Role of Nfu1 and Bol3 in iron-sulfur cluster transfer to mitochondrial clients
Figures
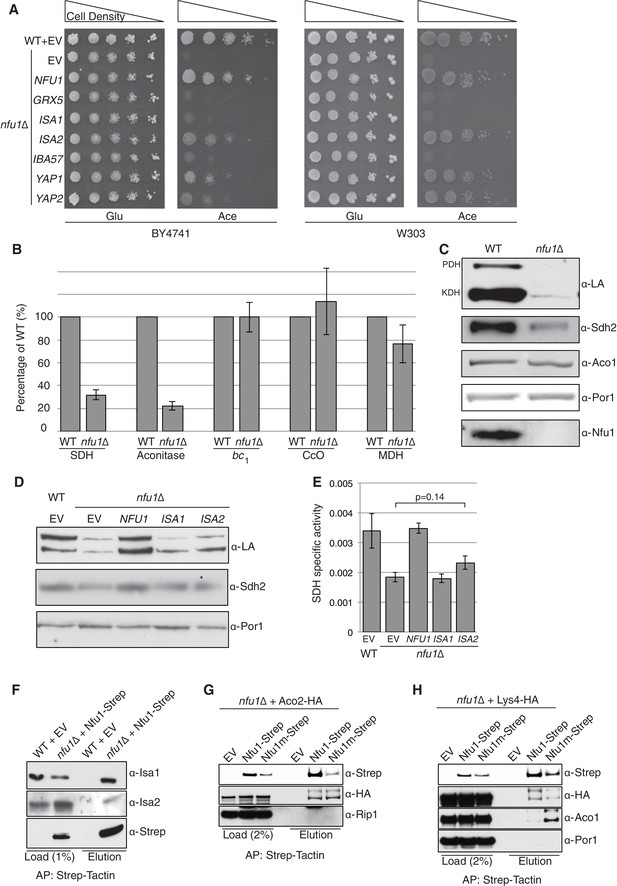
Nfu1 functions with both the ISA [4Fe-4S] assembly complex and [4Fe-4S] client proteins.
Cells lacking Nfu1 exhibit defects in [4Fe-4S] cluster enzymes in mitochondria. (A) Respiratory growth defects revealed by yeast drop-test. Cells harboring empty vectors (EV) or high-copy plasmids expressing designated genes were pre-cultured in liquid synthetic complete (SC) glucose media lacking uracil. Serially diluted cells (10-fold) were spotted on SC media plates at 30°C. Grx5 is a monothiol glutaredoxin involved in mitochondrial Fe-S biogenesis. Isa1, Isa2 and Iba57 are subunits of the ISA scaffold complex required for [4Fe-4S] cluster synthesis. Yap1 is a transcription factor that induces expression of anti-oxidant genes. Glu is 2% glucose and Ace is 2% acetate. (B) The relative activity of aconitase, SDH, cytochrome bc1, cytochrome c oxidase (CcO), and malate dehydrogenase (MDH) were measured in isolated mitochondria from cells cultured in SC media with 2% raffinose. Data are shown as mean ± SE (n = 3) (CcO, n = 4). (C) Steady-state protein levels measured by SDS-PAGE followed by immunoblotting in isolated mitochondria. Anti-LA antibody is an antibody specific to lipoic acid (LA) that is conjugated to proteins. PDH is pyruvate dehydrogenase and KDH is α-ketoglutarate dehydrogenase. Sdh2 is the Fe-S cluster subunit of SDH. Aco1 is mitochondrial aconitase. Por1 is a mitochondrial loading control. (D) Restoration of LA moieties on PDH and KDH shown by SDS-PAGE followed by immunoblotting in isolated mitochondria from nfu1∆ cells over-expressing ISA1 and ISA2. (E) Enzymatic activity of SDH in mitochondria isolated from nfu1∆ cells over-expressing ISA1 and ISA2. Data are shown as mean ± SE (n = 3). (F) Strep-tag affinity purification of Nfu1-Strep revealed the Nfu1 interaction with Isa1 and Isa2. Mitochondria were solubilized with 0.1% n-dodecyl maltoside (DDM). Clarified lysates were incubated with Strep-Tactin superflow beads for 16 hr. After washing, proteins were eluted with 2.5 mM desthiobiotin, and then analyzed by immunoblotting. (G) Strep-tag affinity purification of Nfu1-Strep in the presence of ectopically expressed Aco2-HA. Nfu1m-Strep is the G/T>H mutant described in Figure 4. (H) Strep-tag affinity purification of Nfu1-Strep in the presence of ectopically expressed Lys4-HA. Lys4 and Aco2 are both nuclear DNA-encoded mitochondrial proteins that require a [4Fe-4S] cluster for each function in the lysine biosynthetic pathway in yeast. Nfu1m-Strep is the G/T>H mutant described in Figure 4.
