

高等学校化学学报 ›› 2014, Vol. 35 ›› Issue (4): 776.doi: 10.7503/cjcu20131287
齐艳兵, 朱吉人, 孙尧金, 杜芸, 褚建君, 石婷, 赵一雷( ), 王晓雷(
), 王晓雷( )
)
收稿日期:2013-12-27
出版日期:2014-04-10
发布日期:2014-02-25
作者简介:联系人简介: 赵一雷, 男, 博士, 教授, 博士生导师, 主要从事生物分子结构计算和动力学研究. E-mail: 基金资助:
QI Yanbing, ZHU Jiren, SUN Yaojin, DU Yun, CHU Jianjun, SHI Ting, ZHAO Yilei*( ), WANG Xiaolei*(
), WANG Xiaolei*( )
)
Received:2013-12-27
Online:2014-04-10
Published:2014-02-25
Contact:
ZHAO Yilei,WANG Xiaolei
E-mail:yileizhao@sjtu.edu.cn;thundawner@gmail.com
Supported by:摘要:
通过生物信息学分析、 分子动力学模拟及量子化学计算, 对21种邻对位取代酚类模式底物与漆酶的结合能力以及反应活性进行了探讨. 生物信息学结构比对分析发现漆酶的活性口袋含有Asp/Glu206, Asn/His208, Asn264, Gly392和His458等保守的氨基酸残基(氨基酸残基编号以Trametes versicolor漆酶为例, PDB: 1KYA); 采用MM-GBSA方法计算了21种酚类模式底物与T. versicolor漆酶的结合自由能. 分子力学计算结果表明, 漆酶与底物的结合力主要来自Asp206和Asn264等残基与底物分子形成的分子间氢键, 并且Phe265残基和酚类底物的芳香环形成π-π相互作用. 量子化学计算表明, 芳环上取代基的推拉电子效应显著影响协同电子转移的底物去质子化过程, 其中推电子能力较强的—NH2, —OH, —OCH3和—CH=CHCH3等基团能够明显增强酚羟基反应活性, 而吸电子的—CONH2和—Cl则具有相反的效应.
中图分类号:
TrendMD:
齐艳兵, 朱吉人, 孙尧金, 杜芸, 褚建君, 石婷, 赵一雷, 王晓雷. 漆酶与酚类模式底物的结合及反应活性的理论研究. 高等学校化学学报, 2014, 35(4): 776.
QI Yanbing, ZHU Jiren, SUN Yaojin, DU Yun, CHU Jianjun, SHI Ting, ZHAO Yilei, WANG Xiaolei. Theoretical Studies of the Binding-affinity and Reactivity Between Laccase and Phenolic Substrates†. Chem. J. Chinese Universities, 2014, 35(4): 776.
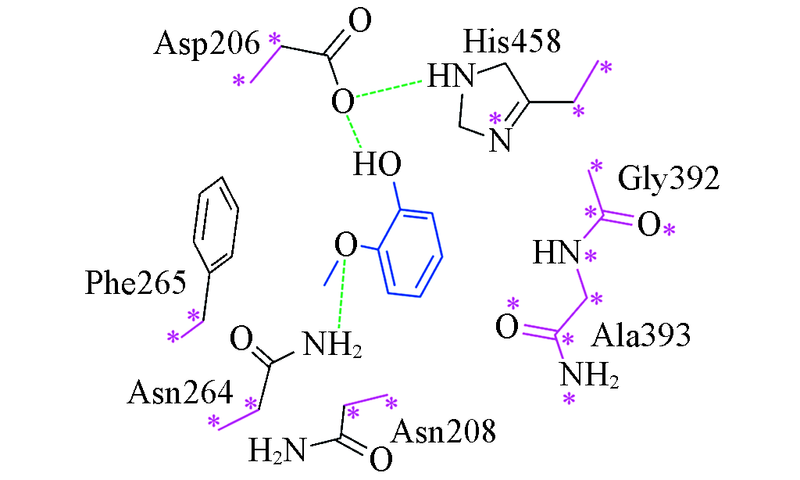
Fig.3 Constructed laccase-substrate model calculated in quantum chemistrySubstrate in blue, constrained heavy atoms in purple and labeled with asterisk, the green dash line represent hydrogen bonding interaction.
