YAP/TAZ initiate and maintain Schwann cell myelination
Figures
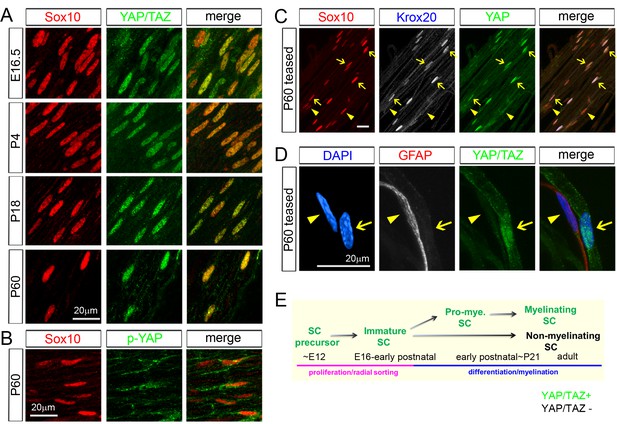
YAP/TAZ are largely nuclear in proliferating, differentiating and mature myelinating SCs.
YAP/TAZ localization was investigated in sciatic nerve cryosections (A, B) or teased fibers (C, D). SC nuclei are marked by Sox10 (red) or DAPI (blue). (A) YAP/TAZ (green) are in SC nuclei throughout development, from E16.5 to P60. (B) YAP phosphorylated on Ser 112 (p-YAP, green) is largely absent from SC nuclei at P60. (C) Co-localization of YAP (green) in the nuclei of myelinating SCs marked by Krox20 (white) at P60. Arrows point to mSC nuclei that contain YAP and Krox20; arrowheads point to non-myelinating SC nuclei, which lack Krox20 and YAP. (D) YAP/TAZ (green) are present in the nucleus of a mSC, associated with a large axon, but is absent from the nucleus of a non-myelinating SC, marked by GFAP (red), at P60. Arrow points to myelinating SC nucleus; arrowhead points to non-myelinating SC nucleus. (E) Diagram summarizing the developmental stages in which YAP/TAZ are present or absent from the SC nucleus. The following figure supplements are available for Figure 1.
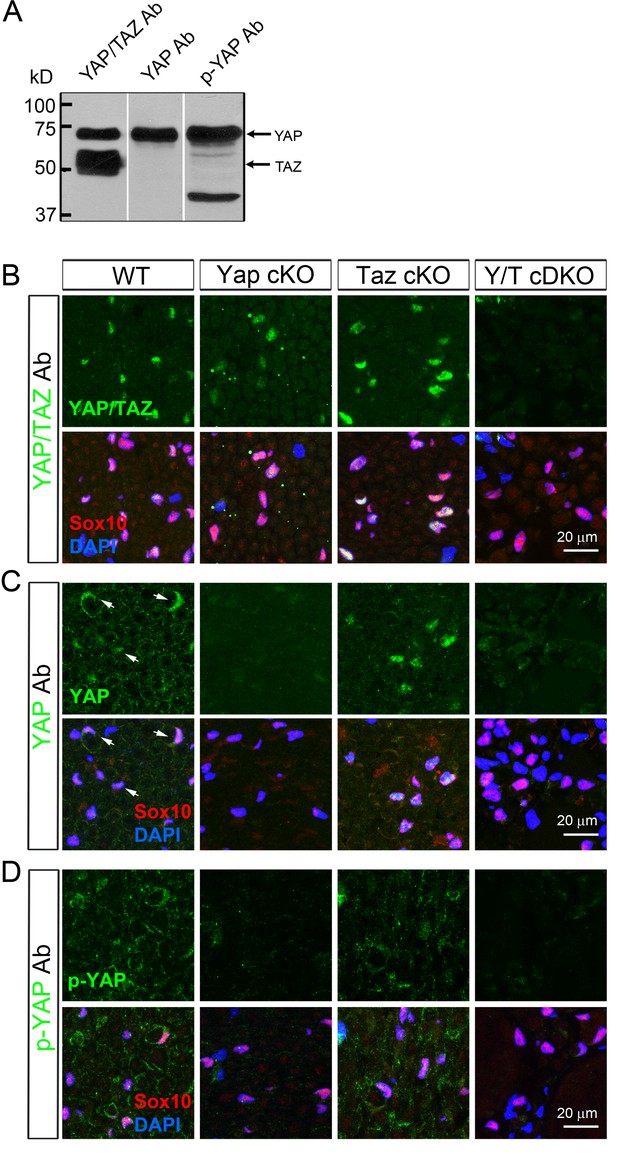
Selection of specific antibodies for immunohistochemical analysis of YAP and TAZ.
(A) Western blotting of WT P23 sciatic nerve lysates, showing three different antibodies specifically binding to both YAP/TAZ, only YAP, and only to phosphorylated YAP. (B–D) These antibodies were further tested in transverse sciatic nerve sections of adult WT, Yap cKO, Taz cKO and Yap/Taz cDKO mice. Sections were co-stained with anti-Sox10 (red) to mark SC nuclei, and DAPI to label all cellular nuclei. (B) An antibody specifically recognizing both YAP and TAZ. Its immunoreactivity completely disappears only in cDKO SCs. (C) An antibody selectively binding to YAP. Its immunoreactivity is eliminated in YAP cKO SCs and Yap/Taz cDKO SCs, but not in Taz cKO SCs. Arrows in WT denote examples of SC nuclei exhibiting YAP immunoreactivity. (D) An antibody (S112) selectively binding to phosphorylated YAP (p-YAP). Its immunoreactivity is largely cytoplasmic in WT, and is eliminated in Yap cKO SCs and Yap/Taz cDKO SCs, but not in Taz cKO.
