INTRODUCTION
Indonesia archipelago is known for its indigenous natural resources, such as marine sponge, which shows high potency of bioactive compounds (Latifah et al., 2021). Around 15,000 species of marine sponges are known to exist, and they remain desirable for use in the pharmaceutical industry (Lim et al., 2014). Sponges do not have an innate immune system and can only protect themselves by producing metabolites that serve as a kind of self-defence and allow them to adapt to their surroundings (Calcabrini et al., 2017; Rocha et al., 2001). Kangean Island is part of the Indonesian Coral Triangle area located in East Java Province, with various types of marine sponges. It contains a wide variety of marine natural products with a high benefit value (Lorig-Roach et al., 2018; Pawlik and McMurray, 2020). Most benefits are antiinflammatory, antifungal, antibacterial, and anticancer agents. One of the sponge species belonging to the Callyspongiidae family, which is widely distributed in this marine environment, has active chemicals with the potential to develop into future treatments for severe diseases like cancer. Callyspongia sp. is a marine sponge that generates secondary metabolites such as steroids, alkaloids, flavonoids, and terpenoids that can be used as antibacterial agents (Wao and Priska, 2021). It is well known that natural products from marine sponges have antioxidant, antiproliferative, and cytotoxic activities since the first discovery of the nucleotides spongouridine and spongotimidine from the marine sponge Cryptotethia crypta. Their synthetic derivative compounds are used to treat breast, lung, pancreatic, and bladder cancers (Kumar and Adki, 2018). Secondary metabolites obtained from sponges of the Callyspongiidae family in their extract and compound have shown antioxidant (Arunachalam and Amirtham Jacob Appadorai, 2013), cytotoxic, and anticancer activities (Youssef et al., 2003).
The hydro-ethanoic extract of sponge Callyspongia sp. from East Java, Indonesia, showed the ability to trap 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radicals at IC50 181 μg/mL, toxic to tumor cell line with IC50 below 50 μg/mL against HT-29 and T47D cells (Abdillah et al., 2013). Aqueous ethanol extract from Callyspongia crassa and Callyspongia siphonella (C. siphonella) showed inhibition against the growth of MCF-7 and Caco-2 cancer cells (Ibrahim et al., 2017). Furthermore, the extract of C. siphonella extracted with various semi-polar solvents showed antiproliferative and proapoptotic activity against HepG-2, MCF-7, and Caco-2 cells (Rady and Bashar, 2020). Two oxindole brominated alkaloid compounds from the Red Sea sponge C. siphonella reveal a strong cytotoxic effect against different human cancer cell lines, presumably through the induction of necrosis (El-Hawary et al., 2019). In addition, the methanol extract from Callyspongia aerizusa is also active in inhibiting the growth of A549 cells with IC50 9.38 μg/mL (Hadisaputri et al., 2021). Callyspongamide, a compound isolated from Callyspongia fistularis, showed inhibitory activity against HeLa, MCF-7, and A549 cells (Ki et al., 2020; Youssef et al., 2003). Polyacetylene diol compounds separation results from EtOAc extract Callyspongia sp. showed antiproliferative properties against HL-60 cells with IC50 values below 10 μg/mL (Umeyama et al., 2010).
Moreover, the organic extracts of marine spongesmay be a viable source of toxic metabolites produced by related bacteria as well as secondary metabolites for the development of novel therapeutics for both human and veterinary illnesses. In light of the enormous potential of marine resources in Indonesia, Kangean Island is one of the islands with various marine, including sponges. The marine sponge Callyspongia affinis, a species of sponge that is only found in the Kangean archipelago of Indonesia, so there are no research reports related to its metabolites and potential benefits. This article aims to learn more about the biological activity of the extract, which includes antioxidant, toxic, and anticancer properties linked to the presence of secondary metabolites that make up the extract.
MATERIALS AND METHODS
Sponge material
The sponges were collected by scuba diving (3–10 m depth) from Kangean Island in the Province of East Java, Indonesia. The samples were identified at the Sepuluh Nopember Institute of Technology’s Zoology and Animal Engineering Laboratory, Surabaya, Indonesia, using scanning and optical microscope studies on skeletal slides and fragmented spicule mounts by the keys of John N. A. Hooper’s sponge guide (Erpenbeck et al., 2020; Umeyama et al., 2010) and identified as C. affinis. The samples were put in plastic bags containing seawater, transferred to the laboratory on ice, and then stored at −10°C.
