Impact of nucleic acid and methylated H3K9 binding activities of Suv39h1 on its heterochromatin assembly
Figures
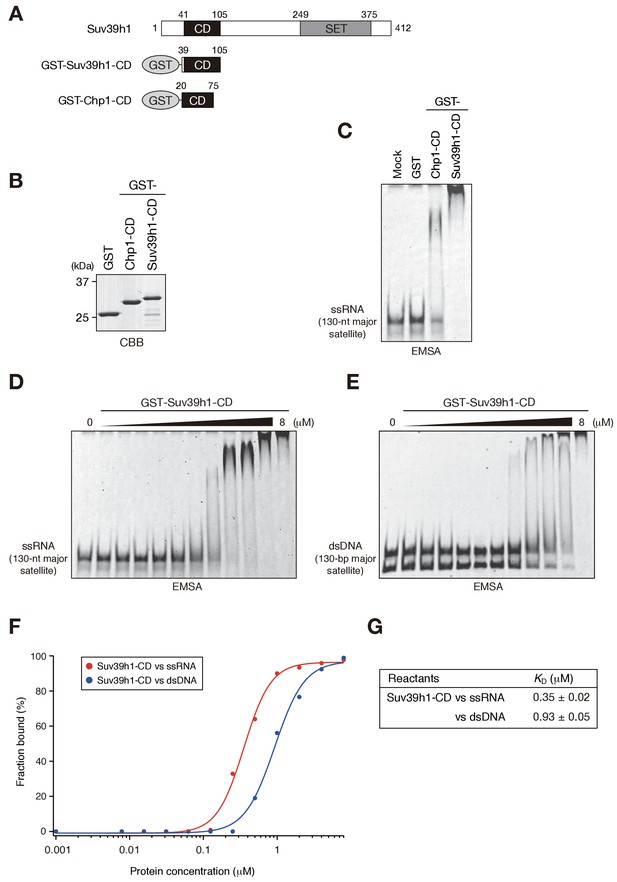
Suv39h1-CD can bind nucleic acids.
(A) Schematic of full-length Suv39h1, GST-fused Suv39h1-CD (39–105), and S. pombe Chp1-CD (20–75). (B) The recombinant GST-fused proteins used in (C); these proteins were visualized by CBB staining. (C) An EMSA using GST-fused proteins. 8 μM of GST or GST-fusion was used in one assay. Fluorescein-labeled 130-nt major satellite ssRNA was used as a probe. (D and E) Titration EMSAs using GST-Suv39h1-CD (0–8 μM with 0.5-fold dilutions) incubated with (D) 130-nt ssRNA or (E) 130 bp dsDNA. DNA probe was detected as doublet bands in gels, because the number of the fluorescent dye, which was conjugated at the single or both 5’-ends, could slightly affect the migration. (F) The binding isotherm of Suv39h1-CD to ssRNA and dsDNA; plots were calculated from the unbound fractions. (G) The dissociation constants measured by titration ssRNA and dsDNA EMSA experiments (D and E).
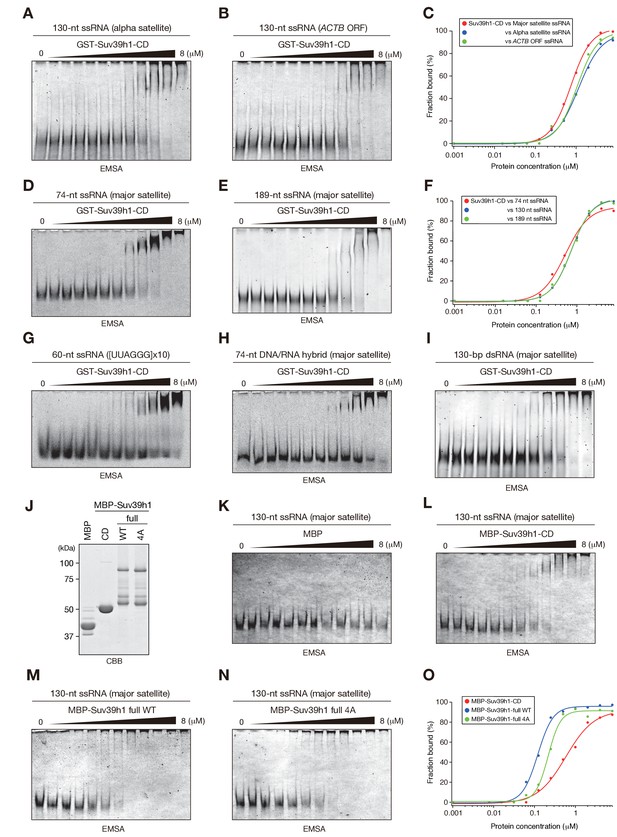
Characteristics of Suv39h1-full-length and -CD’s RNA binding.
