INTRODUCTION
Breast cancer is one of the most dangerous cancers affecting the female population worldwide, which accounts for more than 500,000 deaths every year (Torre et al., 2015). Though the etiology of breast cancer includes multiple factors such as environmental, genetic, social, demographic, and hormonal, the role of oxidative stress in the occurrence of breast cancer cannot be ruled out (Rockenbach et al., 2011). During the aerobic oxidative process of energy production by the metabolism of carbohydrates, proteins, and lipids, the highly reactive oxygen species (ROS) such as superoxide (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals are produced which are efficiently scavenged by the endogenous antioxidant defense systems in the form of enzymes namely superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), glutathione reductase (GR), glutathione S-transferase (GST), and non-enzymatic antioxidant vitamin C, vitamin E, and glutathione (Amin et al., 2012). Oxidative stress is encountered by the cells whenever the critical balance between the production of ROS and their potential scavenging by this defense system is lost. This leads to the destruction of nuclear as well as mitochondrial DNA, resulting in a mutation that in turn activates oncogenes and inactivates the tumor suppressor genes. According to Grinan-Lison et al. (2021), antioxidants have the potential to combat breast cancer carcinogens as well as inhibit tumor progression which makes their usage as adjuvants in cancer chemotherapy.
In spite of the availability of advanced treatment in the form of radiation, chemotherapy, and surgery, still, breast cancer management remains a critical health problem worldwide due to the expensive nature, side effects, and resistance to anticancer drugs by cancer cells. It is widely accepted that plant-derived natural products are excellent sources of anticancer drugs. The biologically active phytochemicals present in the natural crude extracts have anticancer potential in modulating the signaling cascades leading to either cell cycle progression or apoptosis and may be developed into a new anticancer drug with minimal or no side effects. Excoecaria agallocha L (EEEA) which belongs to the family of Euphorbiacea is a mangrove tree widely grown in Asia, Africa, India, and Northwest Australia. The medicinal properties such as antinociceptive, gastro-protective, antimicrobial, antioxidant, anti-hyperglycemic, and anticancer activities are reported for the whole plant (Simlai and Roy, 2013). Kumar Reddy et al. (2019) have shown the anticancer activity of leaves of this plant in the human breast cancer (MCF-7) cell line. According to Sultana et al. (2022) the bioactive compound Bergenin present in the EEEA has the ability to arrest the SiHa cervical cancer cells through autophagy and apoptosis mechanism in the G2/M phase cell cycle. With the above scenario, this investigation is undertaken to study the anti-proliferative potentials of ethanolic EEEA leaves in terms of their restoration of altered antioxidant status in breast cancer MCF-7 cell line.
MATERIALS AND METHODS
Chemicals and reagents
The fine chemicals such as oxidized glutathione (GSSG), fetal bovine serum, nicotinamide adenine dinucleotide phosphate (NADPH), 1-chloro-2, 4, dinitrobenzene, and epinephrine were purchased from HiMedia Chemicals Private Limited. The MCF-7 cell line was obtained from National Centre for Cell Science, Pune, India. Other chemicals such as acids bases and analytical grade chemicals used in this study were obtained from SD fine chemicals limited.
Collection and preparation of ethanolic extract EEEA
The mangrove plant EEEA leaves were collected from the Pichavaram mangrove forest from the southeast coast of Tamil Nadu, India. The leaves were authenticated by the herbaria of the Centre of Advanced Study in Marine Biology, Annamalai University, Tamil Nadu, India. The EEEA leaves were shade dried after cleaning with water and a coarse powder was prepared using a blender. Using 90% v/v ethanol, the extract of EEEA was prepared by cold maceration process at room temperature. The extract was subjected to filtration and excess solvent present in the EEEA extract was removed using rota flash evaporator and the final sample yield was calculated. The yield of the EEEA extract was found to be 2.12% w/w which was stored at 4°C until further use.
Treatment and maintenance of MCF-7 cell lines with EEEA extract
The MCF-7 cell line which was primarily passaged was seeded in a 25-cm2 tissue culture flask containing DMEM medium, 10% FBS with an antibiotic solution containing penicillin (100U/ml), streptomycin (100μg/ml), and amphotericin B (2.5μg/ml) and maintained in a humidified 5% CO2 incubator (NBS Eppendorf, Germany) at 37°C for 24 hours. Once 70% confluence of cells is attained in the 6-well plate (approximately 4 × 105 cells), the cells were divided into four groups and the following treatments were given. Group-I control cells without any treatment, group-II cells were treated with EEEA 25 μg/ml, and group–III cells were treated with EEEA 50μg/ml. Group-IV cells were treated with doxorubicin (10μg/ml), the positive control used in this study. The treated cells were incubated at 37°C for 24 hours in a CO2 incubator.