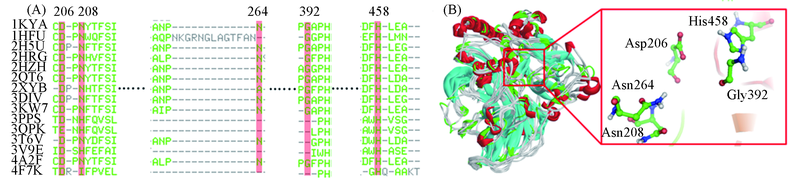
Fig.4 Sequence alignment of the laccases from 15 different organisms(A) and superimposition of the 15 laccase structures and diagram for the common structure of their active pockets(B)
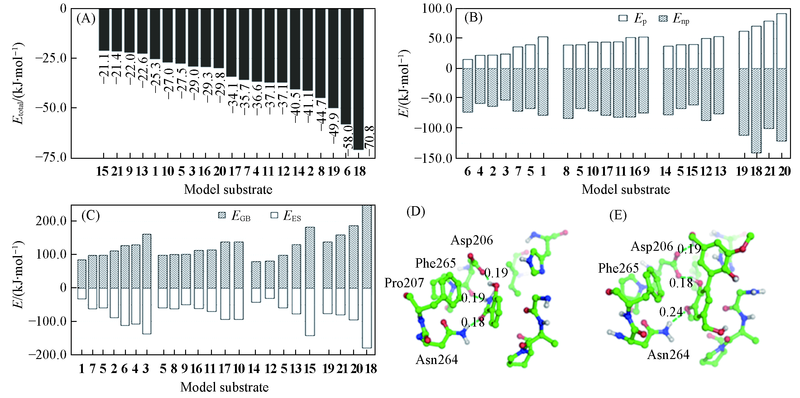
Fig.5 Analysis of binding affinity(A)The total binding energy between laccase and substrates; (B) the polar(Ep) and non-polar(Enp) interaction components;(C) the electrostatic contribution to the solvation free energy calculated by GB(EGB) and electrostatic energy(EES); (D) the complex structure of laccase and substrate 6; (E) the complex structure of laccase and substrate 18. Distance in nm.
| Substrate | ΔEb/(kJ·mol-1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leu164 | Asp206 | Asn208 | Phe239 | Asn264 | Phe265 | Pro391 | Gly392 | Ala393 | Pro394 | Hie458 | |
| 1 | -1.34 | 9.45 | -1.34 | -0.42 | -3.51 | -3.55 | -0.29 | -2.80 | 0 | -2.76 | -1.00 |
| 2 | -2.42 | -1.42 | -0.59 | -0.29 | -1.67 | -4.05 | -0.38 | -2.13 | -0.79 | -1.80 | -1.76 |
| 3 | -0.84 | -8.99 | -1.96 | -0.67 | -3.64 | -3.30 | -0.25 | -0.84 | -1.42 | -1.21 | -1.55 |
| 4 | -1.09 | -2.26 | -0.96 | -0.42 | -2.55 | -3.64 | -0.29 | -1.38 | -0.67 | -1.88 | -1.05 |
| 5 | -0.75 | 9.15 | -2.80 | -0.67 | -5.27 | -3.34 | -0.25 | -1.21 | -1.00 | -2.22 | -0.79 |
| 6 | -0.84 | -4.39 | -4.68 | -0.96 | -6.73 | -4.39 | -0.25 | -0.96 | -0.42 | -2.72 | -2.09 |
| 7 | -2.63 | 2.30 | -0.50 | -0.33 | -0.84 | -4.05 | -1.05 | -2.34 | -0.92 | -2.22 | -2.68 |
| 8 | -1.46 | 6.44 | -2.01 | -0.50 | -5.73 | -4.56 | -0.25 | -3.39 | -0.67 | -3.97 | -0.75 |
| 9 | -2.13 | 9.78 | -0.96 | -0.50 | -5.35 | -3.01 | -0.38 | -3.55 | -0.38 | -2.51 | -0.54 |
| 10 | -1.71 | 8.40 | -0.84 | -0.46 | -6.10 | -3.51 | -0.33 | -3.55 | -2.97 | -2.93 | -0.71 |
| 11 | -1.34 | 6.77 | -2.05 | -0.46 | -6.10 | -4.35 | -0.25 | -2.88 | -1.25 | -4.22 | -1.09 |
| 12 | -2.63 | 7.15 | -2.05 | -0.54 | -3.89 | -3.68 | -0.46 | -4.39 | -0.29 | -2.88 | -0.88 |
| 13 | -2.26 | 9.24 | -0.92 | -0.42 | -4.47 | -3.14 | -0.50 | -4.47 | 0.04 | -1.71 | -0.71 |
| 14 | -2.34 | 3.76 | -1.09 | -0.46 | -1.25 | -4.60 | -0.42 | -2.63 | -0.42 | -2.55 | -1.30 |