(A), (B), (D), (E), and G–I) EMSAs were conducted with GST-fused Suv39h1-CD (39–105) and the following probes: (A) 130-nt alfa satellite ssRNA, (B) 130-nt ACTB mRNA, (D) 74-nt major satellite ssRNA, (E) 189-nt major satellite ssRNA, (G) 60-nt (UUAGGG)x10 ssRNA, (H) 74-nt major satellite DNA/RNA hybrid, or (I) 130-nt major satellite dsRNA. (C and F) The binding isotherms of Suv39h1-CD to various ssRNA probes. (J) MBP-fusion proteins used in (K–N) were resolved by SDS-PAGE and visualized by Coomassie staining. (K–N) EMSAs were conducted with MBP (K), MBP-fused Suv39h1-CD (39-105) (L), MBP-fused full-length Suv39h1-WT (M) or 4A (N) using 130-nt major satellite ssRNA as the probe. (O) The binding isotherms of MBP-fused Suv39h1 proteins to 130-nt major satellite ssRNA.
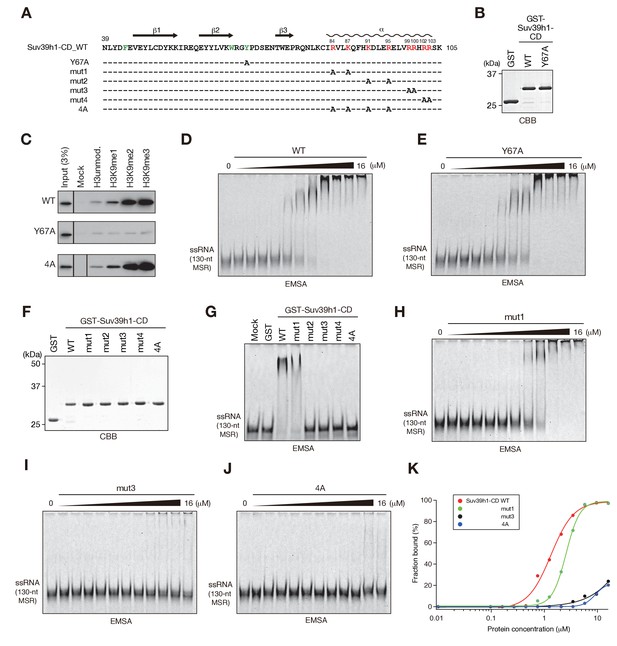
The residues required for Suv39h1-CD to bind RNA.
(A) The alignment of wild-type (WT) and mutant Suv39h1-CD (39–105). The upper lines indicate secondary structure. The positively charged amino acids in the C-terminal α-helix are indicated in red. The aromatic cage residues responsible for Suv39h1-CD’s recognition of H3K9me3 were indicated in green. (B, F) The recombinant proteins used in (C–E and G–J); the proteins were visualized by CBB staining. (C) An in vitro peptide-binding assay using wild-type (WT) and mutant (Y67A or 4A) Suv39h1-CDs. Biotin-tagged H3 (1–21) peptide (100 pmol) was incubated with GST-Suv39h1-CD (1 pmol), and the pulled-down proteins were analyzed by western blotting with an anti-GST antibody. (D, E, H–J) Titration EMSAs using serially diluted GST-fused WT or mutant Suv39h1-CD. (G) An EMSA using GST-fused WT or mutant Suv39h1-CD. (K) Binding isotherms of WT and mutant Suv39h1-CD proteins for ssRNA.