Cell lysate preparation for antioxidant assay
Trypsinization was carried out in the treated cells using Trypsin-EDTA Solution, which was collected in the Eppendorf tubes and centrifuged for 5 minutes at 3,000 rpm to collect the pellet. The collected pellet was resuspended in 200 μl of lysis buffer (0.1M Tris, 0.2M EDTA, 2M NaCl, 0.5% Triton). The cell lysate prepared was incubated for 20 minutes at 4°C and was used for the analysis of antioxidant enzymes.
Estimation of enzymatic and non-enzymatic antioxidants
The following enzymatic antioxidants such as CAT (Sinha, 1972), SOD (Marklund and Marklund, 1974), GR (Dobler and Anderson, 1981), GPX (Necheles et al., 1968), and GST (Habig et al., 1974) were analyzed in the cell lysate using established protocols. The non-enzymatic antioxidants like GSH (Sedlak and Lindsay, 1968), ascorbic acid (Omaye et al., 1979), and α-Tocopherol (Desai, 1984) were also measured in the cell lysate. All the enzymatic and non-enzymatic parameters were estimated using the Shimadzu spectrophotometer, UV-1601 model at specific wavelengths.
Statistical analysis
All the above-mentioned experiments were performed in triplicates and statistical significance was calculated using the latest SPSS version. To test the statistical significance for comparison, ANOVA was used followed by Dunnett’s t-test. p-values < 0.05 were considered significant.
RESULTS
Enzymatic antioxidants
The levels of antioxidant enzymes like CAT, GST, GR, and GPx in control, EEEA, and Doxorubicin-treated MCF-7 cell lines are depicted in Table 1. The enzymes such as SOD, GST, GR, and GPx were significantly decreased (p < 0.01) and CAT is increased in the untreated control cell lines when compared to the EEEA and doxorubicin-treated groups. In this study, the cells treated with 50 μg/ml EEEA extract exhibited higher enzymatic antioxidant levels proving the fact that the phytochemicals present in the extract could scavenge the free radicals thereby reducing the further complications of oxidative stress encountered by the cancer cells.
Non-enzymatic antioxidants
Table 2 depicts the levels of non-enzymatic antioxidants such as glutathione, vitamin C, and vitamin A in different cell line groups. In accordance with the enzymatic antioxidants, the levels of vitamin C and vitamin A were significantly (p < 0.01, 0.05) decreased and glutathione levels were increased in untreated control MCF-7 cell lines. The EEEA extract treatment restores the levels of these altered non-enzymatic antioxidants and it was more pronounced in the group II cells which were treated with the concentration of EEEA 50μg/ml of the extract.
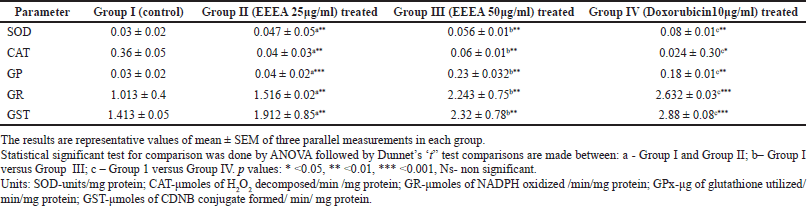 | Table 1. Levels of enzymatic antioxidants in different experimental groups of MCF-7 cell lines. [Click here to view] |
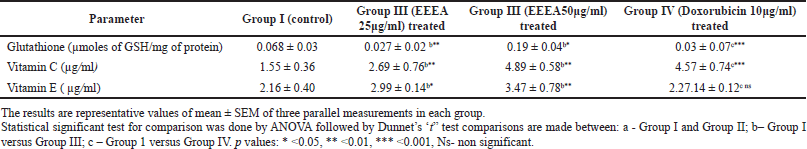 | Table 2. Levels of non enzymatic antioxidants in different experimental groups of MCF-7 cell lines. [Click here to view] |
DISCUSSION
Cancer is a group of much-feared diseases characterized by uncontrolled growth and the spread of abnormal cells leading to mortality (Akim et al., 2011). In spite of current advanced treatment by several classes of anticancer drugs, all have clinical limitations and adverse side effects. Therefore, the search for a new anticancer drug from natural products represents an area of great research interest to provide a successful and novel antioxidant and anticancer molecule with a mechanism of action to combat oxidative stress as well as kill cancer cells. Currently, about 60% of anticancer drugs used for cancer chemotherapy are derived from medicinal plants and still many plants with anticancer potential have to be properly investigated for anticancer drug development (Cragg and Newman, 2005). Batsa and Periyasamy (2013) have shown that the methanolic leaf extract of EEEA at lower concentrations exhibited high anticancer activity.