| 15 | -2.51 | -6.94 | -0.75 | -0.42 | -1.59 | -3.55 | -0.46 | -2.88 | -0.59 | -1.80 | -1.00 |
| 16 | -1.30 | 8.74 | -1.76 | -0.46 | -6.23 | -4.01 | -0.33 | -2.84 | -0.04 | -3.05 | -0.88 |
| 17 | -1.50 | 8.28 | -1.00 | -0.46 | -4.72 | -4.85 | -0.25 | -2.30 | -1.30 | -3.55 | -0.88 |
| 18 | -2.30 | 10.24 | -0.88 | -0.46 | -2.93 | -6.60 | -5.77 | -5.64 | -3.68 | -3.59 | -5.98 |
| 19 | -8.53 | 8.40 | -0.13 | -0.21 | 0.04 | -3.05 | -4.81 | -6.60 | 1.34 | -1.67 | -4.39 |
| 20 | -4.97 | 8.07 | -0.75 | -0.38 | -4.18 | -5.31 | -0.92 | -4.93 | -1.38 | -5.94 | -3.72 |
| 21 | -2.68 | 9.91 | -1.38 | -0.42 | -5.31 | -7.77 | -1.21 | -3.68 | -3.47 | -3.64 | -3.59 |
| Average | -2.26 | 4.85 | -1.38 | -0.46 | -3.93 | -4.22 | -0.92 | -3.09 | -0.96 | -2.80 | -1.76 |
Table 1 Energy decomposition of the binding affinity between the amino acid(AA) residues and phenolic substrates
| Substrate | ΔEb/(kJ·mol-1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leu164 | Asp206 | Asn208 | Phe239 | Asn264 | Phe265 | Pro391 | Gly392 | Ala393 | Pro394 | Hie458 | |
| 1 | -1.34 | 9.45 | -1.34 | -0.42 | -3.51 | -3.55 | -0.29 | -2.80 | 0 | -2.76 | -1.00 |
| 2 | -2.42 | -1.42 | -0.59 | -0.29 | -1.67 | -4.05 | -0.38 | -2.13 | -0.79 | -1.80 | -1.76 |
| 3 | -0.84 | -8.99 | -1.96 | -0.67 | -3.64 | -3.30 | -0.25 | -0.84 | -1.42 | -1.21 | -1.55 |
| 4 | -1.09 | -2.26 | -0.96 | -0.42 | -2.55 | -3.64 | -0.29 | -1.38 | -0.67 | -1.88 | -1.05 |
| 5 | -0.75 | 9.15 | -2.80 | -0.67 | -5.27 | -3.34 | -0.25 | -1.21 | -1.00 | -2.22 | -0.79 |
| 6 | -0.84 | -4.39 | -4.68 | -0.96 | -6.73 | -4.39 | -0.25 | -0.96 | -0.42 | -2.72 | -2.09 |
| 7 | -2.63 | 2.30 | -0.50 | -0.33 | -0.84 | -4.05 | -1.05 | -2.34 | -0.92 | -2.22 | -2.68 |
| 8 | -1.46 | 6.44 | -2.01 | -0.50 | -5.73 | -4.56 | -0.25 | -3.39 | -0.67 | -3.97 | -0.75 |
| 9 | -2.13 | 9.78 | -0.96 | -0.50 | -5.35 | -3.01 | -0.38 | -3.55 | -0.38 | -2.51 | -0.54 |
| 10 | -1.71 | 8.40 | -0.84 | -0.46 | -6.10 | -3.51 | -0.33 | -3.55 | -2.97 | -2.93 | -0.71 |
| 11 | -1.34 | 6.77 | -2.05 | -0.46 | -6.10 | -4.35 | -0.25 | -2.88 | -1.25 | -4.22 | -1.09 |
| 12 | -2.63 | 7.15 | -2.05 | -0.54 | -3.89 | -3.68 | -0.46 | -4.39 | -0.29 | -2.88 | -0.88 |