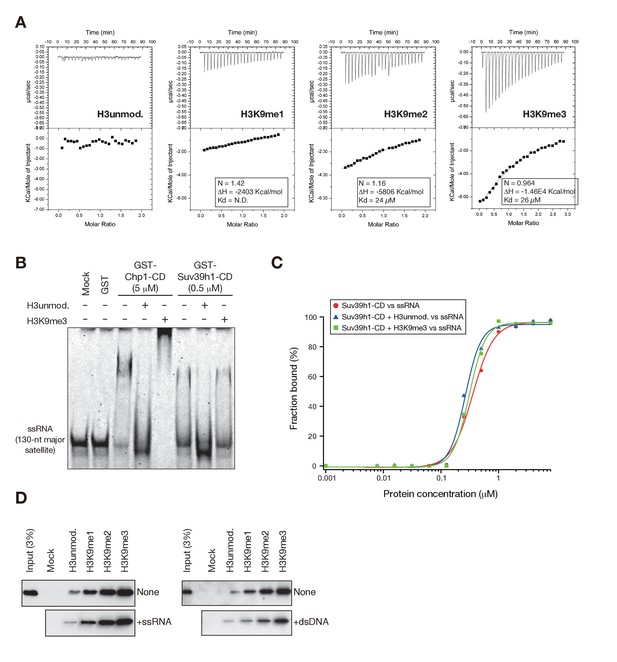
Suv39h1-CD’s RNA binding is independent of its H3K9me3 recognition.
(A) Isothermal titration calorimetry (ITC) showing the binding affinities of GST-Suv39h1-CD (39–96) for unmodified H3K9, H3K9me1, H3K9me2, and H3K9me3 peptides. (B) EMSAs using GST-fused S. pombe Chp1-CD and Suv39h1-CD; GST-fused proteins were incubated with a 130-nt ssRNA probe in the presence or absence of equal molar quantities of unmodified and K9-trimethylated H3 peptides. (C) Binding isotherms of GST-Suv39h1-CD and ssRNA in the presence or absence of equal molar amounts of the indicated H3 peptides. (D) In vitro peptide-binding assay using WT Suv39h1-CD in the presence of major satellite ssRNA or dsDNA.
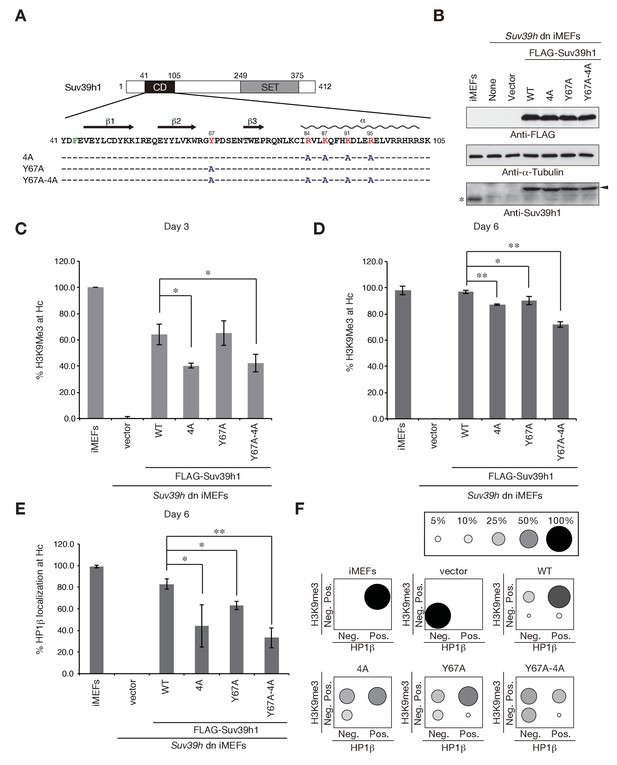
Suv39h1-CD’s nucleic acid binding and H3K9me binding are both crucial for pericentric heterochromatin assembly.
(A) Alignment of wild-type (WT) and mutant full-length Suv39h1. Boxes outline the chromodomain (shaded black), and the SET domain (shaded gray). (B) Suv39h1 protein expression level. *Endogenous Suv39h1; arrowhead: FLAG-Suv39h1. (C–D) Kinetic comparison of the appearance ratio of the cells containing H3K9me3+ DAPI-dense foci after induction of WT and mutant Suv39h1 in Suv39h dn iMEFs. Immunohistochemical staining for H3K9me3 in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged 4A, Y67A, Y67A-4A, or WT Suv39h1 at (C) 3 days or (D) 6 days post-infection. The H3K9me3-positive cells within a population of 100 or more cells were counted (n = 3, mean ± SD, *p<0.05, **p<0.001). (E) The rate of HP1β heterochromatin accumulation in WT and mutant Suv39h1 during heterochromatin establishment. Immunohistochemical staining for HP1β in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged 4A, Y67A, Y67A-4A, or WT Suv39h1 at 6 days post-infection. (F) HP1β accumulated at heterochromatin in cells where H3K9me3 was restored. The radii of the circles indicate the number of cells in each category. Immunohistochemical staining for HP1β in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged 4A, Y67A, Y67A-4A, or WT Suv39h1 at 6 days post-infection. The cells with HP1β heterochromatin accumulation within a population of 100 cells or more were counted (n = 3, mean ± SD, *p<0.05, **p<0.005). The radii of the circles indicate the % ratio of cells in each category.