ROS are produced in the mitochondria due to the metabolic reactions that occur in the respiratory chain and will have varied biological features. At high concentrations of ROS, they induce apoptosis or necrosis thereby inducing cell death. At particular concentrations, they act like endogenous carcinogens to induce DNA mutation, inflammation, oncogenic stimulation, increased metabolic activity, and mitochondrial damage leading to carcinogenesis (Fang et al., 2009). Therefore, it is an accepted fact that cancer cells show increased oxidative stress. There are several antioxidant defense systems in terms of endogenous and exogenous antioxidants to combat the oxidative stress encountered by cancer cells. The antioxidants in the form of enzymes, such as SOD, CAT, GPx, GR, and GST constitute the first line of the endogenous antioxidant enzyme system. The exogenous antioxidants are molecules mainly supplied from an external source either in the diet or as supplements, and also plays important role in reducing oxidative stress. According to Gulcin (2020), the use of antioxidants as dietary supplements has the potential to revert the oxidative stress induced by radio- and chemotherapy.
In our present investigation, there is an increase in the level of CAT, and a decrease in the levels of SOD, GPx, GR, and GST was observed in the untreated MCF-7 cell lines. Mates and Sanchez-Jimenez (1999) also reported a decreased level of SOD in the MCF-7 cell lines. SOD constitutes one of the main cellular antioxidant enzymes that catalysis the conversion of O2- to H2O2 which in turn gets converted into water with the help of CAT. The increased CAT in this investigation may be due to the low levels of H2O2 because of the decreased activity of SOD. The same results were observed in the studies by Kattan et al. (2008). In this present investigation, the increased levels of O2- radicals due to the decreased concentration SOD enzyme may have a proliferative effect in the MCF-7 cells. The treatment with our EEEA extract corrected the levels of the SOD and CAT thereby scavenging the excess levels of ROS, leading the MCF-7 cells toward apoptosis.
There is another parallel GSH-dependent antioxidant enzyme system namely GST, GPX, and GR that are involved in the detoxification mechanisms using GSH as co-substrate. GST mainly catalysis the transfer of electrophilic xenobiotics to reduced glutathione (GSH) thereby converting it into GSSG. The enzyme GPx in turn reduces hydroperoxides as well as H2O2 by utilizing GSH and converting it to GSSG. The GSSH produced due to the above-mentioned reactions, in turn, regains its original reduced (GSH) state by GR, an NADPH-dependent oxidoreductase. In this investigation, the control cells showed an increased concentration of GSH and decreased levels of GST and GPX. The increased GSH levels may be due to their underutilization by these enzymes.
It is also observed that the levels of non-enzymatic antioxidants such as vitamin A and vitamin C levels were restored toward normalcy in the EEEA-extract-treated MCF -7 cells. Based on the meta-analysis studies by Harris et al. (2014), vitamin C supplementation in newly diagnosed cancer patients improves cancer survival. According to Milani et al. (2017), vitamin A has the ability to inhibit tumor growth factor signaling inflammatory cytokines and cell cycle progression in breast cancer cells. The various phytoconstituents such as diterpenoids, triterpenoids, flavonoids, alkaloids, anthraquinone, phytosterol, fixed oil, tannin, phorbol esters, free amino acids, mucilage, glycosides, carbohydrates, and lignin present in EEEA extract as reported by Deepa and Padmaja (2014) are responsible in reverting this altered enzymatic antioxidants levels in the MCF-7 cells thereby regulating the oxidative stress occurring in the tumor cells.
Phytochemical studies revealed the presence of diterpenoids and Anjaneyulu and Rao (2000) isolated five diterpenoids (agallochins A–E) from the leaves of EEEA. Several preclinical studies have proved that terpenoids may be a potential therapeutic agent in treating several types of cancer including breast cancer (Rabi and Gupta, 2008). These terpenoids have the ability to regulate various transcription and growth factors of intracellular signaling mechanisms thereby inhibiting the initiation and promotion of carcinogenesis as well as their invasion and metastasis, inducing apoptosis and suppressing tumor angiogenesis (Deepa and Padmaja, 2014).
CONCLUSION
In this study, it may be concluded that there was a restoration of antioxidant levels in the ethanolic extract of EEEA-treated MCF-7 cell line. This may be because the plant extract has the ability either to scavenge the free radicals produced in the cancer cells or the phytoconstituents present in the extract have the potential to increase the endogenous antioxidant system thereby reducing the oxidative stress encountered by the cancer cells. Extensive research both in genetic and in vivo studies is required to understand the role of EEEA extract as an antioxidant in the form of an adjuvant to prevent complications due to chemotherapy.