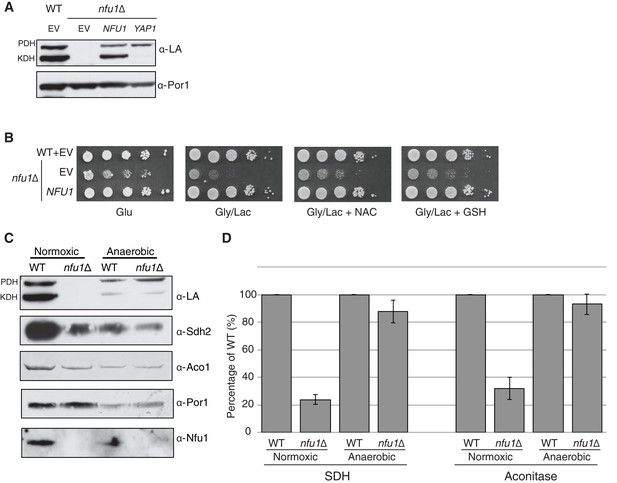
Nfu1 has a heighted importance during times of oxidative stress and is expendable in anoxic conditions.
Defects in cells lacking Nfu1 are pronounced under oxidative stress conditions. (A) Steady-state levels of proteins in isolated mitochondria from nfu1∆ cells harboring high-copy NFU1 plasmids or YAP1 plasmids. (B) Yeast drop-test with 5 mM n-acetyl cysteine (NAC) and 2 mM glutathione (GSH). Gly/Lac is SC medium with 2% glycerol and 2% lactate as carbon sources. (C) Steady-state levels of proteins in isolated mitochondria from cells cultured under normoxic conditions or anaerobic conditions. (D) Relative activity of SDH and aconitase in mitochondria from panel C. Data are shown as mean ± SE (n = 3).
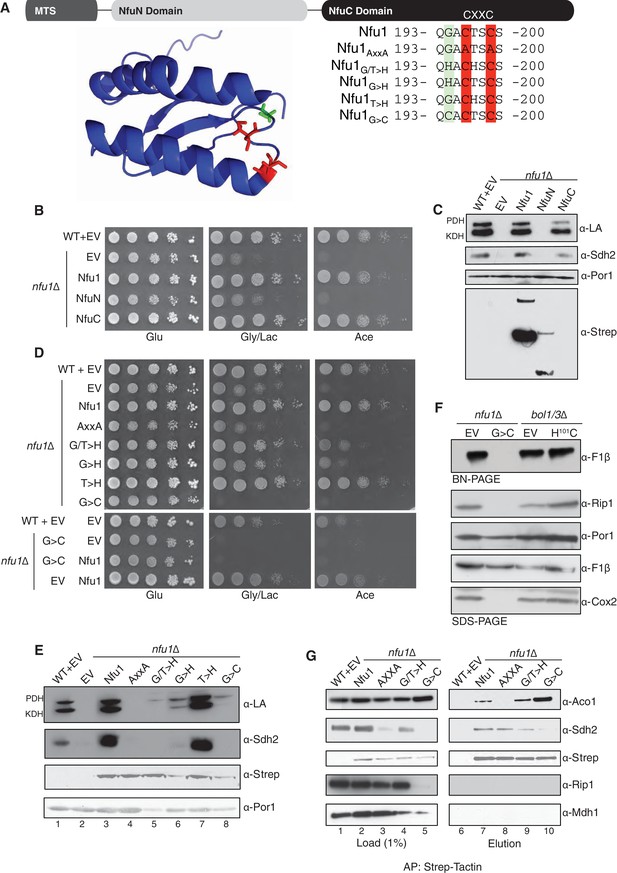
The CxxC motif of C-terminal domain of Nfu1 is essential for function.