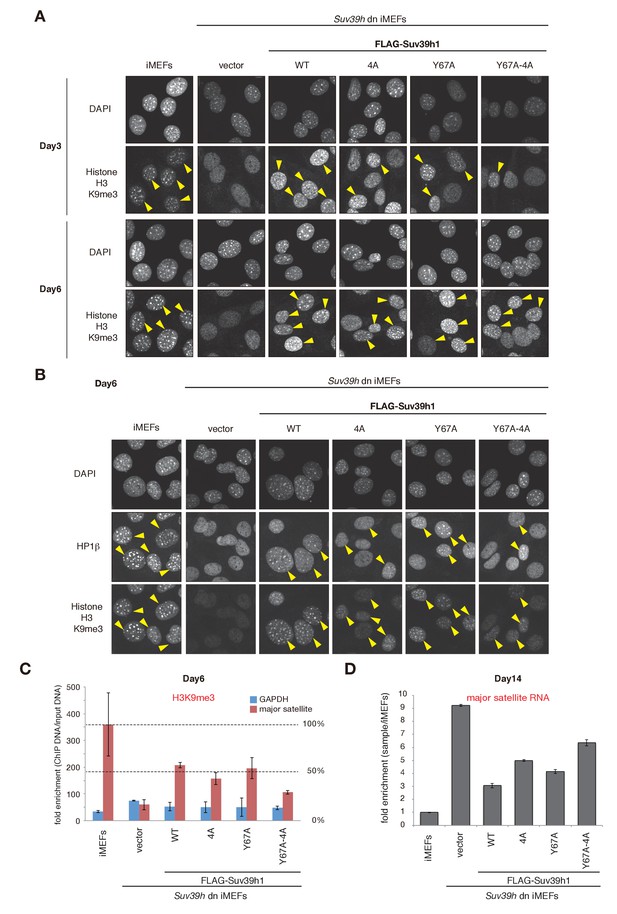
H3K9me3 or HP1β in wild-type and mutant Suv39h1 during heterochromatin assembly.
(A) Immunohistochemical staining for H3K9me3 in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged WT or mutant (4A, Y67A, or Y67A-4A) Suv39h1 at 3 and 6 days post-infection. Arrowheads indicate H3K9me3 fluorescence signals. (B) HP1β and H3K9me3 in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged WT or mutant (4A, Y67A, or Y67A-4A) Suv39h1 during heterochromatin assembly, at 6 days post-infection. An anti-HP1β antibody (BMP002: MBL) and anti-H3K9Me3 antibody (clone 2F3) were used for co-immunofluorescence. Arrowheads indicate HP1β heterochromatin accumulation. (C) Native chromatin immunoprecipitation (ChIP) assays showing H3K9me3 enrichment at major satellite repeats in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged WT or mutant (4A, Y67A, or Y67A-4A) Suv39h1 at 6 days post-infection (n = 2, mean ± SD,). Anti-H3K9me3 antibody (clone 2F3) was used. (D) qRT-PCR assays for major satellite RNA transcripts in Suv39h dn iMEFs expressing FLAG-tagged WT or mutant (4A, Y67A, or Y67A-4A) Suv39h1 at 14 days post-infection. Representative results of two independent biological replicates are shown.