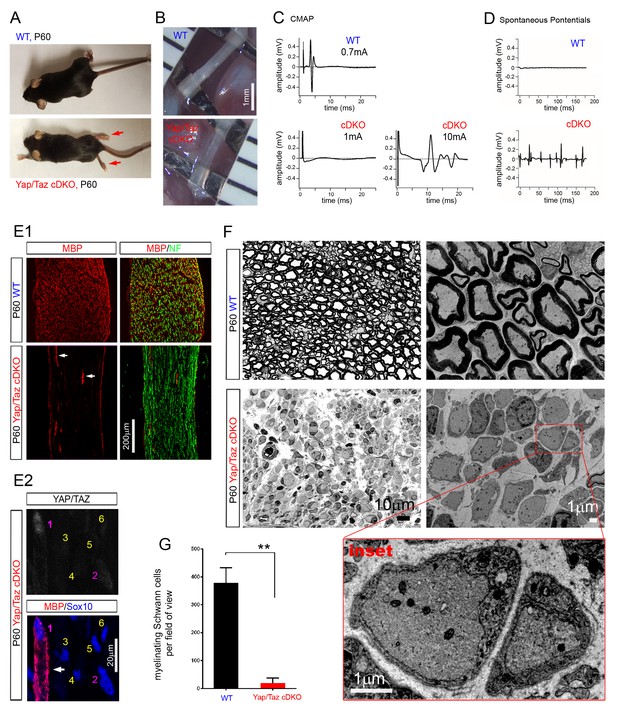
YAP/TAZ are required for myelination of sorted axons.
(A) Living control and Yap/Taz DKO mice, showing splayed paralyzed hindlimbs (arrows) and muscle wasting of P60 Yap/Taz cDKO mice. (B) Control sciatic nerve is white and opaque, while cDKO sciatic nerve is translucent. (C) CMAPs generated by stimulation of the sciatic nerve. (D) Spontaneously generated abnormal potentials in cDKO footpad muscles. (E1, E2) Low and high magnification views of longitudinal sections of WT and P60 cDKO sciatic nerves. Arrows denote mSCs exhibiting strong MBP and YAP/TAZ immunoreactivity. (E1) cDKO nerves rarely contain mSCs (arrows). mSCs are marked with MBP (red), and axons are marked with neurofilament (green). (E2) An example of an mSC (ie, MBP+) in P60 cDKO, which shows strong YAP/TAZ immunoreactivity (#1 SC; arrow). Note that adjacent SCs show low (#2 SC) or no YAP/TAZ immunoreactivity (#3–6 SCs), and they do not express MBP. (F) Left half panels: semi-thin transverse sciatic nerve sections showing mSCs in control but not in cDKO. Right half panels: TEM of transverse nerve sections showing myelination of large axons in control (upper panel), but many large axons arrested at the 1:1 promyelinating stage in cDKO. Lower panel: Inset of the same cDKO panel, showing two large axons surrounded by cDKO SCs arrested at the promyelinating stage. (G) Quantification of myelinating Schwann cells per field of view in WT and cDKO semi-thin sections. n = 3 mice per genotype. **p=0.0034, unpaired Student’s t-test. The following figure supplements are available for Figure 2.
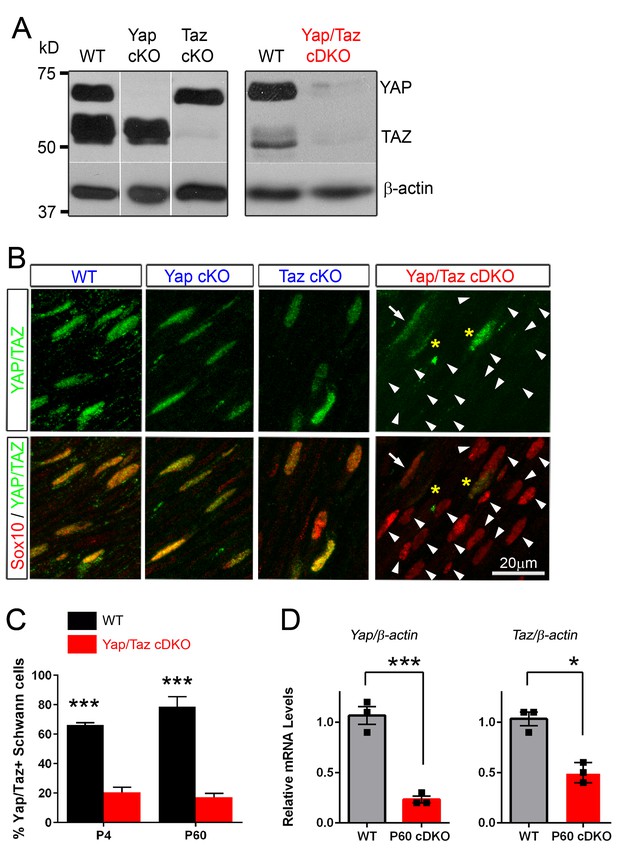
Recombination efficiency in Yap cKO, Taz cKO and Yap/Taz cDKO.