The CxxC motif is critical for Nfu1 function. (A) A schematic representation of Nfu1 domains. MTS, the mitochondrial targeting sequence; NfuN, the N-terminal domain of Nfu1; NfuC, the C-terminal domain harboring the highly conserved CxxC motif. The human NfuC tertiary structure (PDB: 2M5O) and primary sequences showing the CxxC motif (red) and adjacent amino acids indicated in partial sequences (green). (B) The respiratory growth defect of nfu1∆ cells was rescued with NfuC. Nfu1, NfuN, and NfuC were all fused with a C-terminal Strep-tag and expressed exogenously using low-copy plasmids. (C) Restoration of Nfu1 target proteins by NfuC expression in nfu1∆ cells. (D) Respiratory growths of nfu1∆ cells that express Nfu1 sequence variants were tested. All variants were fused with a Strep-tag and expressed on low-copy plasmids. (E) Steady-state levels of LA-conjugated proteins and Sdh2 in nfu1∆ cells that express Nfu1 variants. (F) BN-PAGE and SDS-PAGE analysis of [4Fe-4S] cluster independent enzymes in the dominant negative backgrounds nfu1Δ + G>C and bol1/3Δ + H101C. (G) Strep-tag purification of Nfu1 sequence variants as described in Figure 3B immunoblotting for [4Fe-4S] cluster client proteins Aco1 and Sdh2.
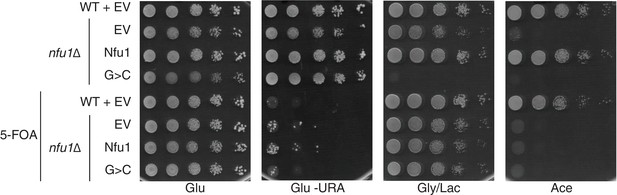
Yeast growth tests evaluating respiratory growth (Gly/Lac) of nfu1Δ + G>C cells following treatment with 5’-Fluoroorotic acid (5-FOA) to show cells have not lost their mitochondrial DNA (rho-).
5-FOA was used to induce shedding of the pRS416 vector. Glu-URA is SC media with 2% glucose lacking Uracil to show the successful loss of the pRS416 vector.
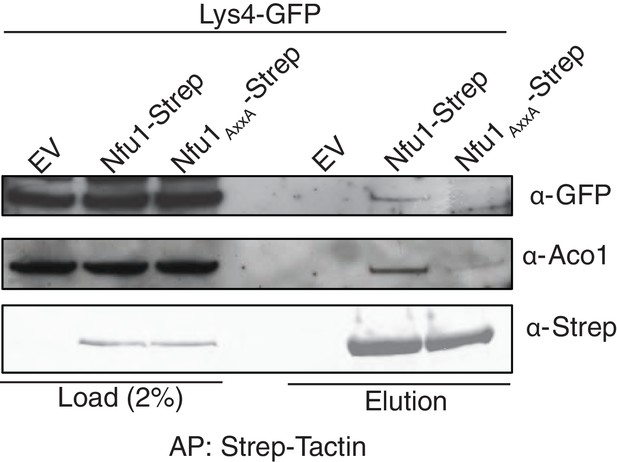
Affinity purification using Strep-Tactin to immobilize Strep tagged Nfu1 and the Nfu1 AxxA variant expressed ectopically in the BY4743 background with a single copy of Lys4 chromosomally tagged with GFP.