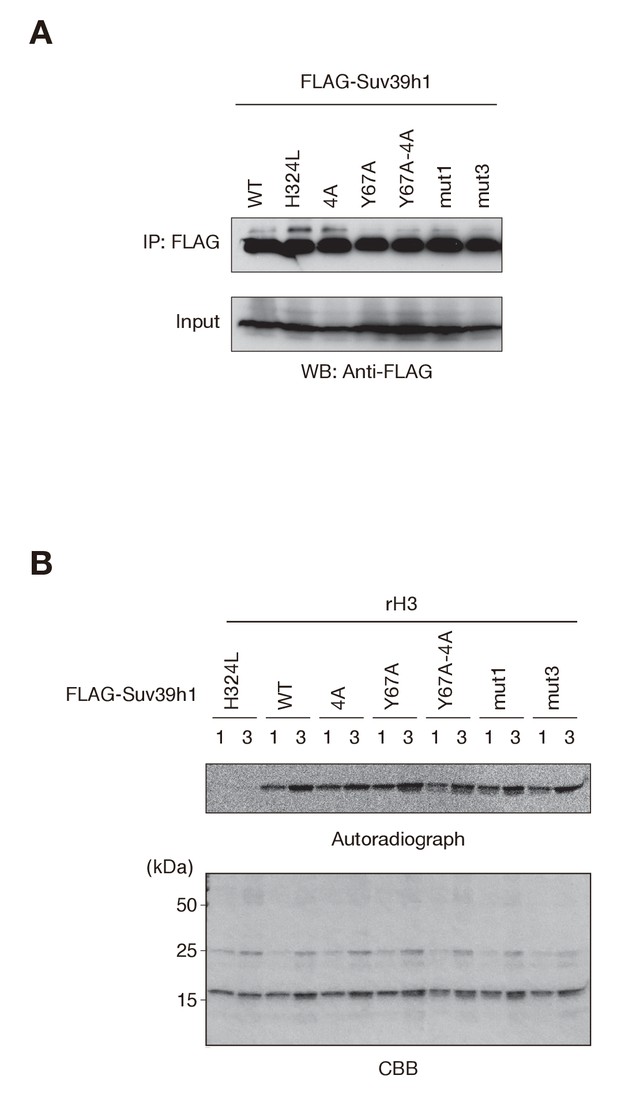
Comparable histone H3 methylation by wild-type and mutant Suv39h1 in vitro.
(A) Western blots of purified FLAG-tagged wild-type (WT) or mutant (mut) Suv39h1. (B) FLAG-tagged WT or mutant Suv39h1 was incubated with 14C-labeled SAM. The proteins were separated by SDS-PAGE and stained with Coomassie Blue (bottom). Autoradiography was detected with the BAS-5000 Image Analyzer (top).
Cell proliferation rates of Suv39h dn iMEFs were not changed by expressing FLAG-tagged WT or mutant Suv39h1.
Suv39h dn iMEFs stained by CellTrace Far Red Cell Proliferation Kit were infected with retroviruses for FLAG-Suv39h1 (wt or mutant)-IRES-GFP. Cell proliferation rates were analyzed by FACS. This panel shows representative results of two independent experiments.
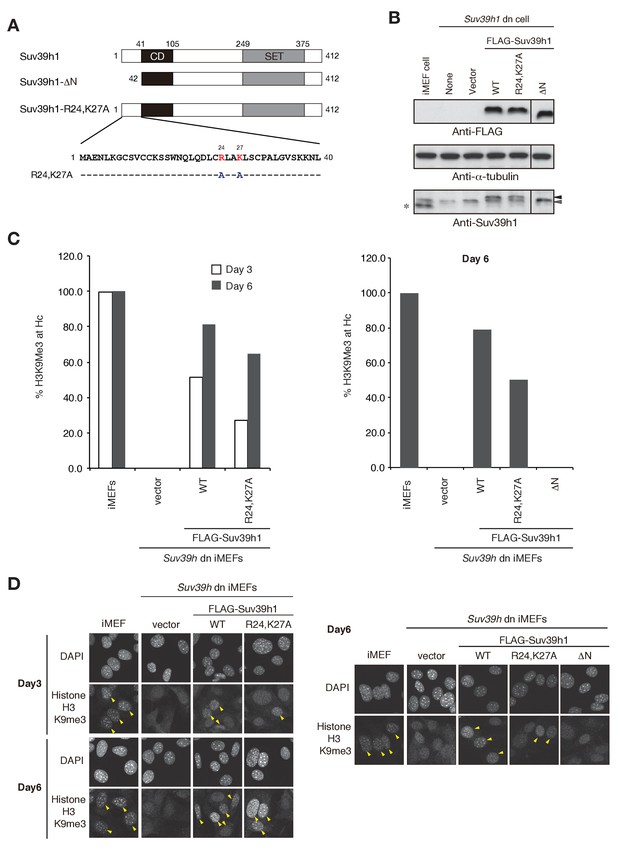
Suv39h1’s N-terminal region also contributes to Suv39h1-mediated pericentric H3K9me3 formation.