(A) Western blotting of sciatic nerve lysates from P60 mice of the indicated genotypes, showing efficient, selective deletion of YAP in Yap cKO, TAZ in Taz cKO, and both YAP/TAZ in Yap/Taz cDKO. Blots in the left panels were exposed for longer than the blot in the right panels. (B) Immunohistochemical analysis showing SC nuclei (red) of Yap cKO and Taz cKO that retain YAP/TAZ immunostaining (green) because SCs express both YAP and TAZ. YAP/TAZ are completely eliminated from most cDKO SCs (arrowheads); occasional SCs exhibit low or faint YAP/TAZ immunoreactivity (for example, arrow). Asterisks indicate non-Schwann cells with YAP/TAZ immunostaining. (C) Bar graph showing percentage of SCs immunopositive for YAP/TAZ at P4 and P60 in WT and cDKO. Most SCs counted as immunopositive in cDKO exhibited only weak or faint YAP/TAZ immunostaining. n = 2 per genotype (P4 WT, P60 WT, cDKO) or n = 3 per genotype (P4 cDKO) mice per genotype. P4: ***p=0.0007; P60: p=0.0003, 2-way ANOVA with Sidak’s multiple comparison test. (D) Quantitative RT-PCR showing marked reduction of Yap/Taz mRNA expression in cDKO mice. Expression of Yap and Taz is normalized to that of β-actin as an internal control, and WT expression is arbitrarily given the value 1. n = 3 mice of each genotype. ***p=0.0009, *p=0.0215, unpaired Student’s t-test.
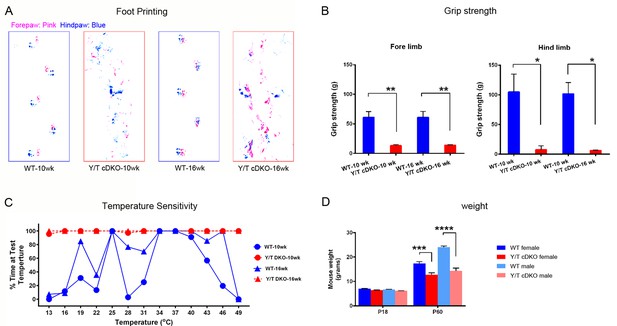
Behavioral tests of motor and sensory function of adult Yap/Taz cDKO mice.
Behavioral tests were conducted on WT or age-matched Yap/Taz cDKO mice. (A) Foot printing showing uncoordinated gait of 10 week- and 16 week-old cDKO mice. (B) Grip strength test, showing markedly impaired motor function in both fore- and hind limbs of 10 week- and 16 week-old cDKO mice. Forelimbs, 10-week: n = 3 mice per genotype, **p=0.0028; 16-week: n = 3 mice per genotype, **p=0.0030. Hindlimbs, 10-week: n = 3 mice per genotype, *p=0.0105; 16-week: n = 3 mice per genotype, *p=0.0113. 2-way ANOVA with Tukey’s multiple comparisons test. (C) Thermosensitivity analysis showing impaired sensory function in 10 week- and 16 week-old cDKO mice. (D) Bar graph showing weight reduction of cDKO mice compared to control mice. n = 4 (P60 WT females, Y/T cDKO females, WT males) or n = 2 (P18 all genotypes, and P60 Y/T cDKO males) mice per genotype, ****p<0.0001, ***p=0.0005, 2-way ANOVA with Tukey’s multiple comparisons test.
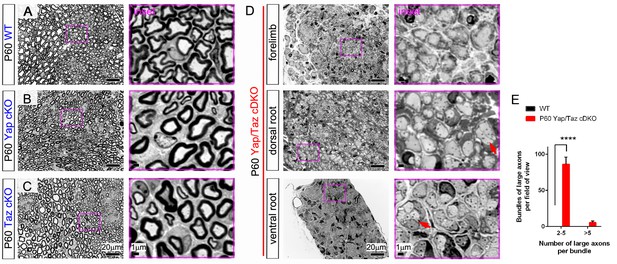
Complete amyelination of forelimb peripheral nerves and nerve roots in P60 Yap/Taz cDKO.
Semi-thin sections of sciatic nerves of WT, Yap cKO and Taz cKO and of forelimb nerve, dorsal root and ventral root of cDKO were stained with toluidine blue. Insets in pink boxes are enlarged areas of left panels. (A) Control sciatic nerve showing normal myelination. (B) P60 Yap cKO nerves showing completely normal myelination. (C) P60 Taz cKO nerves showing nearly normal myelination. Also infrequent small bundles of non-myelinated axons (not shown). (D) A forelimb nerve, dorsal root and ventral root of P60 cDKO showing incomplete but extensive radial sorting and virtual amyelination. Red arrows indicate small bundles of unmyelinated axons frequently observed in cDKO nerves. (E) Quantification of small (2–5 axons) and large (>5 axons) bundles containing large-caliber axons in semi-thin transverse sciatic nerve sections of WT and Yap/Taz cDKO mice at P60. n = 3 mice per genotype. ****p<0.0001, 2-way ANOVA with Sidak’s multiple comparison test.
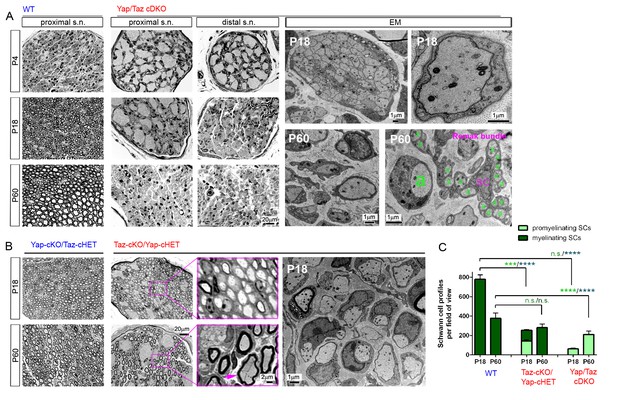
YAP/TAZ are required for timely axon sorting.