https://doi.org/10.7554/eLife.15991.007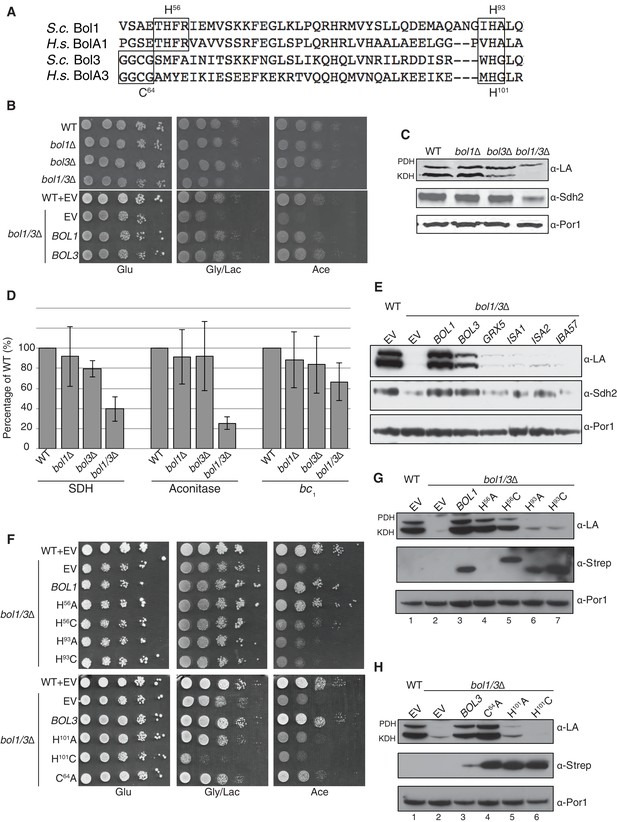
The Mitochondrial Bol1 and Bol3 proteins function in Fe-S biogenesis.
Bol1 and Bol3 play roles in Fe-S cluster biogenesis in mitochondria (A) Partial sequences of yeast and human mitochondrial BolA proteins. Boxed are conserved motifs with proposed ISC ligands that were mutated in this work. (B) Respiratory growth defects of bol1∆ cells, bol3∆ cells and bol1∆bol3∆ double mutants and complementation by plasmid-borne BOL1 or BOL3. (C) Steady-state levels of LA-conjugated proteins and Sdh2 in cells lacking Bol1 and/or Bol3. (D) Relative activity of SDH, cytochrome bc1 complex and aconitase were measured. Data are shown as mean ± SE (n=3). (E) Observation of LA moieties on PDH and KDH and Sdh2 steady-state levels by SDS-PAGE followed by immunoblotting in isolated mitochondria from bol1/3∆ cells over-expressing the indicated Fe-S cluster gene. (F) Respiratory function of Bol1 and Bol3 sequence variants in conserved residues were examined by yeast drop-test. All Bol1 variants were fused with a C-terminal Strep-tag and expressed on low-copy plasmids. All Bol3 variants were fused with a N-terminal Strep-tag between the MTS and the remainder of the protein and expressed on low-copy plasmids. (G and H) Steady-state levels of LA-conjugated proteins in cells lacking Bol1 and Bol3 with Bol1 variants (G) and Bol3 variants (H) exogenously expressed.
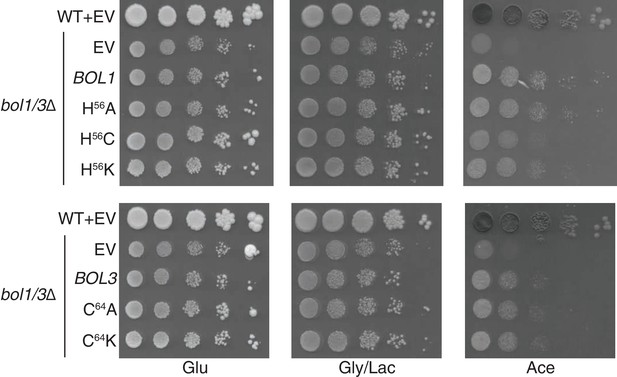
Yeast growth tests evaluating the viability of cells expressing mitochondrial Bol1 and Bol3 N-terminal ligands mutated to lysine in the bol1/3Δ background.
