Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium
Figures
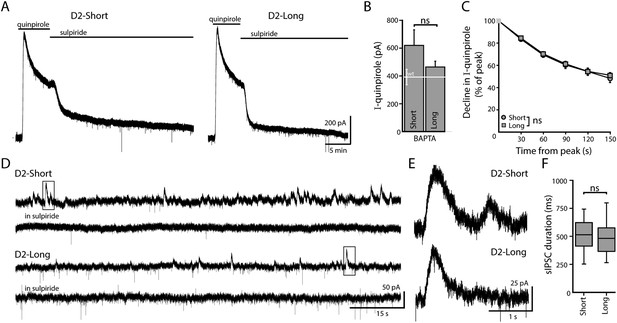
When virally expressed in midbrain dopamine neurons, D2S and D2L function as autoreceptors.
(A) Representative traces of whole-cell voltage clamp recordings, using a BAPTA-containing internal solution, of the outward current in D2S and D2L neurons induced by bath application of quinpirole (30 μM), which was reversed by sulpiride (600 nM). (B) The amplitude of quinpirole-induced currents in D2S and D2L neurons using BAPTA internal did not differ (n = 12–14, unpaired t test), shown in reference to the amplitude of the quinpirole-induced currents in WT neurons (white line). (C) There was no difference between D2S and D2L in the decline of the D2 receptor-dependent current in the continued presence of quinpirole using BAPTA internal (two-way ANOVA). (D, E) Representative traces of spontaneous D2-sIPSCs mediated by D2S and D2L receptors, blocked by sulpiride. Inset boxes are shown enlarged in (E). The frequency and amplitude of D2S- and D2L-sIPSCs were not analyzed since these parameters may be influenced by the expression of D2 receptors in presynaptic dopamine neurons, which cannot be confirmed. (F) The duration of D2S-sIPSCs and D2L-sIPSCs did not differ (n = 84–100 sIPSCs, Mann–Whitney U test). ns indicates not significant.
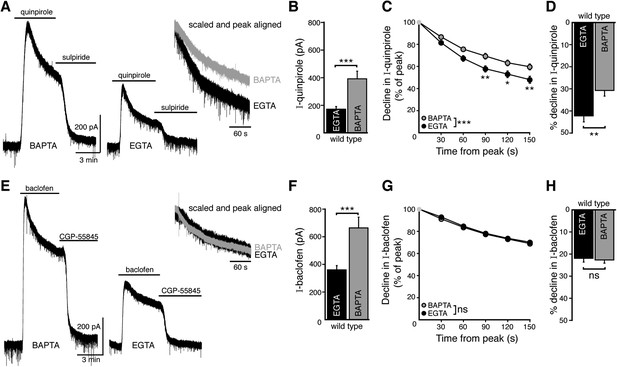
Weak intracellular calcium buffering reveals calcium-dependent desensitization of D2 autoreceptor-dependent GIRK currents in wild type dopamine neurons.
(A) Representative traces of whole-cell voltage clamp recordings of the outward current induced by bath application of quinpirole (10 μM) that was reversed by sulpiride (600 nM), using a BAPTA or EGTA-containing internal solution. (B) The amplitude of the quinpirole-induced current was larger using BAPTA than EGTA internal (n = 15 each, unpaired t test). (C, D) The decline in quinpirole-induced current was faster using EGTA internal compared to BAPTA (C: two-way ANOVA followed by Bonferroni, D: unpaired t test) (E) Representative traces of whole-cell voltage clamp recordings of the outward current induced by bath application of baclofen (30 μM) which was reversed by CGP-55845 (200 nM), using BAPTA or EGTA internal. (F) The amplitude of the baclofen-induced current was larger using BAPTA than EGTA internal (n = 14–16, unpaired t test). (G, H) There was no difference in the decline in baclofen-induced current recorded with EGTA and BAPTA internals (G: two-way ANOVA, D: unpaired t test). ns indicates not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
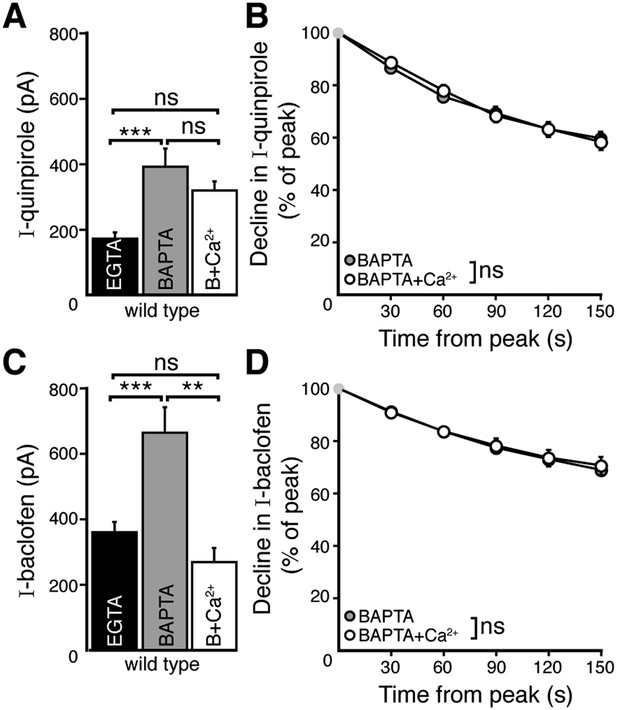
Increasing resting free internal calcium does not enhance desensitization of D2 autoreceptor-dependent GIRK currents.