ACKNOWLEDGMENTS
We thank Er. A.C.S. Arun Kumar, President, Dr. M.G.R Educational and Research Institute University for providing the necessary facilities.
AUTHORS’ CONTRIBUTIONS
All the authors contributed to the data collection and preparation of the manuscript.
CONFLICT OF INTERESTS
The authors declared that there was no conflict of interest in the study.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akim AM, Ling LC, Rahmat A, Zakaria ZA. Antioxidant and anti proliferative activities of Roselle juice on Caov3, MCF7, MDA MB231 and HeLa cancer cell lines. Afr J Pharm Pharmacol, 2011; 5:957–65.
Amin KA, Mohamed BA, El-Wakil MAM, Ibrahem SO. Impact of breast cancer and combination chemotherapy on oxidative stress, hepatic and cardiac markers. J Breast Cancer, 2012; 15(3):306–12. CrossRef
Anjaneyulu ASR, Rao VL. Five diterpenoids (agallochins A–E) from the mangrove plant Excoecaria agallocha Linn. Phytochemistry, 2000; 55(8):891–901. CrossRef
Batsa AJ, Periyasamy K. Anticancer activity of Excoecaria agallocha leaf extract in cell line model. Int J Pharm Biol Sci, 2013; 3:392–8.
Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol, 2005; 100:72–9. CrossRef
Deepa M, Padmaja CK. Preliminary phytochemical analysis and thin layer chromatography of the extracts of Excoecaria agallocha L. Int J Pharm Sci Res, 2014; 5:4535–42.
Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol, 1984; 105(1):138–47 CrossRef
Dobler RE, Anderson BM. Simultaneous inactivation of the catalytic activities of yeast glutathione reductase by N–alkyl melimides. Biochim Biophys Acta, 1981; 659(1):70–85. CrossRef
Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev, 2009; 61:290–302. CrossRef
Grinan-Lison C, Blaya-Canovas JL, Lopez-Tejada A, Ávalos-Moreno M, Navarro-Ocon A, Cara FE, Gonzalez-Gonzalez A, Lorente JA, Marchal JA, Granados-Principal S. Antioxidants for the treatment of breast cancer: are we there yet? Antioxidants, 2021; 10:205. CrossRef
Gulcin ?. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol, 2020; 94:651–715. CrossRef
Habig WH, Pabst MJ, Jakoby WB. Glutathione –S- transferases. The first enzymatic step in mercapturic acid formation. J Biolog Chemis, 1974; 249:7130. CrossRef
Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer, 2014; 50:1223–31. CrossRef
Mates JM, Sanchez-Jimenez F. Antioxidant enzymes and their implications in pthophysiologic, processes. Front Biosci, 1999; 23(2):11–22.
Kattan Z, Minig V, Leroy P, Dauça M, Becuwe P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res Treat, 2008; 108:203–15. CrossRef
Kumar Reddy PR, Durairaj P, Thirunavukkarasu P, Hari R. Effect of ethanolic extract of Exocaria agallocha leaves on the cytotoxic activity and cell cycle arrest of human breast cancer cell lines- MCF-7. Pharmacogn Mag, 2019; 15(S2):46–51. CrossRef
Marklund S. Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem, 1974; 47:469–74. CrossRef
Milani A, Basirnejad M, Bolhassani A, Shahbazi S. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol, 2017; 174:1290–324. CrossRef
Necheles TF, Boles TA, Allen DM. Erythrocyte glutathione – peroxidase deficiency and haemolytic disease of newborn. J Pediatric, 1968; 72:319. CrossRef
Omaye ST, David JT, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol, 1979; 62(6):3–11.
Rabi T, Gupta S. Dietary terpenoids and prostate cancer chemoprevention. Front Biosci, 2008; 13:3457–69. CrossRef
Rockenbach G, Di Pietro PF, Ambrosi C, Boaventura BC, Vieira FG, Crippa CG, Da Silva EL, Fausto MA. Dietary intake and oxidative stress in breast cancer: before and after treatments. Nutr Hosp, 2011; 26(4):737–44.
Simlai A, Roy A. Biological activities and chemical constituents of some mangrove species from Sundarban estuary: an overview. Pharmacogn Rev, 2013; 7(14):170–8. CrossRef
Sinha AK. Colorimetric assay of catalase. Anal Biochem, 1972; 47:389. CrossRef
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with ellman’s reagent. Anal Biochem, 1968; 25(2):192–205 CrossRef
Sultana T, Mitra AK, Das S. Evaluation of anticancer potential of Excoecaria agallocha (L.) leaf extract on human cervical cancer (SiHa) cell line and assessing the underlying mechanism of action. Futur J Pharm Sci, 2022; 8:3. CrossRef
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin, 2015; 65(2):87–108. CrossRef