(A) Left nine panels: Semi-thin transverse sections of proximal control sciatic nerve and proximal and distal cDKO nerves at P4, P18 and P60. Right four panels: TEM of transverse cDKO nerve sections at P18 and P60. At P18, some immature SCs associate with unsorted axon bundles (left panel), but other SCs are promyelinating (right panel). At P60, many large axons are sorted but arrested at the 1:1 promyelinating stage (left and right panels, marked by large ‘a’), while small axons are fully surrounded by the cytoplasm of non-myelinating SCs in extended Remak bundles. s.n. = sciatic nerve; a = axon; SC = non-myelinating SC. (B) Transverse sections of Yap-cKO/Taz-cHET and Taz-cKO/Yap-cHET sciatic nerves at P18 and P60. Left-most two small panels: Semi-thin sections of Yap-cKO/Taz-cHET. Right-most four small panels: Semi-thin sections of Taz-cKO/Yap-cHET. Large panel: TEM of Taz-cKO/Yap-cHET, showing single large axons associated with SCs transiently arrested at the promyelinating stage. (C) Quantification of myelinating vs promyelinating Schwann cells in transverse semi-thin sections of WT vs Taz cKO/Yap cHET and Yap/Taz cDKO sciatic nerves at P18 and P60. n = 3 mice per genotype, except for WT P60 (n = 2). P value significance is shown in pale green for promyelinating Schwann cells and in dark green for myelinating Schwann cells. ***p<0.001, ****p<0.0001, n.s. = non-significant, 2-way ANOVA with Sidak’s multiple comparison test.
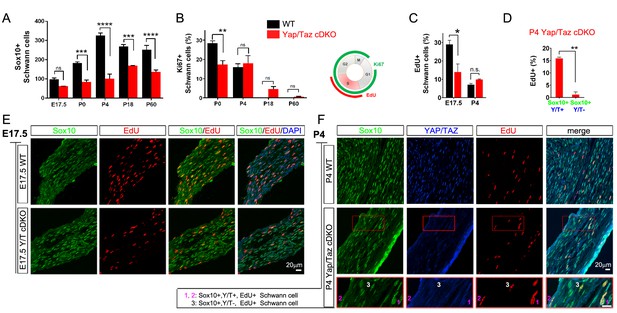
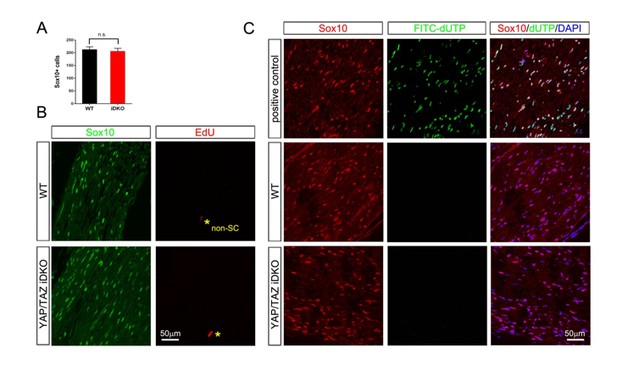
YAP/TAZ are required for proper proliferation of immature SCs.
(A) Quantitation of total SCs (Sox10+ nuclei) per transverse sciatic nerve section in WT and Yap/Taz cDKO mice. n = 3 mice per genotype. ***p<0.001, ****p<0.0001, n.s. = non-significant, 2-way ANOVA with Sidak’s multiple comparison test. (B) Percentage of proliferating SCs as determined by co-staining for Ki67 and Sox10. n = 3 mice per genotype. **p<0.01, n.s. = non-significant, 2-way ANOVA with Sidak’s multiple comparison test. (C) Quantitation of proliferating SCs in longitudinal sciatic nerve sections at E17.5 and P4, as determined by co-staining for EdU and Sox10. n = 3 (E17.5) or 2 (P4) mice per genotype. *p=0.0192, n.s. = non-significant, 2-way ANOVA with Sidak’s multiple comparison test. (D) Quantification of proliferation of SCs in cDKO sciatic nerves at P4; SC nuclei were separately binned as containing detectable YAP/TAZ (Sox10+; Y/T+) or no detectable YAP/TAZ (Sox10+; Y/T-), before counting the % of each subset that was EdU+. n = 2 mice per genotype. **p=0.0044, unpaired Student’s t-test. (E, F) Identification of proliferating SCs in longitudinal WT and cDKO sciatic nerve sections. SC nuclei are marked by Sox10 (green), proliferating cell nuclei by EdU incorporation (red), and total cell nuclei by DAPI (blue). (F, bottom insert panel) Identification of proliferating YAP/TAZ+ and YAP/TAZ- SCs at P4, showing examples of EdU+ (proliferating) SCs with no (#3) or weak (#1,#2) YAP/TAZ immunoreactivity. The following figure supplements are available for Figure 4.
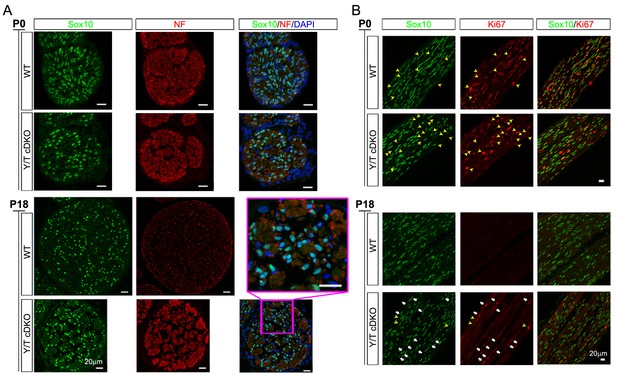
Schwann cell proliferation during postnatal development.