(A) The amplitude of the quinpirole (10 μM)-induced current using BAPTA+Ca2+ internal solution was not different from the amplitudes using BAPTA or EGTA internal solutions (n = 7–15, ANOVA followed by Bonferroni). (B) Increasing resting free calcium with BAPTA+Ca2+ had no effect on the decline in quinpirole-induced current (two-way ANOVA). (C) Increasing resting free calcium with BAPTA+Ca2+ internal decreased the amplitude of the baclofen (30 μM)-induced current, making it no greater than the amplitude recorded using EGTA internal (ANOVA followed by Bonferroni). (D) Increasing resting free calcium with BAPTA+Ca2+ had no effect on the decline in baclofen-induced current (two-way ANOVA). Additional experiments that demonstrate BAPTA+Ca2+ internal increased resting free calcium can be found in Figure 3—figure supplement 1. ns indicates not significant, **p < 0.01, ***p < 0.001.
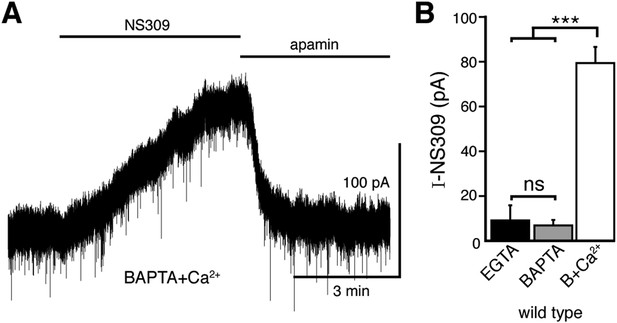
The positive modulator of the SK channel, NS309, produces an outward current when using the BAPTA+Ca2+ internal solution.
(A) Representative trace of whole-cell voltage clamp recordings of the outward current induced by bath application of NS309 (10 μM), which was reversed by apamin (200 nM), using a BAPTA+Ca2+ internal. (B) NS309 produced a current using a BAPTA+Ca2+, but not BAPTA or EGTA internal (n = 5–6, ANOVA followed by Bonferroni). ns indicates not significant, ***p < 0.001.
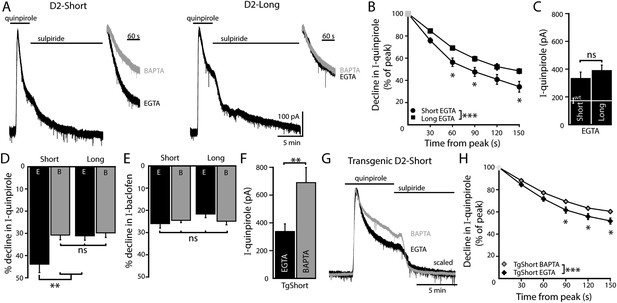
D2S but not D2L receptor-GIRK currents exhibit calcium-dependent desensitization.