https://doi.org/10.7554/eLife.15991.009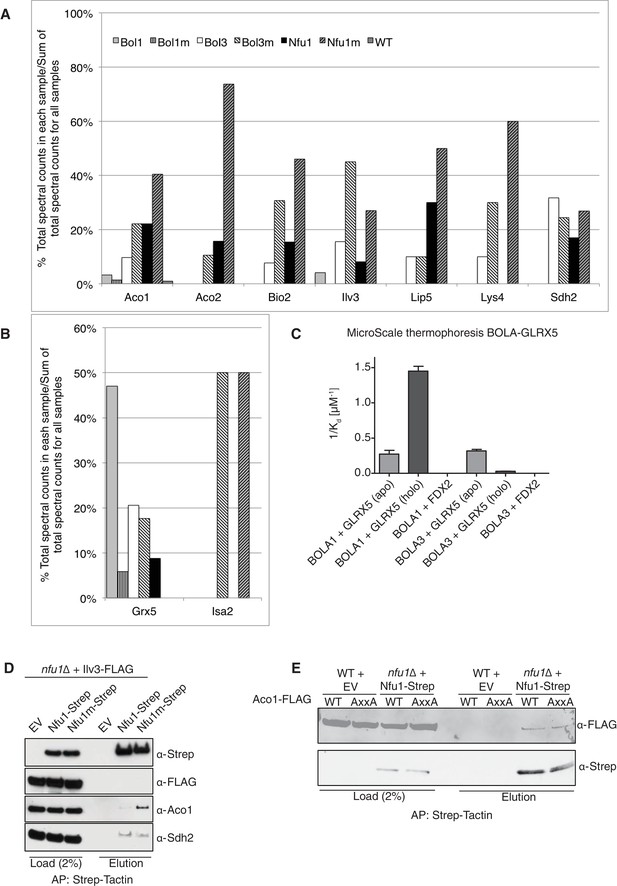
Proteomic analysis of Nfu1, Bol1 and Bol3 establishes function within mitochondrial Fe-S for Bol1 and Bol3.
(A and B) Percentages of spectral counts identified by MS proteomics. Percentages were calculated by the number of spectral counts identified for a denoted protein in an individual Strep-tagged protein divided by the total number of spectral counts for that protein identified from all seven samples. Strep-tagged proteins were expressed from low-copy plasmids in corresponding single deletion mutants. Samples were Strep-affinity purified as in Figure 3. Bol1m is the H93C variant. Bol3m is the H101C variant. Nfu1m is the G/T>H variant. WT is wild-type BY4741 expressing an empty vector. All were fused with a C-terminal Strep-tag. WT is BY4741 wild type harboring a low-copy empty plasmid. (C) Human GLRX5 or NFU1 were used in apo- and holo- form and mixed at increasing concentrations with 200 nM fluorescently labelled BOLA1 or BOLA3. Microscale thermophoresis were performed and dissociation constants (Kd) were determined. Error bars indicate the SD (n=3). (D) Strep-tag affinity purification of Nfu1-Strep in the presence of ectopically expressed Ilv3-FLAG. (E) Affinity purification using Strep-Tactin agarose beads to purify Nfu1-Strep from an nfu1∆ background expressing either WT Aco1 or Aco1 AxxA mutant.
-
Figure 5—source data 1
(Table 1) Spectral counts, unique peptides, and coverage of mitochondrial Fe-S client proteins, bait proteins, and Fe-S assembly machinery identified by MS proteomics.