Preparation and partition of methanol extract
The frozen sponge was thawed, carefully cleansed with 70% alcohol, dried, cut into small pieces, and ground to create a finely powdered sample for 1.91 kg. The powdered sample was dissolved in MeOH under sonication and evaporated using a rotary vacuum evaporator to obtain 75.15 g of brownish-green concentrated extracts. The MeOH extract was partitioned with n-hexane:water and the n-hexane fraction (3.46 g) was obtained. The water fraction was partitioned with EtOAc and BuOH, respectively, in order to obtain EtOAc (19.06 g), BuOH (3.05 g), and water (29.97 g) fractions.
Metabolites analysis
Metabolites analysis was analyzed on the C. affinis crude extract on a high-performance liquid chromatography Thermo Scientific Dionex Ultimate 3000 RSLCnano with a micro flow meter system coupled to an HRMS Thermo Scientific Q Exactive, full scan at 70,000 resolutions and 17,500 resolutions of data-dependent MS2. Chromatographic separation was carried out on a Hypersil GOLD aQ (50 × 1 mm × 1.9 u particle size). A linear binary solvent gradient of 0.1% formic acid in water (v/v) as solvent A and acetonitrile as solvent B was performed over 30 minutes at a flow rate of 40 μl per minute. The column temperature was 30°C, and the injection volume was 2 μl. The raw data were transformed into positive and negative ionization files using MS Converter and subjected using mzCloud MS/MS Library Compound Discoverer software.
DPPH assay
DPPH radicals have an absorption maximum of 515 nm. When an antioxidant reduces DPPH radicals, the color of the solution changes, and the reaction may be easily tracked using a spectrophotometer (UV–Vis Shimadzu). The previously mentioned approach was modified to test the extract’s capacity to eradicate free radicals (Oogarah et al., 2020). Determination procedures were as follows: 0.1 mM DPPH solution was prepared by dissolving 3.94 mg DPPH powder in 100 ml MeOH. A methanolic solution of the sample was prepared by dissolving 2.5 mg samples in 10 ml MeOH. After mixing 0.5 ml of DPPH solution with 20 μl of test extract at various concentrations, the mixture’s absorbance was measured at 515 nm after 20 minutes at 37°C (A). Daily measurements were taken at the same wavelength on a blank sample that contained 20 μl of the aforementioned DPPH solution. Three duplicates of the experiment were performed. The amount of radical scavenging activity was determined using the following formula: Inhibition (%) is calculated using the formula [(Ab − As)/Ab] × 100, where Ab and As represent the absorbance of the reaction solutions containing the blank and the samples, respectively.
Brine shrimp lethality test
This test was used to predict the cytotoxic activity of MeOH extract and fraction of n-hexane, EtOAc, BuOH, and water. Artemia salina L. nauplii (Artemiidae) were employed in brine shrimp lethality test. For 48 hours at 28°C, brine shrimp eggs were incubated in 500 ml of filtered seawater with continual aeration. The active egg cells nauplii were gathered and used for testing. After that, the active nauplii of the egg cell were collected and used for testing. One mg of each extract dissolved into 1 mL of Dimethyl Sulfoxide (DMSO) and solutions of varying concentrations of 150, 100, 75, 50, 25, 10, and 5 μg/mL in triplicate were obtained by the serial dilution using seawater. Fifteen nauplii were then inserted into each vial and kept at room temperature for 24 hours.The vials were inspected using a magnifying glass to count the number of survived nauplii. This data calculated the percent of the lethality of the brine shrimp nauplii for each concentration. As negative controls, filtered seawater and DMSO were utilized. The LC50 value was calculated using the curve method based on the probit analysis (Chakraborty and Francis, 2021; Meyer et al., 1982).