(A) Examples of transverse sections of P0 and P18 WT and cDKO sciatic nerves immunostained to quantify SCs. SC nuclei (green, marked by Sox10). The tibial nerve is delineated by anti-neurofilament (NF) staining (red), and all nuclei in the nerve are identified by DAPI staining (blue). Inset shows close-up of Yap/Taz cDKO tibial nerve at P18. n = 3 mice per genotype; three tibial locations per mouse; three adjacent sections per location. (B) Postnatal proliferation of SCs, marked by Sox10 (green) and assessed by co-staining with anti-Ki67 (red). White arrows denote Ki67+ and Sox10+ SCs in P18 cDKO. Yellow arrowheads denote non-SCs that are Ki67+ (that is, proliferating) in P0 WT, P0 cDKO, and P18 cDKO mice.
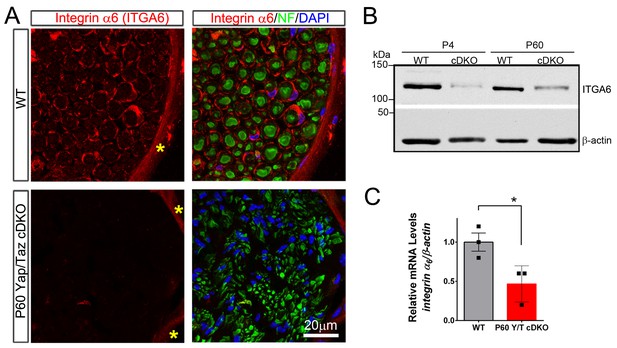
YAP/TAZ are required for integrin α6 expression in Schwann cells.
(A) Transverse sections of P60 WT and Yap/Taz cDKO sciatic nerves immunostained for Integrin α6 (red), neurofilament (green) and DAPI (blue). Integrin α6 localizes to Schwann cells and perineurial cells in WT, but only perineurial cells in Yap/Taz-cDKO. Asterisks denote perineurium. (B) Western blotting of P4 and P60 WT and Yap/Taz cDKO sciatic nerve lysates, using anti-integrin α6, and anti-βactin as a loading control. n = 3 experiments. (C) Quantitative RT-PCR using Integrin α6 and β-actin-specific primers and total RNA isolated from P60 WT and Yap/Taz cDKO sciatic nerves. Expression of Integrin α6 is normalized to that of β-actin as an internal control, and WT expression is arbitrarily given the value 1. n = 3 mice per genotype. *p<0.05, unpaired Student’s t-test.
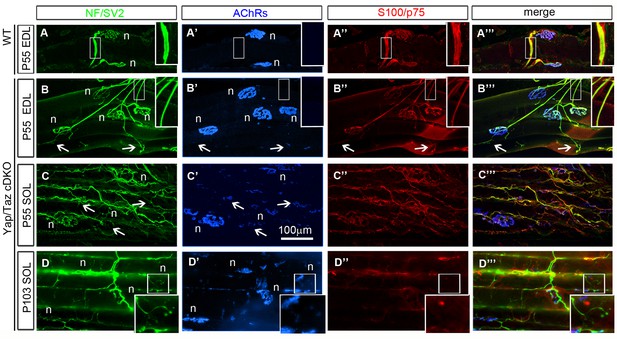
Neuromuscular junctions of Yap/Taz cDKO mice.
(A–A’’’) Two representative junctions from a P55 extensor digitorum longus (EDL) muscle of WT mouse. NF and SV2 immunostaining (green) reveal pre-terminal axons (inset) and axon terminals at neuromuscular junctions (NMJ, (n), also identified by clustered muscle acetylcholine receptors (AChRs, blue). S100 and p75 immunostaining reveals myelinating Schwann cells surrounding pre-terminal axons and terminal Schwann cells that cap axon terminals. (B–B’’’) Four representative NMJs in a P55 EDL of cDKO mouse. All muscle fibers are innervated by axons and SCs showing no signs of degeneration. Pre-terminal axons are thinner than control but remain in contact with cDKO SCs arrested at the promyelinating stage (compare insets). Axon terminals and terminal SCs frequently extend processes that form satellite synaptic contacts (arrows), as commonly observed in paralyzed muscles. (C–C’’’) Three representative NMJs in a P55 soleus muscle of cDKO mouse. Terminal sprouting and satellite extrasynaptic contacts are much more extensive in a slow-twitch soleus muscle. Nevertheless, cDKO SCs remain tightly associated with pre-terminal axons and terminal sprouts, showing their ability to extend processes and survive. (D–D’’’) In muscles of P105 Yap/Taz cDKO mouse, axons and SCs frequently retract from NMJs, exhibiting postsynaptic disassembly of AChRs (boxed NMJ) characteristic of chronically paralyzed muscles.
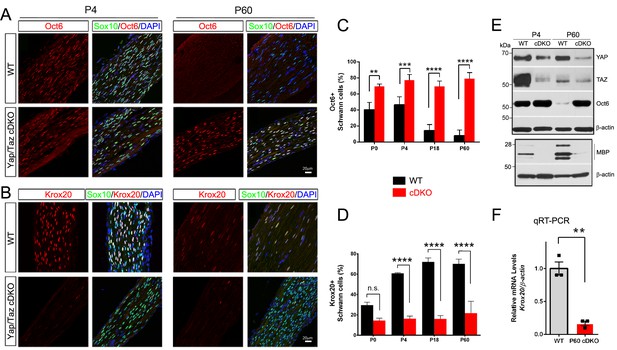
YAP/TAZ are required for Krox20 upregulation in differentiating Schwann cells.