(A) Alignment of wild-type (WT) and mutant full-length Suv39h1. Boxes outline the chromodomain (shaded black), and the SET domain (shaded gray). (B) Suv39h1 protein expression level. *Endogenous Suv39h1; black arrowhead: FLAG-Suv39h1 WT or R24,K27A; gray arrowhead: FLAG-Suv39h1-DN. (C) Comparison of the rate of increase of H3K9me3 in WT and mutant Suv39h1 during the establishment of heterochromatin. Immunohistochemical staining for H3K9me3 in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged R24,K27A, DN, or WT Suv39h1 at 3 days (white bar) or 6 days (gray bar) post-infection. The H3K9me3-positive cells within a population of 100 or more cells were counted. Right and left panel show representative results of two independent experiments. (D) Immunohistochemical staining for H3K9me3 in WT iMEFs, Suv39h dn iMEFs, or Suv39h dn iMEFs expressing FLAG-tagged WT or mutant (R24,K27A or DN) Suv39h1 at 3 or 6 days post-infection. Arrowheads indicate H3K9me3 fluorescence signals. Right and left panel show representative results of two independent experiments.
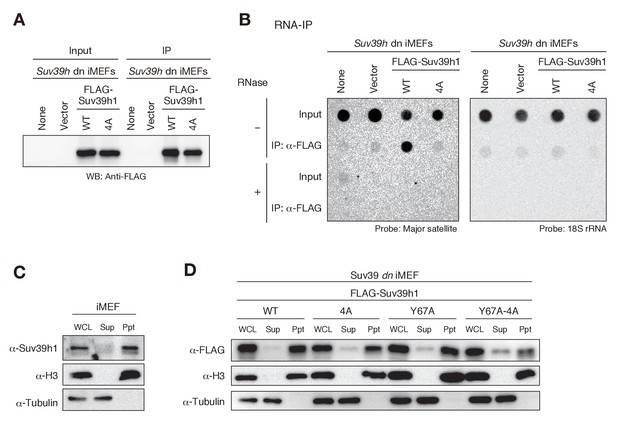
Suv39h1-CD’s nucleic acid binding is required for its interaction with major satellite RNAs in vivo.
(A) Whole cell lysates (input) of Suv39h dn iMEFs expressing FLAG-tagged wild-type (WT) or mutant (4A) Suv39h1 and FLAG immunoprecipitates (IP) were subjected to immunoblotting using an anti-FLAG M2 antibody. Parental Suv39h dn iMEFs (none) and Suv39h dn iMEFs with an empty vector (vector) were used as controls. (B) Dot-blot analysis of immunoprecipitated RNAs. RNAs associated with WT or mutant (4A) Suv39h1 in Suv39h dn iMEFs were precipitated with the anti-FLAG M2 antibody and subjected to dot-blot analysis using a labeled probe for major satellite repeats (left) and 18S rRNA (right). (C, D) Chromatin fractionation assays were performed using control iMEFs (C) and Suv39h dn iMEFs expressing FLAG-tagged WT or mutant Suv39h1 (D). Soluble (Sup) and insoluble chromatin-enriched (Ppt) fractions were resolved by SDS-PAGE and analyzed by immunoblotting. For comparison, anti-α-tubulin and anti-histone H3 antibodies were used to detect soluble- and chromatin-associated proteins, respectively.
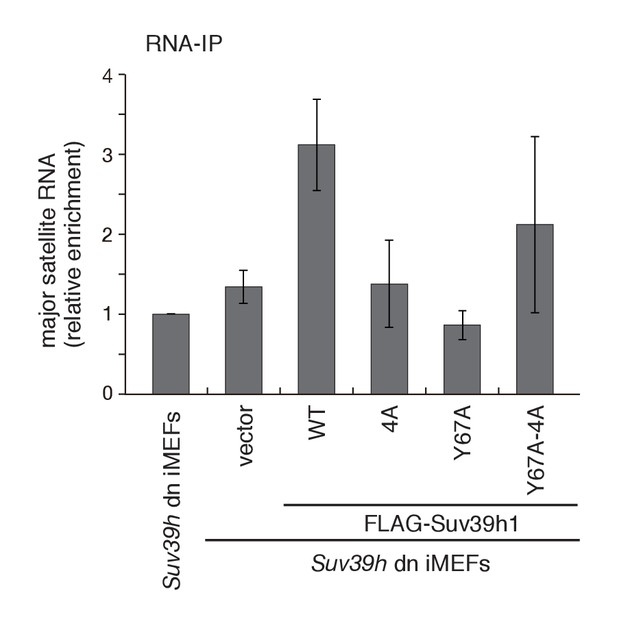
Both Suv39h1-CD’s nucleic acid- and H3K9me3 binding activities are required for its interaction with major satellite RNAs in vivo.