| 13 | -2.26 | 9.24 | -0.92 | -0.42 | -4.47 | -3.14 | -0.50 | -4.47 | 0.04 | -1.71 | -0.71 |
| 14 | -2.34 | 3.76 | -1.09 | -0.46 | -1.25 | -4.60 | -0.42 | -2.63 | -0.42 | -2.55 | -1.30 |
| 15 | -2.51 | -6.94 | -0.75 | -0.42 | -1.59 | -3.55 | -0.46 | -2.88 | -0.59 | -1.80 | -1.00 |
| 16 | -1.30 | 8.74 | -1.76 | -0.46 | -6.23 | -4.01 | -0.33 | -2.84 | -0.04 | -3.05 | -0.88 |
| 17 | -1.50 | 8.28 | -1.00 | -0.46 | -4.72 | -4.85 | -0.25 | -2.30 | -1.30 | -3.55 | -0.88 |
| 18 | -2.30 | 10.24 | -0.88 | -0.46 | -2.93 | -6.60 | -5.77 | -5.64 | -3.68 | -3.59 | -5.98 |
| 19 | -8.53 | 8.40 | -0.13 | -0.21 | 0.04 | -3.05 | -4.81 | -6.60 | 1.34 | -1.67 | -4.39 |
| 20 | -4.97 | 8.07 | -0.75 | -0.38 | -4.18 | -5.31 | -0.92 | -4.93 | -1.38 | -5.94 | -3.72 |
| 21 | -2.68 | 9.91 | -1.38 | -0.42 | -5.31 | -7.77 | -1.21 | -3.68 | -3.47 | -3.64 | -3.59 |
| Average | -2.26 | 4.85 | -1.38 | -0.46 | -3.93 | -4.22 | -0.92 | -3.09 | -0.96 | -2.80 | -1.76 |
| Position | Model | R(2-ortho) | R(6-ortho) | R(para) | ΔΔG/(kJ·mol-1) | ΔΔG'/(kJ·mol-1) | Taft σ* | σpara |
|---|---|---|---|---|---|---|---|---|
| 2-ortho- | 4 | NH2 | H | H | 0.00 | 0.62 | ||
| 3 | OH | H | H | 39.33 | 1.34 | |||
| 5 | OCH3 | H | H | 40.84 | 1.81 | |||
| 7 | CH=CHCH3 | H | H | 43.72 | 0.36 | |||
| 1 | H | H | H | 55.76 | 0.49 | |||
| 2 | Cl | H | H | 78.50 | 2.96 | |||
| 6 | CONH2 | H | H | 98.69 | 1.68 | |||
| ortho, | 9 | OCH3 | H | NH2 | -8.78 | -49.62a | -0.66 | |
| para- | 10 | OCH3 | H | OH | 19.31 | -21.53a | -0.37 | |
| 8 | OCH3 | H | Cl | 46.44 | 5.60a | 0.23 | ||
| 11 | OCH3 | H | CONH2 | 53.88 | 13.04a | 0.36 | ||
| 2,6- | 12 | OCH3 | OCH3 | H | -19.77 | -60.61a | 3.62 | |
| 13 | OCH3 | OH | H | -0.84 | -41.67a | 3.15 | ||
| 15 | OH | OH | H | 45.48 | 6.14b | 2.68 | ||
| 14 | Cl | Cl | H | 76.37 | -2.17c | 5.92 | ||
| Lignin | 21 | OCH3 | H | CHR''OH | 11.75 | |||
| model | 18 | OCH3 | PhR | CH2OH | 25.50 | |||
| compounds | 20 | OCH3 | H | CHR'OH | 37.91 | |||
| 16 | OCH3 | H | CH2OH | 42.85 | ||||
| 17 | OCH3 | H | CHO | 72.31 | ||||
| 19 | OCH3 | H | COR' | 76.95 |
Table 2 Comparison of Gibbs free energy changes among different phenolic substrates