(A) Representative traces of whole-cell voltage clamp recordings of the outward current in D2S and D2L neurons induced by bath application of quinpirole (30 μM) which was reversed by sulpiride (600 nM), using EGTA internal, compared with the BAPTA trace shown in Figure 1A (scaled and peak-aligned). (B, D) Using EGTA internal, the decline in quinpirole-induced current was greater in D2S than D2L neurons (B: two-way ANOVA followed by Bonferroni, D: n = 16 each, one-way ANOVA followed by Fisher's LSD). (C) The amplitude of quinpirole-induced currents in D2S and D2L neurons using EGTA internal did not differ (n = 16–17, unpaired t test), shown in reference to the amplitude of the quinpirole-induced currents in WT neurons (white line). (D) In D2S neurons the decline in quinpirole-induced current was greater using EGTA internal compared to BAPTA, but not in D2L neurons (n = 12–16, one-way ANOVA followed by Fisher's LSD). The time course of the decline can be found in Figure 4—figure supplement 1. (E) There was no difference in the decline in baclofen-induced current recorded with EGTA or BAPTA internal in either splice variant (n = 11–19, one-way ANOVA). (F) In neurons from transgenic D2S mice, the amplitude of the quinpirole-induced current was larger using BAPTA than EGTA internal (n = 7–8, unpaired t test). (G, H) Representative scaled and peak-aligned traces of whole-cell voltage clamp recordings from neurons from transgenic D2S mice of the outward currents induced by bath application of quinpirole (10 μM), which were reversed by sulpiride. The decline in quinpirole-induced current was greater using EGTA internal compared to BAPTA (two-way ANOVA followed by Bonferroni). ns indicates not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
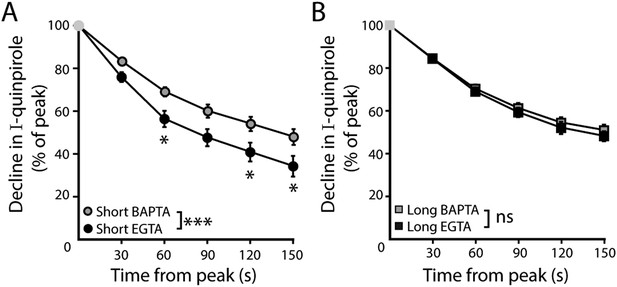
Time course of desensitization of D2 receptor splice variant-GIRK currents.
(A) In D2S neurons, the decline in quinpirole (30 μM)-induced current was greater using EGTA internal compared to BAPTA (n = 10–16). (B) In D2L neurons, the decline in quinpirole-induced current using EGTA internal was no different from BAPTA internal (n = 10–16). Two-way ANOVAs followed by Bonferroni). ns indicates not significant, *p < 0.05, ***p < 0.001.
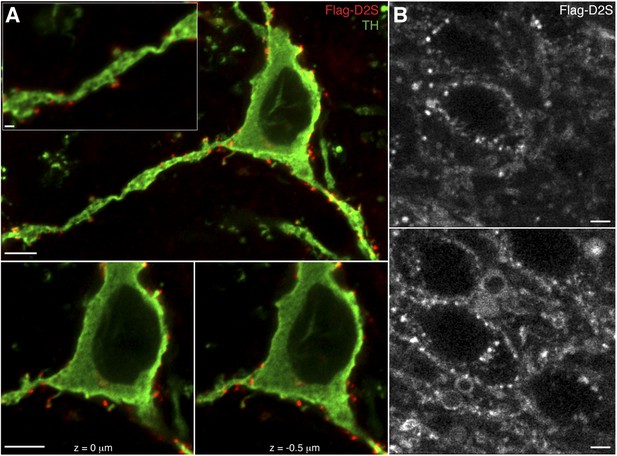
Expression and labeling of Flag-D2S receptors in dopamine neurons.
(A) Representative confocal microscopy images of Flag-D2S receptors clustered on the soma, dendrites, and spine-like structures of dopamine neurons, labeled by incubation of live slices in Alexa Fluor-594 conjugated anti-Flag M1 antibody (red, Flag-D2S), then post-fixed and immunostained for tyrosine hydroxylase (green, TH), scale bars: 1 μm (upper left inset) and 5 μm. (B) Representative two-photon microscopy images of live dopamine neurons, where Flag-D2S receptors were labeled by incubation of live slices in Alexa Fluor-594 conjugated anti-Flag M1 antibody (Flag-D2S), scale bars: 5 μm.
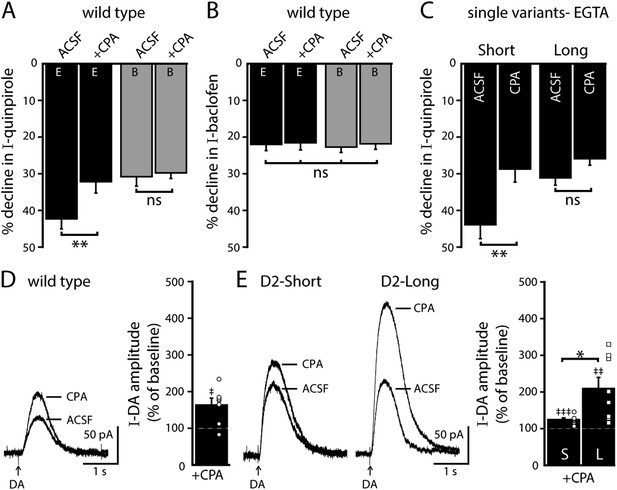
Depleting intracellular calcium stores differentially modifies D2S and D2L receptor-dependent GIRK conductance.