(A) Identification of Oct6+ SC nuclei at P4 and P60 in WT and Yap/Taz cDKO, as determined by co-staining for Oct6 (red), Sox10 (SC nuclei; green) and DAPI (all cell nuclei; blue). (B) Identification of Krox20+ SC nuclei at P4 and P60 in WT and Yap/Taz cDKO, as determined by co-staining for Krox20 (red), Sox10 (SC nuclei, green) and DAPI (all cell nuclei). (C) Quantification of Oct6 expression in SC nuclei. n = 4 mice per genotype (WT and cDKO P4) or n = 3 mice per genotype (WT and cDKO P0, (P18 and P60). **p<0.01 (P0), ***p<0.001, ****p<0.0001, 2-way ANOVA with Sidak’s multiple comparison test. (D) Quantification of Krox20 expression in SC nuclei. n = 3 mice per genotype (WT P0, WT P4, mutant P18) or n = 2 mice per genotype (mutant P0, mutant P4, WT P18, WT and mutant P60). ****p<0.0001, n.s. = non-significant, 2-way ANOVA, with Sidak’s multiple comparison test. (E) Western blotting of P4 and P60 WT and Yap/Taz cDKO sciatic nerve lysates, using the indicated antibodies and anti-βactin as a loading control. n = 3 experiments. (F) Quantitative RT-PCR using Krox20 and β-actin-specific primers and total RNA isolated from WT and Yap/Taz cDKO P60 sciatic nerves. Expression of Krox20 is normalized to that of β-actin as an internal control, and WT expression is arbitrarily given the value 1. n = 3 mice per genotype. **p<0.01, unpaired Student’s t-test.
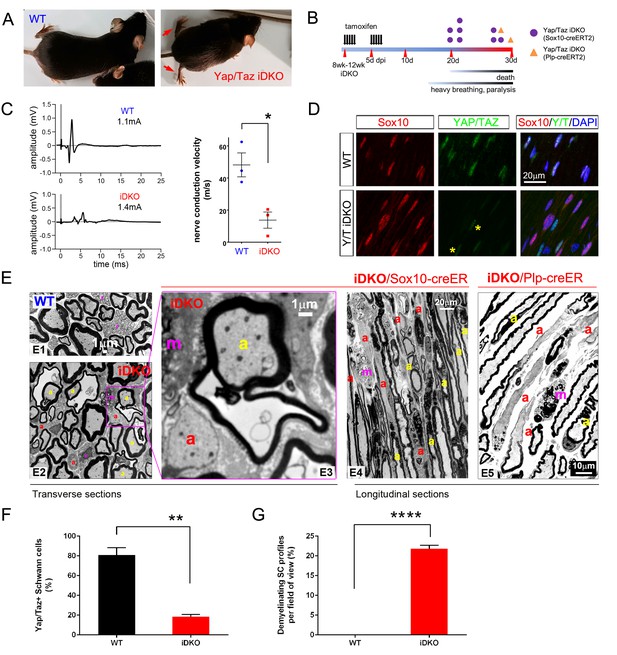
YAP/TAZ are required for myelin maintenance.
(A) Living eight week-old WT and Yap/Taz iDKO (Sox10-Cre-ERT2) mice 20 days post-first tamoxifen injection. Arrows indicate abnormal splayed gait. (B) Cartoon showing timeline of tamoxifen injection and time of sacrifice/ death of iDKO mice due to severity of symptoms. Purple dots: Sox10-creERT2; Yapfl/fl; Tazfl/fl; Orange dots: Plp1-creERT2; Yapfl/fl; Tazfl/fl iDKO mice. (C) Representative images of CMAPs generated in WT and iDKO mice. Right panel: Nerve conduction velocity, n = 3 mice per genotype. *p=0.0186, unpaired Student’s t-test. (D) Longitudinal cryosections of sciatic nerves of 11 week-old WT and iDKO (Sox10-Cre-ERT2) mice, 20 days after first tamoxifen injection, showing loss of YAP/TAZ (green) in iDKO but not WT SC nuclei, marked by Sox10 (red). All cell nuclei are marked by DAPI staining (blue). Asterisks mark lack of deletion of YAP/TAZ in non-SCs. n = 2 mice per genotype; two sections per mouse. (E) Transverse sciatic nerve (E1-3) and longitudinal ventral root (E4, E5) sections from 11 week old WT, iDKO (Sox10-Cre-ERT2; E2–E4) and iDKO (Plp1-Cre-ERT2; E5) mice, 20 days after first tamoxifen injection. (E1–E3) TEM of WT (E1) and iDKO (E2, E3) sciatic nerves. Axons with abnormal myelin profiles are marked with a yellow ‘a’; completely demyelinated axons are marked with a red ‘a’; myelin-laden macrophages are marked with a red ‘m’. n = 3 mice of each genotype. (E4–E5) Semi-thin ventral root sections, showing loss of myelin and loosened myelin sheaths in iDKO (Sox10-Cre-ERT2) and iDKO (Plp1-Cre-ERT2) mice. Demyelinated axons are marked by ‘a’ and myelin-filled macrophages are marked by ‘m’. Note the demyelinated internodes (marked by sets of ‘a’) contiguous with normally myelinated internodes. (F) Bar graph showing percentage of SCs immunopositive for YAP/TAZ 20 days after first tamoxifen injection, in WT and iDKO (Sox10-Cre-ERT2) sciatic nerve. (G) Quantification of demyelinating SC profiles 20 days after first tamoxifen injection, in transverse sections of WT and iDKO (Sox10-Cre-ERT2) sciatic nerve. n = 3 mice per genotype, ****p<0.0001, unpaired Student’s t-test. The following figure supplements are available for Figure 6.