| Position | Model | R(2-ortho) | R(6-ortho) | R(para) | ΔΔG/(kJ·mol-1) | ΔΔG'/(kJ·mol-1) | Taft σ* | σpara |
|---|---|---|---|---|---|---|---|---|
| 2-ortho- | 4 | NH2 | H | H | 0.00 | 0.62 | ||
| 3 | OH | H | H | 39.33 | 1.34 | |||
| 5 | OCH3 | H | H | 40.84 | 1.81 | |||
| 7 | CH=CHCH3 | H | H | 43.72 | 0.36 | |||
| 1 | H | H | H | 55.76 | 0.49 | |||
| 2 | Cl | H | H | 78.50 | 2.96 | |||
| 6 | CONH2 | H | H | 98.69 | 1.68 | |||
| ortho, | 9 | OCH3 | H | NH2 | -8.78 | -49.62a | -0.66 | |
| para- | 10 | OCH3 | H | OH | 19.31 | -21.53a | -0.37 | |
| 8 | OCH3 | H | Cl | 46.44 | 5.60a | 0.23 | ||
| 11 | OCH3 | H | CONH2 | 53.88 | 13.04a | 0.36 | ||
| 2,6- | 12 | OCH3 | OCH3 | H | -19.77 | -60.61a | 3.62 | |
| 13 | OCH3 | OH | H | -0.84 | -41.67a | 3.15 | ||
| 15 | OH | OH | H | 45.48 | 6.14b | 2.68 | ||
| 14 | Cl | Cl | H | 76.37 | -2.17c | 5.92 | ||
| Lignin | 21 | OCH3 | H | CHR''OH | 11.75 | |||
| model | 18 | OCH3 | PhR | CH2OH | 25.50 | |||
| compounds | 20 | OCH3 | H | CHR'OH | 37.91 | |||
| 16 | OCH3 | H | CH2OH | 42.85 | ||||
| 17 | OCH3 | H | CHO | 72.31 | ||||
| 19 | OCH3 | H | COR' | 76.95 |
| [1] | Mayer A. M., Staples R. C., Phytochemistry,2002, 60(6), 551—565 |
| [2] | Bao W., O'malley D. M., Whetten R., Sederoff R. R., Science,1993, 260(5108), 672 |
| [3] | Wan Y. Y., Miyakoshi T., Du Y. M., Chen L. J., Hao J. M., Kennedy J. F., Int. J. Biol. Macromol., 2012, 50(3), 530—533 |
| [4] | Giardina P., Faraco V., Pezzella C., Piscitelli A., Vanhulle S., Sannia G., Cellular and Molecular Life Sciences,2010, 67(3), 369—385 |
| [5] | Dai Y., Yin L., Niu J., Environmental Science & Technology,2011, 45(24), 10611—10618 |
| [6] | Lu J., Huang Q., Mao L., Environmental Science & Technology,2009, 43(18), 7062—7067 |
| [7] | Feng Y., Colosi L. M., Gao S., Huang Q., Mao L., Environmental Science & Technology,2012, 47(2), 1001—1008 |
| [8] | Baldrian P., FEMS Microbiology Reviews, 2006, 30(2), 215—242 |
| [9] | Solomon E. I., Sundaram U. M., Machonkin T. E., Chemical Reviews,1996, 96(7), 2563—2606 |
| [10] | Piontek K., Antorini M., Choinowski T., J. Biol. Chem., 2002, 277(40), 37663—37669 |