(A) In wild type neurons, CPA (10 μM, > 20 min) reduced the decline in the quinpirole-induced current using EGTA internal and the effect was prevented with the use of BAPTA internal (n = 11–15, one-way ANOVA followed by Fisher's LSD). (B) CPA had no effect on the decline in baclofen-induced current recorded with EGTA or BAPTA internal in wild type neurons (n = 14–16, one-way ANOVA). (C) In D2S, but not D2L neurons, CPA reduced the decline in quinpirole-induced current using EGTA internal (n = 6–16, one-way ANOVA followed by Fisher's LSD). (D, E) Submaximal D2 receptor-dependent outward currents were produced by iontophoretic application of dopamine once every 50 s while recording with EGTA internal (I-DA, arrows). (D) CPA (10 μM, 25–30 min) augmented I-DA in wild type neurons, shown in representative averaged traces (left) and grouped data (right, n = 7). (E) CPA augmented I-DA in D2S and D2L neurons, shown in representative averaged traces (left) and grouped data (right). The augmentation by CPA was greater in D2L than D2S neurons (n = 7–8, unpaired t test). The time course of the CPA-induced augmentation of I-DA can be found in Figure 5—figure supplement 1. Baseline: mean amplitude of six I-DAs preceding CPA application, ns indicates not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ‡ indicates significance over baseline (within-group comparison, paired t tests).
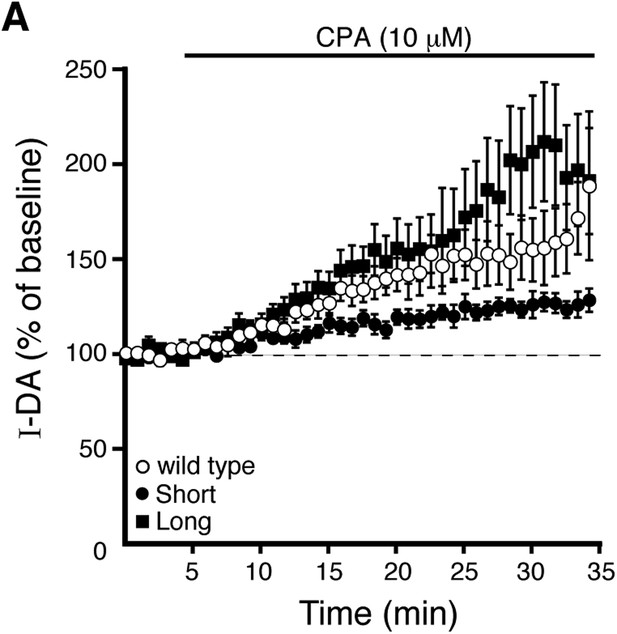
Prolonged CPA application enhances D2 receptor-dependent currents produced by exogenous dopamine.
(A) Submaximal D2 receptor-dependent outward currents (I-DA) were produced once every 50 s by iontophoretic application of dopamine while recording with EGTA internal. Prolonged CPA (10 μM) application enhanced I-DA in wild type (open circles), D2S (black circles), and D2L (black squares) neurons. Baseline: mean amplitude of six I-DAs preceding CPA application.
Blocking L-type calcium channels differentially modifies D2S and D2L receptor-dependent GIRK conductance.
(A, B) In wild type neurons, isradipine (300 nM, > 20 min) had no significant effect on the decline in quinpirole-induced (A) and baclofen-induced current (B) recorded with EGTA and BAPTA internal (quinpirole: n = 11–15, one-way ANOVA followed by Fisher's LSD; baclofen: n = 12–16, one-way ANOVA). (C) In D2S, but not D2L neurons, isradipine reduced the decline in quinpirole-induced current using EGTA internal (n = 6–16, one-way ANOVA followed by Fisher's LSD). (D, E) Submaximal D2 receptor-dependent outward currents were produced by iontophoretic application of dopamine once every 50 s while recording with EGTA internal (I-DA, arrows). (D) Isradipine (300 nM, > 15 min) augmented I-DA in wild type neurons, shown in representative averaged traces (left) and grouped data (right, n = 11). (E) Isradipine augmented I-DA in D2S and D2L neurons, shown in representative averaged traces (left) and grouped data (right). The augmentation by isradipine was greater in D2L than D2S neurons (n = 6–11, unpaired t test). The time course of the isradipine-induced augmentation of I-DA can be found in Figure 6—figure supplement 1. Baseline: mean amplitude of six I-DAs preceding isradipine application, ns indicates not significant, *p < 0.05, **p < 0.01, and ‡ indicates significance over baseline (within-group comparison, paired t tests).