Bol1m is the H93C variant. Bol3m is the H101C variant. Nfu1m is the G/T>H variant. (Table 2) Spectral counts, unique peptides, and coverage of mitochondrial Fe-S client proteins (Aco1 and Lip5), Fe-S assembly machinery protein (Grx5) and the mitochondrial peroxiredoxin Prx1 comparing two unique biological replicates by affinity purification and subsequent MS analysis. Bol1m is the H93C variant. Bol3m is the H101C variant. Nfu1m is the G/T>H variant. Prx1 was a reproducible interactor with Bol1, but the significance of this interaction remains to be established.
- https://doi.org/10.7554/eLife.15991.011
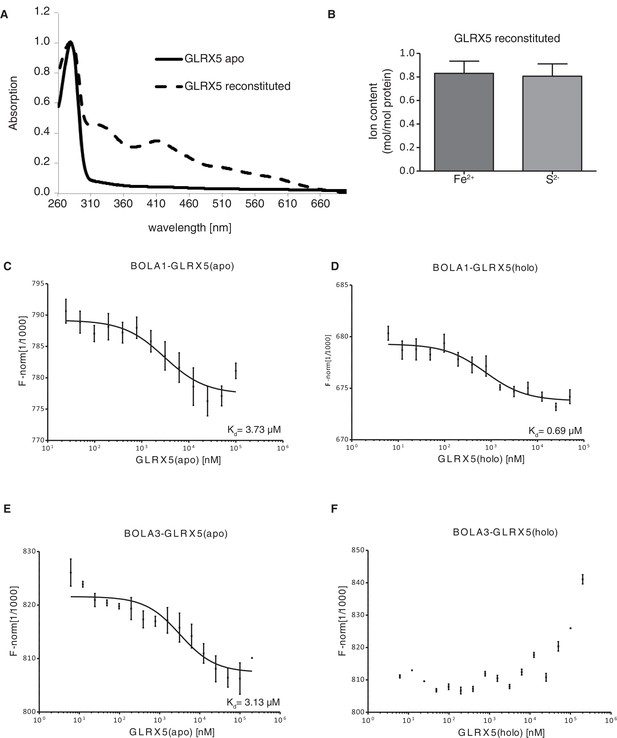
Interaction studies of human BOLA proteins with GLRX5.
(A) UV–visible absorption spectrum of apo-GLRX5 (black line) and chemically reconstituted GLRX5 (dashed line). Reconstituted human GLRX5 (100 µM) showed absorption bands at 320 nm and 425 nm besides the protein absorption at 280 nm, characteristic for the [2Fe–2S] cluster bound to GLRX5. (B) Iron and sulfide determination of chemically reconstituted GLRX5. Reconstituted human GLRX5 contains about 0.85 Fe2+ and 0.8 S2- per monomer indicating a bridging [2Fe-2S] cluster between two GLRX5 monomers. (C–F) Quantification of the interaction between the human apo- or holo-GLRX5 and the BOLA proteins. Microscale thermophoresis was performed using the indicated fluorescently labeled human BOLA proteins and apo-GLRX5 (C;E) or holo-GLRX5 (D;F).
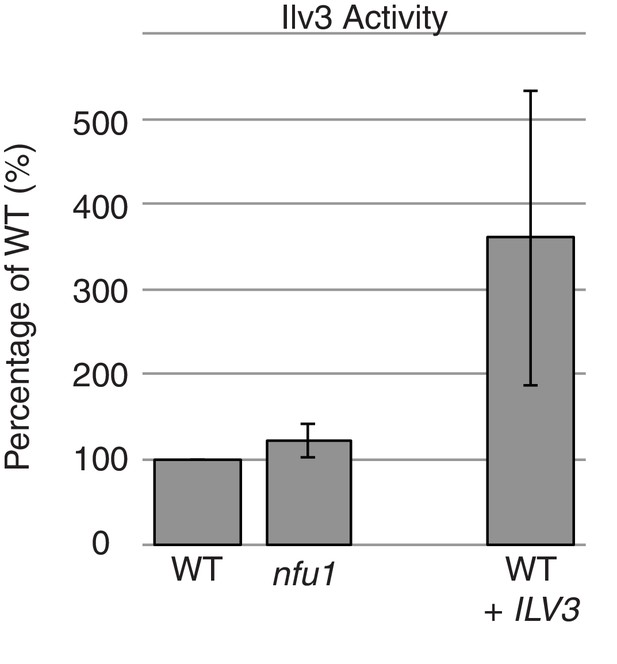
Ilv3 activity assay using wild-type and nfu1Δ purified mitochondria along with wild-type overexpressing Ilv3 as a control.