RNA immunoprecipitation (RNA-IP) experiments using parental Suv39h dn iMEFs and Suv39h dn iMEFs expressing FLAG-tagged WT or mutant (4A, Y67A, or Y67A-4A) Suv39h1 (n = 3, mean ± SD). An anti-FLAG M2 antibody was used to precipitate Suv39h1-interacting RNAs.
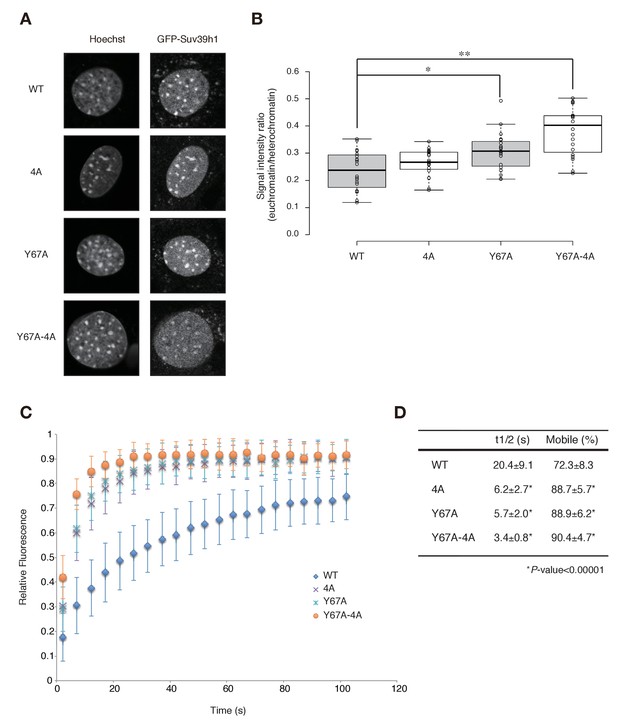
Both Suv39h1-CD’s nucleic acid binding and H3K9me binding contribute to Suv39h1’s retention on heterochromatin.
(A) Subcellular localization of Hoechst staining and wild-type (WT) or mutant GFP-Suv39h1 in Suv39h dn iMEF cells. (B) Box plot showing the signal-intensity ratio between euchromatin and heterochromatin. Center lines show the medians, box limits indicate the 25th and 75th percentiles as determined by R software, whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, and outliers are represented by dots; n = 20 sample points. (*p<0.005, **p<0.00005) (C) FRAP analysis of WT or mutant GFP-Suv39h1 in Suv39h dn iMEFs. The means of the relative intensity in the bleached area are indicated with the SD (n ≥ 29). (D) Quantification and statistical analysis of the FRAP analysis in C; and the mobile population fraction (mobile) and half-time of fluorescence recovery (t1/2).
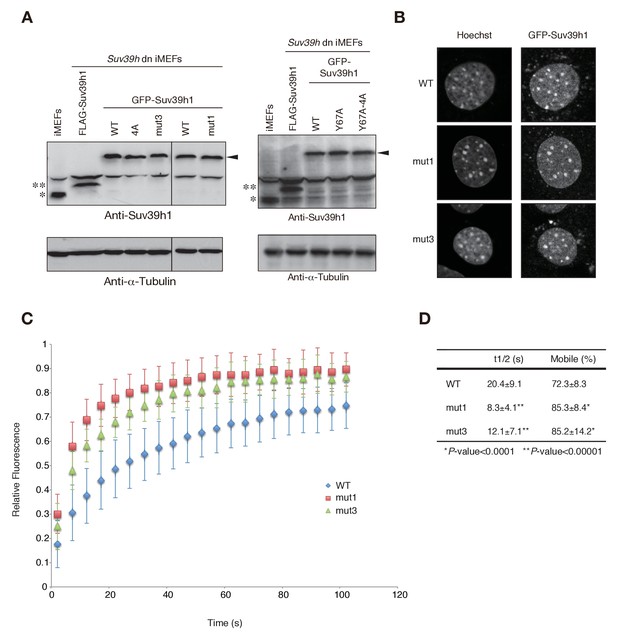
Wild-type and mutant GFP-Suv39h1 analysis.