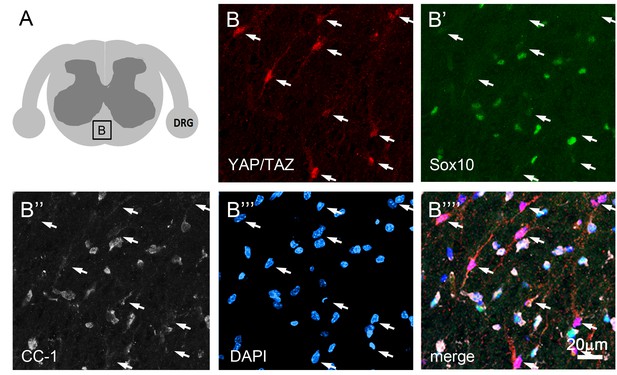
YAP and TAZ are not expressed in mature oligodendrocytes.
(A) Cartoon denoting a spinal cord white matter area of P60 WT mouse from which quadruple immunostaining images of B–B’’’’ were taken. (B’–B””) Cells strongly immunopositive for YAP/TAZ are present (B, arrows), but YAP/TAZ are absent from oligodendrocytes identified by Sox10 (B’, green) and CC1 (B’’, white). All nuclei are marked by DAPI (blue).
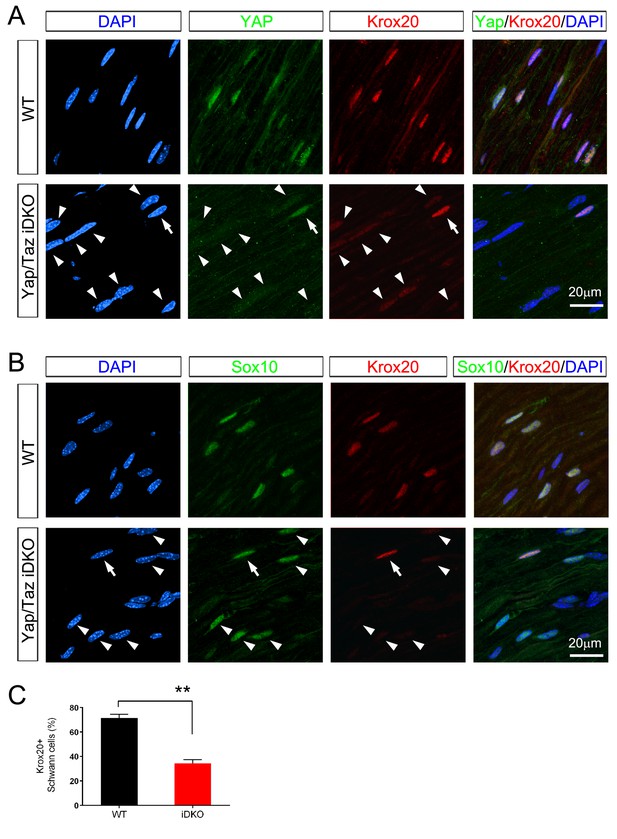
Inducible ablation of Yap/Taz in adult Schwann cells leads to rapid loss of Krox20.
(A, B) Immunostaining of longitudinal cryosections of sciatic nerves of WT and iDKO (Sox10-Cre-ERT2) mice 20 days after first tamoxifen injection. (A) Occasional nuclei, marked with DAPI (blue), exhibit YAP/ TAZ (green) and bright Krox20 (red) immunoreactivity (arrow), while most other nuclei show no YAP/TAZ and faint or no Krox20 immunoreactivity (arrowheads). (B) In iDKO sciatic nerves, occasional SC nuclei (Sox10+; green) contain bright Krox20 (red) immunoreactivity (arrow), but most SC nuclei exhibit faint or no Krox20 immunoreactivity (arrowheads). (C) Quantification of loss of Krox20 immunoreactivity in Schwann cell (Sox10+) nuclei 20 days after first tamoxifen injection. Both bright and faint immunoreactivity were counted as Krox20+. n = 3 mice per genotype, **p<0.01, Student’s unpaired t-test.
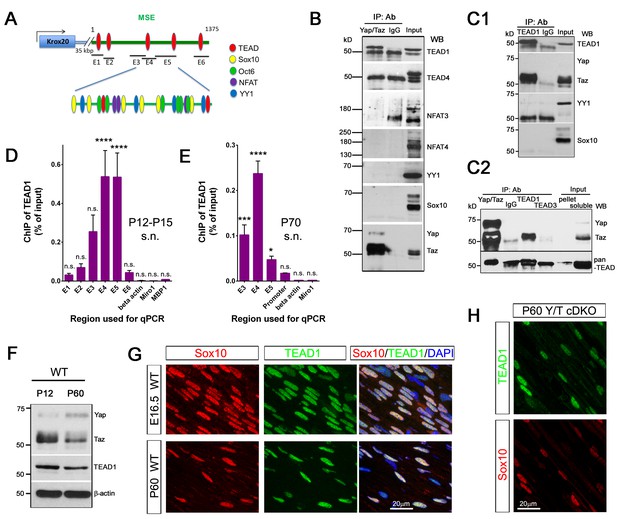
YAP/TAZ-TEAD1 regulate Krox20 transcription during developmental myelination and adult myelin maintenance.