https://doi.org/10.7554/eLife.15991.013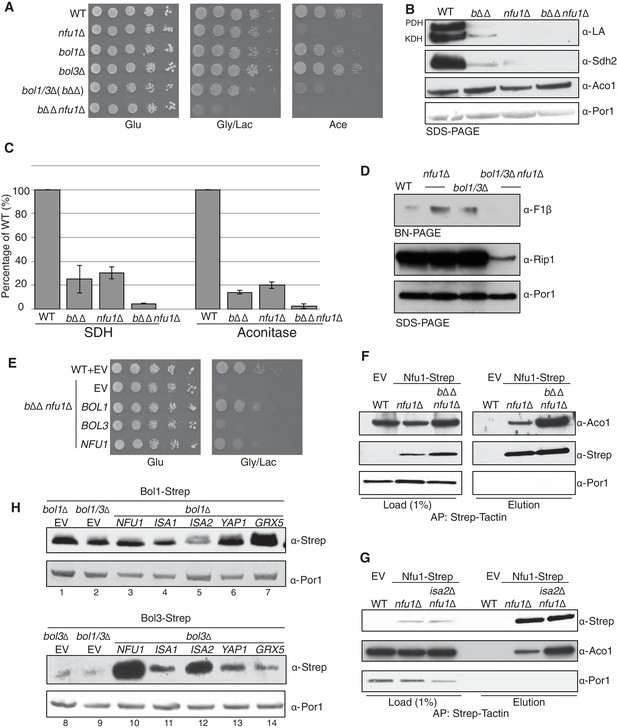
Nfu1 and Bol3 function together in [4Fe-4S] delivery.
(A) Exacerbated respiratory growth defects of bol1∆bol3∆nfu1∆ triple mutants (designated bΔΔnfu1Δ) compared to nfu1∆ single mutants and bol1∆bol3∆ double mutants on non-fermentable carbon sources. (B) Steady-state levels of LA-conjugated proteins and Sdh2 in the absence of Bol1, Bol3 or Nfu1. (C) Relative activity of SDH and aconitase in the absence of Bol1, Bol3 or Nfu1. Data are shown as mean ± SE (n=3) (D) BN-PAGE and SDS-PAGE analysis of [4Fe-4S] cluster independent enzymes in the bΔΔnfu1Δ triple deletion mutant background. F1β is a subunit of ATP synthase. (E) Respiratory growth of bΔΔnfu1Δ triple mutants harboring plasmid-borne BOL1, BOL3 and NFU1, respectively. (F) Strep-tag purification of Nfu1-Strep in the absence of Bol1 and Bol3. (G) Strep-tag purification of Nfu1-Strep in the absence of Isa2. (H) Steady-state levels of Bol1-Strep (upper panel) and Bol3-Strep (bottom panel) in response to overexpression of genes as indicated.