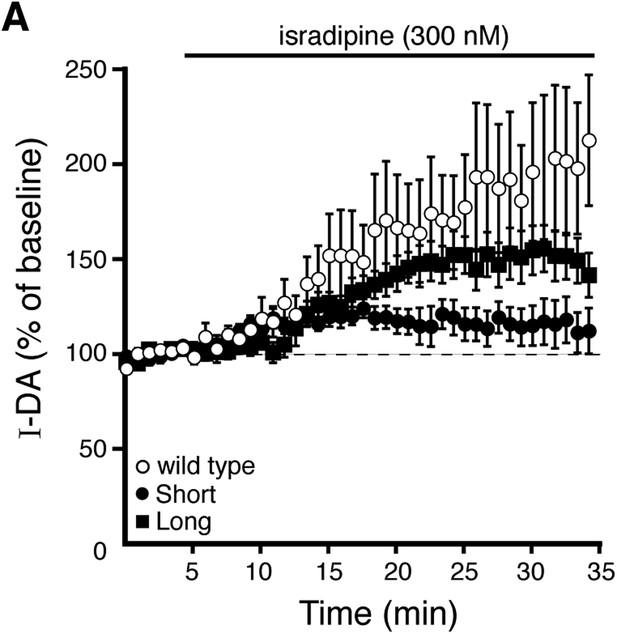
Prolonged isradipine application enhances D2 receptor-dependent currents produced by exogenous dopamine.
(A) Submaximal D2 receptor-dependent outward currents (I-DA) were produced once every 50 s by iontophoretic application of dopamine while recording with EGTA internal. Prolonged isradipine (300 nM) application enhanced I-DA in wild type (open circles), D2S (black circles), and D2L (black squares) neurons. Baseline: mean amplitude of six I-DAs preceding isradipine application.
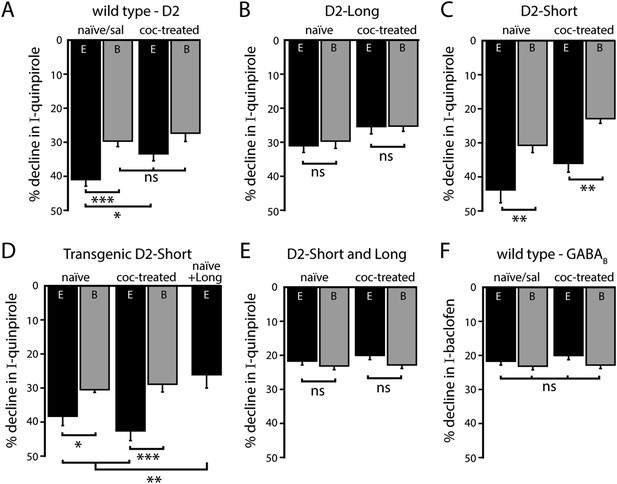
Effects of a single in vivo cocaine exposure on calcium-dependent D2 autoreceptor desensitization.
(A) In neurons from cocaine-treated wild type mice using EGTA internal, the quinpirole-induced current declined less compared to naïve/saline-treated mice, to a level comparable to the decline recorded with BAPTA internal. Cocaine exposure did not alter the decline in the quinpirole-induced current when measured with BAPTA internal (n = 11–26). (B) In D2L neurons after in vivo cocaine exposure, there was no difference in the decline in quinpirole-induced current recorded with EGTA or BAPTA internal (n = 6–7). (C, D) In neurons from (C) AAV-D2S and (D) transgenic D2S mice, the decline in quinpirole-induced current was still greater using EGTA internal compared to BAPTA after in vivo cocaine exposure (C: n = 10 each, D: n = 8–9). (D, E) Co-expression of both splice variants by (D) viral expression of D2L in transgenic D2S mice and (E) infection with a mixture of AAV-D2S and AAV-D2L removed the calcium-dependence of the decline in the quinpirole-induced current (D: n = 5–8, E: n = 6–9) and there was no change after in vivo cocaine (E: n = 7–9). (F) Previous cocaine exposure had no effect on the decline in baclofen-induced current recorded with EGTA or BAPTA internal in wild type neurons (n = 13–27). Comparisons were made with one-way ANOVAs followed when p < 0.05 by Fisher's LSD. ns indicates not significant, *p < 0.05, **p < 0.01, ***p < 0.001.





