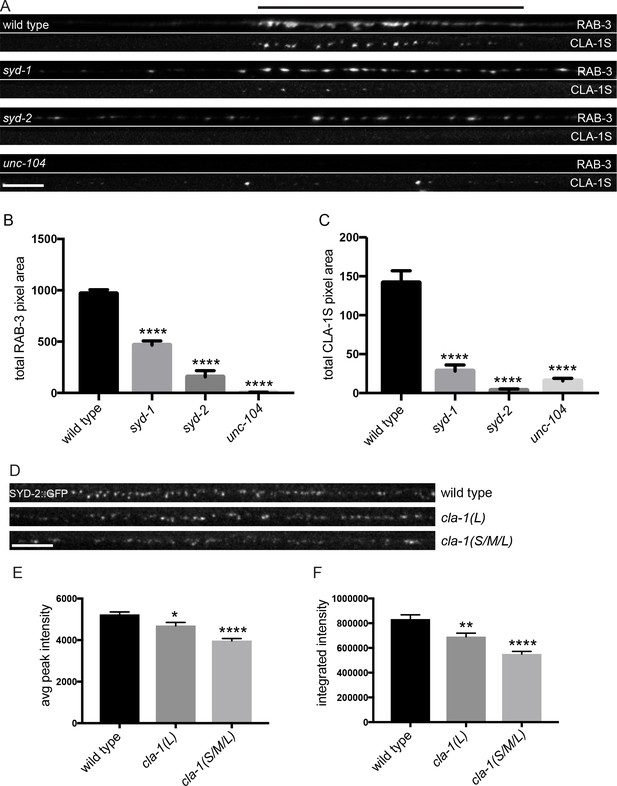
CLA-1S synaptic localization is regulated by UNC-104/Kinesin-3, SYD-2/liprin-α and SYD-1.
(A) CLA-1S::GFP and mCherry::RAB-3 expression in the DA9 motor neuron of the indicated genotypes. syd-1 and syd-2/liprin-α mutants exhibit smaller synaptic vesicle clusters that are distributed throughout the axon, and greatly reduced (in syd-1) or absent (in syd-2) CLA-1S puncta. No synaptic vesicles are detected in unc-104 mutant axons, while the number of CLA-1S puncta is greatly diminished. Scale bar = 5 μm. Line above images indicates wild type synaptic domain. (B) Average total pixel area of mCherry::RAB-3 for wild type animals and various mutants. (C) Average total pixel area of CLA-1S::GFP for wild type animals and various mutants. (D) Endogenous SYD-2::GFP expression in motor neurons of the posterior dorsal nerve cord is reduced in cla-1 mutants. Scale bar = 5 μm. (E) Average SYD-2::GFP puncta intensity is reduced in cla-1 mutants. (F) SYD-2::GFP total (integrated) intensity normalized to length is reduced in cla-1 mutants.
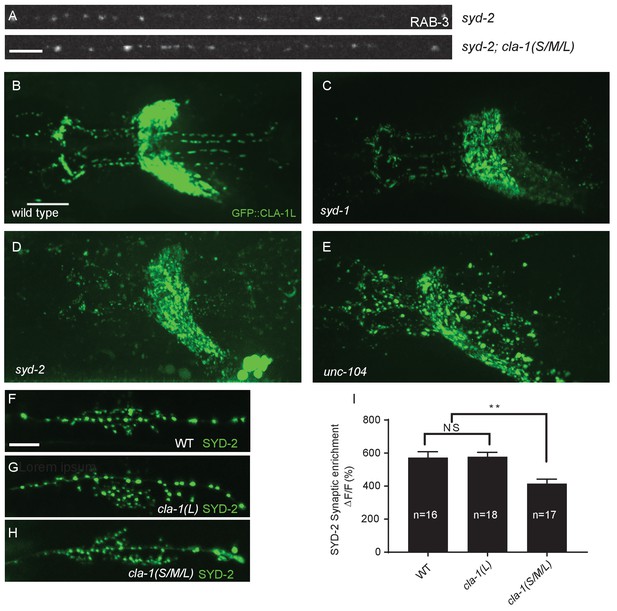
CLA-1L synaptic localization is regulated by UNC-104/Kinesin-3, SYD-2/liprin-α and SYD-1.
(A) Synapse assembly as assessed by GFP::RAB-3 puncta distribution and intensity is unchanged between syd-2 single mutants and syd-2;cla-1(S/M/L) double mutants. Scale bar = 5 μm. (B–E) The pattern of GFP::CLA-1L fluorescence in the nerve ring is perturbed in syd-1 (C), syd-2 (D) and unc-104 (E) mutants compared to wild type (B) animals. Scale bar = 10 μm. (F–H) Active zone marker SYD-2::GFP in the NSM ventral neurite in wild type (F), cla-1(L) (G) and cla-1(S/ML) (H). Scale bar = 5 μm. (I) Synaptic enrichment (ΔF/F) of SYD-2::GFP in NSM is reduced in cla-1(S/M/L) but not cla-1(L) mutants compared to wild type. n = number of animals.
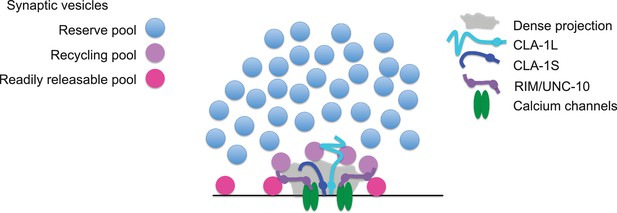
Model for how CLA-1 isoforms may mediate distinct synaptic vesicle interactions.
A model for how CLA-1 S (dark blue) and L (turquoise) isoforms and RIM/UNC-10 (purple) may be organized at the active zone and interact with synaptic vesicles. CLA-1S localizes to the dense projection. The C-terminus of CLA-1L is also anchored at the dense projection, but its N-terminus extends out into the synaptic bouton. Interaction of CLA-1L with synaptic vesicles may facilitate their clustering at the dense projection and release upon repeated stimulation.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29276.019