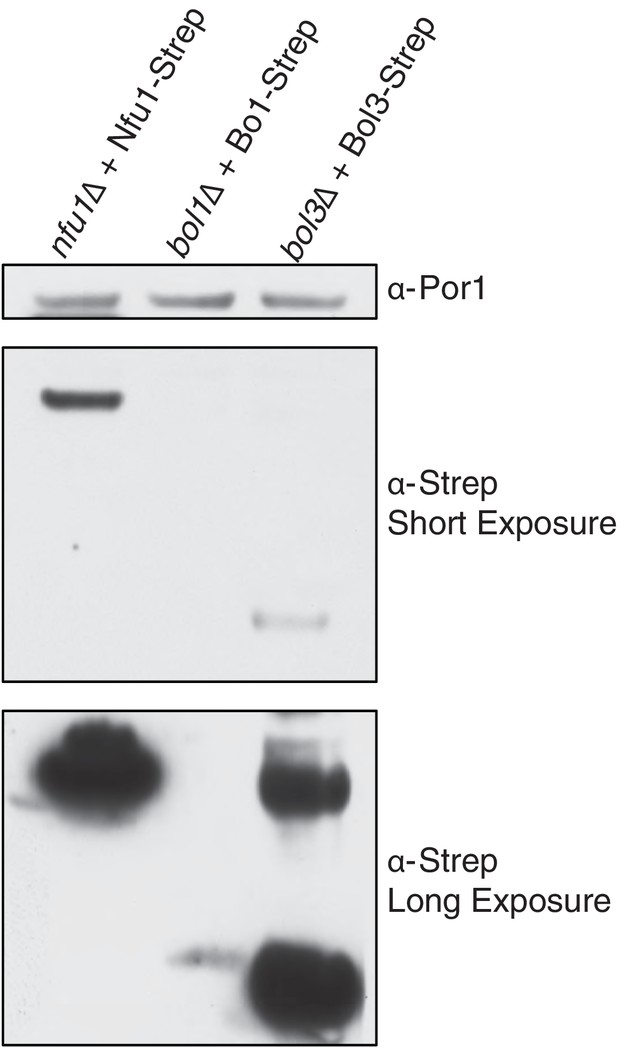
SDS-PAGE followed by immunoblotting to evaluate the different steady state levels of Nfu1-Strep, Bol1-Strep, and Bol3-Strep while being expressed under the same heterologous MET25 promoter and CYC1 terminator.
https://doi.org/10.7554/eLife.15991.015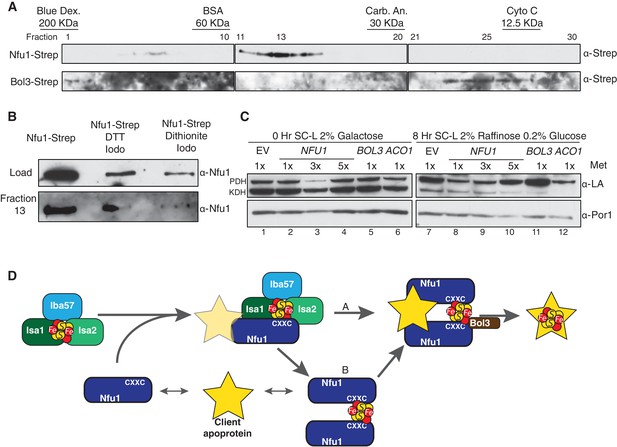
Nfu1 exists as a dimer bridged by a Fe-S cluster.
(A) Immunoblotting of fractions from nfu1∆ + Nfu1-Strep or bol3∆ + Bol3-Strep lysates separated by size exclusion chromatography. Molecular weight standards [bovine serum albumin (BSA), carbonic anhydrase (CA) and cytochrome c] are displayed above the corresponding fractions were used to create a standard curve to calculate apparent molecular weights. Fraction 13 has an apparent molecular weight of 47.6 KDa and Fraction 26 has an apparent molecular weight of 13.2 kDa. (B) Immunoblotting of fraction 13 from nfu1∆ + Nfu1-Strep lysates pretreated with nothing, 100 mM DTT followed by 200 mM iodoacetamide, or 2 mM dithionite followed by 200 mM iodoacetamide were separated by size exclusion chromatography. (C) GAL-NFS1 shutdown was induced over 8 hr with over-expression of NFU1, BOL3, and ACO1 and LA levels were observed by immunoblotting. Nfu1 protein levels were reduced with increasing amounts of methionine by utilizing a MET25 promoter is repressed with excess methionine (1x = 0.6 mM methionine). (D) A working model of late stage mitochondrial iron-sulfur cluster biogenesis and delivery. Two potential pathways of cluster transfer are shown in A and B.