(A) Protein levels of wild-type (WT) or mutant GFP-Suv39h1. *Endogenous Suv39h1; **FLAG-Suv39h1. Arrowhead: GFP-Suv39h1. (B) The subcellular localization of Hoechst staining and WT, mut1, and mut3 GFP-Suv39h1 in Suv39h dn iMEF cells. (C) FRAP analysis of WT, mut1, and mut3 GFP-Suv39h1 in Suv39h dn iMEF cells. The means of the relative intensity in the bleached area are indicated with the SD (n ≥ 11). (D) Quantification and statistical analysis of the FRAP analysis in C, showing the mobile population fraction (mobile) and fluorescence recovery half-time (t1/2).
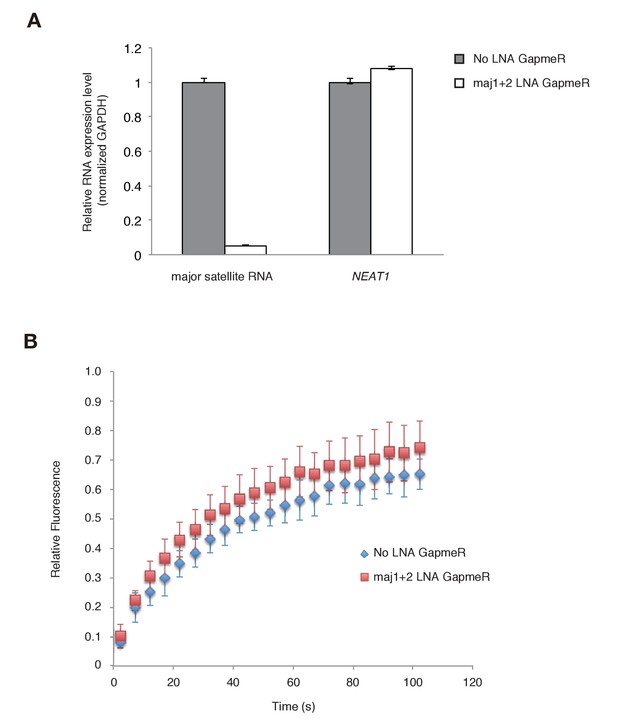
Major satellite RNAs contribute to Suv39h1’s retention on heterochromatin.
(A) qRT-PCR for major satellite RNAs or nuclear non-cording RNA NEAT1 transcripts in Suv39h dn iMEFs expressing GFP-suv39h1 transfected without or with a set of two LNA-DNA GapmeRs directed against the Forward and Reverse major satellite transcripts. (B) FRAP analysis of GFP-Suv39h1 in the described cells (A). The means of the relative intensity in the bleached area are indicated with the SD (n ≥ 13).
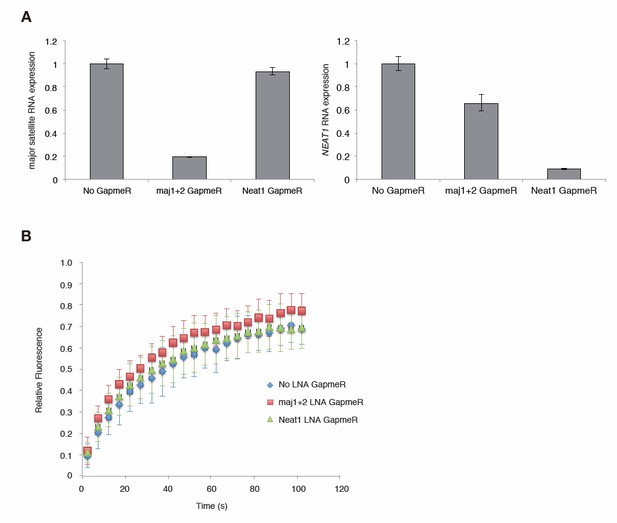
Major satellite RNAs contribute to Suv39h1’ s retention on heterochromatin.
(A) qRT-PCR for major satellite RNAs or nuclear non-cording RNA NEAT1 transcripts in Suv39h dn iMEFs expressing GFP-suv39h1 transfected without or with LNA-DNA GapmeRs directed NEAT1 transcript or set of two LNA-DNA GapmeRs directed against the Forward and Reverse major satellite transcripts. (B) FRAP analysis of GFP-Suv39h1 in the described cells (A). The means of the relative intensity in the bleached area are indicated with the SD (n ≥ 13).