| [11] | Jeon J. R., Chang Y. S., TRENDS in Biotechnology,2013, 31(6), 335—341 |
| [12] | Prasad N. K., Vindal V., Narayana S. L., Kunal S. P., J. Mole. Mode., 2011, 18(5), 2013—2019 |
| [13] | Cambria M. T., Di Marino D., Falconi M., Garavaglia S., Cambria A., J. Biomol. Struct. Dyn., 2010, 27(4), 501—509 |
| [14] | Galli C., Gentili P., Jolivalt C., Madzak C., Vadalà R., Applied Microbiology and Biotechnology,2011, 91(1), 123—131 |
| [15] | Schomburg I., Chang A., Placzek S., Söhngen C., Rother M., Lang M., Munaretto C., Ulas S., Stelzer M., Grote A., Nucleic Acids Research,2013, 41(D1), D764—D772 |
| [16] | Eggert C., Temp U., Dean J. F., Eriksson K. E. L., FEBS Letters,1996, 391(1), 144—148 |
| [17] | Lahtinen M., Kruus K., Boer H., Kemell M., Andberg M., Viikari L., Sipilä J., J. Mole. Cata. B: Enzymatic,2009, 57(1), 204—210 |
| [18] | The PyMOL Molecular Graphics System, Version 1.3r1, Schrödinger LLC, Version 1.3r1, Schrödinger LLC, Portland OR, 2010 |
| [19] | Rastelli G., Rio A. D., Degliesposti G., Sgobba M., J. Comput. Chem., 2010, 31(4), 797—810 |
| [20] | Case D., Darden T., Cheatham III T., Simmerling C., Wang J., Duke R., Luo R., Walker R., Zhang W., Merz K., Amber, Version 10, University of California, San Francisco, 2012 |
| [21] | Mahoney M. W., Jorgensen W. L., J. Chem. Phys., 2000, 112(20), 8910—8922 |
| [22] | Miller B. R. III, McGee T. D. Jr., Swails J. M., Homeyer N., Gohlke H., Roitberg A. E., J. Chem. Theo. Comput., 2012, 8(9), 3314—3321 |
| [23] | Bertrand T., Jolivalt C., Briozzo P., Caminade E., Joly N., Madzak C., Mougin C., Biochemistry,2002, 41(23), 7325—7333 |
| [24] | Frisch M.J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J. A. Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Revision A.02, Gaussian Inc., Wallingford CT, 2009 |
| [25] | Becke A. D., J. Chem. Phys., 1993, 98(2), 1372—1377 |
| [26] | Kallio J., Auer S., Janis J., Andberg M., Kruus K., Rouvinen J., Koivula A., Hakulinen N., J. Mole. Bio., 2009, 392(4), 895—909 |
| [27] | Tadesse M. A., D’Annibale A., Galli C., Gentili P., Sergi F., Org. Biomol. Chem., 2008, 6(5), 868—878 |
| [28] | Dean J.A., Lange’s Handbook of Chemistry, McGraw-Hill Inc., New York, 1985, 1348—1352 |