(A) Schematic showing potential TEAD binding sites in the Krox20 MSE, in relation to published binding sites for known MSE-binding transcription factors. The regions of the MSE covered by primer sets E1-E6 used for ChIP-qPCR are indicated. (B, C1, C2) Immunoprecipitation of P12-P15 WT sciatic nerve lysates with anti-YAP/TAZ (B), anti-TEAD1 (C1), anti-YAP/TAZ, anti-TEAD1 and anti-TEAD3 (C2), plus control Rb IgG (B, C1, C2). Immunoprecipitates were blotted with the indicated antibodies. In (C2), upper blot was stripped and reprobed with anti-pan-TEAD (lower blot): the band in the Rb IgG lane, migrating similarly to TEAD proteins, is non-specific. n = 3 (B) or n = 2 (C1, C2) experiments. (D, E) ChIP-qPCR of P12-P15 sciatic nerves (D) or P70 sciatic nerves (E), using anti-TEAD1 antibody or control Rb IgG. Graphs show the amount of qPCR product obtained with the indicated MSE-specific primer sets or negative control primer sets after TEAD1 ChIP. Amounts are given as % of qPCR product obtained with the same primers using input chromatin. Amounts obtained with the same primer sets after control Rb IgG ChIP were all less than 0.003% of input, and are not shown. (D) n = 5 (E1, E2, E4, E6), n = 4 ((E5), n = 3 (β-actin), n = 2 (E3) independent ChIP assays. (E) n = 2 independent ChIP assays. P-values are given for TEAD1 ChIP versus Rb IgG ChIP for each primer set. ****p<0.0001, ***p=0.0001, *p=0.0498, n.s. = non-significant, 2- way ANOVA with Sidak’s multiple comparison test. Bar represents mean ± SEM. (F) Western blotting of sciatic nerve lysates from WT of P12 or P60 mice, showing persistent expression of TEAD1 and YAP/TAZ. (G) Immunostaining of longitudinal sections of WT sciatic nerves, showing that TEAD1 (green) is present in all nuclei (marked by Sox10, red) of proliferating and differentiated SCs. (H) Longitudinal nerve sections of P60 Yap/Taz cDKO, showing that TEAD1 (green) is present in all cDKO SC nuclei (marked by Sox10, red). The following figure supplements are available for Figure 8.
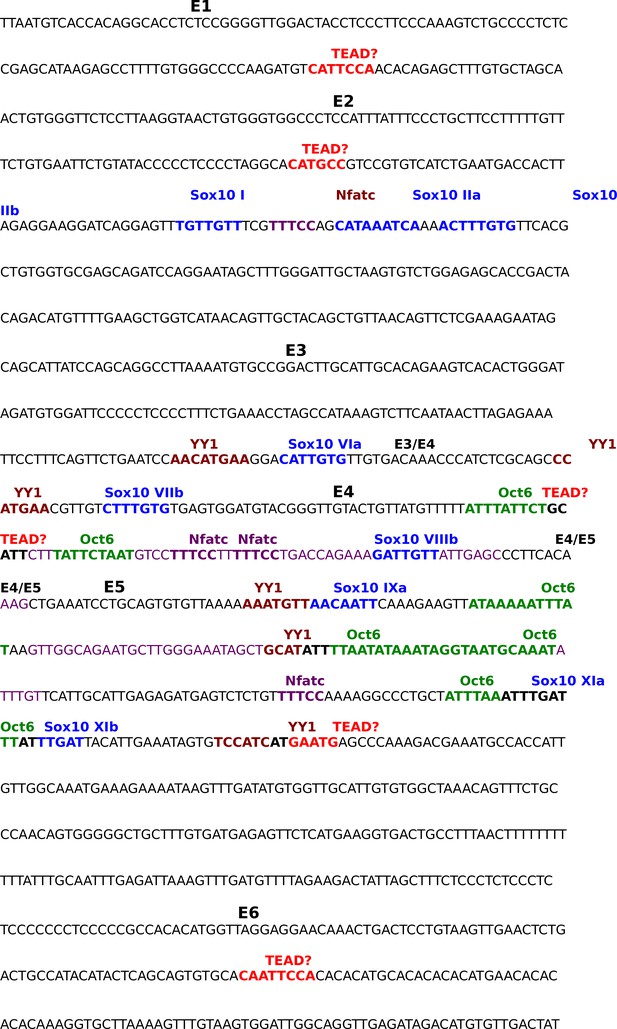
Annotated sequence of the Krox20 Myelin Specific Enhancer.
Sequence shown is the 1 kb distal myelin specific enhancer (MSE) of the mouse Krox20 gene. Sequences covered by primer pairs E1 to E6 used for ChIP qPCR are alternately highlighted in yellow or blue; overlap between primer pair sequences is highlighted in grey. Color-coding- and the method of identification of previously-identified transcription factor binding sites in the MSE are as follows, Sox10: blue, EMSA (Reiprich et al., 2010); YY1: brown, ChIP qPCR (He et al., 2007); Nfatc: purple, EMSA (Kao et al., 2009); Oct6: green, foot printing (Ghislain and Charnay, 2006). Potential TEAD binding sites identified by our own sequence analysis are highlighted in red.
Videos
A movie showing a P60 Yap/Taz cDKO mouse.
https://doi.org/10.7554/eLife.20982.008A movie showing an adult Yap/Taz iDKO and a control littermate mouse.
The iDKO mouse was recorded at 20 days after the first tamoxifen administration.