| [29] | Galli C., Madzak C., Vadalà R., Jolivalt C., Gentili P., Chem. Bio. Chem., 2013, 14(8), 2500—2505 |
| [30] | Weinberg D. R., Gagliardi C. J., Hull J. F., Murphy C. F., Kent C. A., Westlake B. C., Paul A., Ess D. H., McCafferty D. G., Meyer T. J., Chemical Reviews,2012, 112(7), 4016—4093 |
| [31] | Hammes-Schiffer S., Proceedings of the National Academy of Sciences, 2011, 108(21), 8531—8532 |
| [32] | Madzak C., Mimmi M., Caminade E., Brault A., Baumberger S., Briozzo P., Mougin C., Jolivalt C., Protein Engineering Design and Selection,2006, 19(2), 77—84 |
| [1] | 周宁, 唐小华, 曹红, 查飞, 李春, 谢春燕, 徐明平, 孙艺格. 石榴状凝胶微球固定化漆酶的制备、 表征及降解双酚A[J]. 高等学校化学学报, 2022, 43(2): 20210705. |
| [2] | 温炜, 黄达锭, 鲍劲霄, 张增辉. PD-1与单克隆抗体残基特异性结合机制的计算丙氨酸扫描研究[J]. 高等学校化学学报, 2021, 42(7): 2161. |
| [3] | 李心怡, 刘永军. 人工设计逆醛缩酶RA95.5-8F催化β-羟基酮C—C裂解的理论研究[J]. 高等学校化学学报, 2021, 42(7): 2306. |
| [4] | 李康明, 李延赛, 易阳杰, 徐雷涛, 叶姣, 欧晓明, 李建明, 胡艾希. 5-吡唑甲酰胺类衍生物的设计、 合成与生物活性[J]. 高等学校化学学报, 2020, 41(4): 716. |
| [5] | 闫松, 张成武, 袁芳, 秦传玉. 氮掺杂碳纳米管的合成及活化过一硫酸盐的性能与机理[J]. 高等学校化学学报, 2020, 41(11): 2503. |
| [6] | 马玉聪, 樊保民, 王满曼, 杨彪, 郝华, 孙辉, 张慧娟. 曲唑酮的两步法制备及对碳钢的缓蚀机理[J]. 高等学校化学学报, 2019, 40(8): 1706. |
| [7] | 李庆, 易平贵, 陶洪文, 李洋洋, 张志于, 彭文宇, 李玉茹. 溶剂效应和取代基效应对2-(2-氨基苯基)苯并噻唑光谱性质及激发态分子内质子转移的影响[J]. 高等学校化学学报, 2019, 40(7): 1425. |
| [8] | 段秉怡, 王宇, 郭宁宁, 王润伟, 张宗弢, 裘式纶. 蛋黄-壳结构Fe3O4@SiO2@PMO磁性微球的制备及对漆酶的固定化[J]. 高等学校化学学报, 2019, 40(2): 210. |
| [9] | 董秋辰,张光华,张万斌,张雪,刘晶. 甲基丙烯酸二甲氨基乙酯类离子液体对Q235钢的缓蚀性能[J]. 高等学校化学学报, 2019, 40(12): 2556. |
| [10] | 董秋辰,张光华,张万斌,刘晶. 喹啉型双子季铵盐缓蚀剂的实验及理论分析[J]. 高等学校化学学报, 2019, 40(10): 2195. |
| [11] | 马玉聪, 樊保民, 郝华, 吕金玉, 冯云皓, 杨彪. 十八胺基分子组装体在碳钢表面的作用机理与模拟[J]. 高等学校化学学报, 2019, 40(1): 96. |
| [12] | 朱镜璇, 于正飞, 刘野, 詹冬玲, 韩佳睿, 田晓翩, 韩葳葳. 基于分子动力学模拟提高嗜热磷酸三酯酶样内酯酶非特异性底物活力的理论研究[J]. 高等学校化学学报, 2019, 40(1): 138. |
| [13] | 赵邦屯, 陶晶晶, 陈小纪, 付慧敏, 朱卫民. 含噻吩和吡啶基的插烯式四硫富瓦烯衍生物的合成、 结构和电化学性质[J]. 高等学校化学学报, 2018, 39(7): 1449. |
| [14] | 郭睿, 李云鹏, 土瑞香, 宋博, 郭煜. 3-丁基-5,5-二甲基海因咪唑季铵盐对HCl溶液中Q235钢的缓蚀性能[J]. 高等学校化学学报, 2018, 39(5): 1018. |
| [15] | 周丽婷, 雷小洋, 王渭娜, 陈东平, 刘峰毅, 王文亮. Criegee中间体RCHOO(R=H,CH3)与NO2反应机理及大气中HNO3的形成[J]. 高等学校化学学报, 2018, 39(5): 956. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||