MTT assay
MTT is a colorimetric assay that can evaluate the antiproliferative effect of C. affinis extracts. Two cell lines, MCF-7 as a human breast cancer cell line and HeLa as a cervix cell line, have been produced for cytotoxic testing using MTT. All cell lines were acquired from the Brawijaya University Life Science Central Laboratory. HeLa and MCF-7 were cultured in RPMI-1650 (Gibco, USA) medium. After mild trypsinization with 0.25% trypsin-EDTA/Ethylenediaminetetraacetic acid (Gibco, USA), all cells were subcultured, and the vitality and quantity of cells were assessed. The cells were incubated overnight and seeded in 96-well plates at a density of 5 × 103 cells/well in a 100 μl medium. All media contained 10% fetal bovine serum (FBS) (Gibco, USA) and 1% streptomycin-penicillin (Gibco, USA). The cell line was kept at 37°C, with a 5% CO2 atmosphere. The samples were dissolved to create a stock solution in 1% DMSO to give a 2,000 mg/l concentration. Cells incubated for 24 hours were then divided into cell control and media control (blank) groups. The medium is removed and washed using phosphate-buffered saline (PBS) sterile, then each well is added 100 μL PBS. Each extract was added to each well to produce various concentrations of 50, 100, 200, 400, 600, and 800 μg/mL, and then it was incubated for 24 hours at 37°C, with 5% CO2. Using sterile PBS, cells that had been cultured and incubated for 24 hours were disseminated throughout the medium. Then in each well was added 100 μl of MTT (5 mg/ml) in a culture medium followed by 4 hours of incubation at 37°C, with 5% CO2. To dissolve the formazan crystals, 100 l of DMSO was added to each well and incubated at room temperature for 30 minutes. Determine the optical density (OD) of each well using an ELISA reader at 595 nm.
The cell viability percentage was then calculated using the following formula:
Flow cytometry assay
HeLa and MCF-7cells were cultured in RPMI-1640 (Gibco, USA) with 10% of FBS in the presence of different concentrations (50, 100, 200, 400, 600, and 800 μg/mL) to determine the effect of the sponge extracts on apoptosis. After 24 hours, the cells were taken out, washed in PBS, and stained with 50 μl Annexin V–PI solution in PBS (50 μg/mL) according to the manufacturer’s instruction (Invitrogen, Life Technologies, IN, USA). For 40 minutes, the cells were incubated at room temperature. The cells were re-suspended in 500 μl PBS for running flow cytometry using BD FACS Calibur Flow Cytometer. The data were analyzed using the Cell Quest Program.
Statistical methods
The mean and standard deviation were used to express all experimental results. Using one-way analysis of variance and Dunnett’s post-test with GraphPad Prism version 8.00, GraphPad Prism Software, statistically significant differences between means were evaluated (San Diego, CA). Data with a p-value less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Metabolites analysis
The MeOH extract was partitioned into hexane, EtOAc, BuOH, and aqueous fractions to obtain non-polar, semi-polar, and polar metabolites, giving different molecular structures. Thus, the toxicity, antioxidative, and apoptotic properties of each fraction could be studied and compared with the MeOH extracts.
Table 1 shows data from the chromatogram peaks of each fraction, including retention time, molecular weight, and chemical formulas of compounds. The chemical formula was obtained from the molecular ion analysis of each compound’s mass spectra based on the output of the Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS) In general, the metabolites in each fraction are organic molecules containing heteroatoms such as N, O, and a small number of halides such as F, Br, or Cl. The O and N atoms can form functional groups such as lactam amide, phenolic, alcohol, acid, ester, or amines bonded in aliphatic or aromatic chains. The presence of organo halides compounds was also shown in semi-polar and polar fractions. The semi-polar fractions, EtOAc and BuOH, and the polar aqueous fraction contained organochlorine compounds. The sulfide groups are also found in the compounds that make up the semi-polar and polar fractions. The presence of O atoms in the molecular structure of the fraction may form hydroxyl groups of alcohol or phenolic hydroxyl OH−groups, ether, or ester as found in the Callypyrone A and B structures (Chakraborty and Francis, 2021). The presence of nitrogen could be derived from alkaloids which can be presented in the form of primary, secondary, or tertiary amines. Most combinations of O and N form an amide group, as found in the structures of Callyspongiamide (Kapojos et al., 2018), Callyptide (Shaala et al., 2016), and Callyaerins (Ibrahim et al., 2010).
Semi-polar fractions such as EtOAc and BuOH contain fluorinated organic compounds. The existence of this flour is a new thing in reporting the content of secondary metabolites in marine sponges, especially Callyspongia species. In the EtOAc fraction, there were three fluorinated organic compounds with the formulas of C23H43N7O3F, C27H50N2FS, and C28H57NO3FS, while in the BuOH fraction there are C16H18O2F, C16H18OF, and C19H27NFS.
DPPH assay
The ability to scavenge DPPH radicals from each fraction is shown in Table 2. The ability to scavenge radicals is directly related to the antioxidant properties of each fraction. The stronger the ability to scavenge radicals is (marked by a decrease in the absorbance of DPPH), the stronger the antioxidant properties are. The EtOAc fraction showed the highest radical scavenging ability with an IC50 value of 27.617 μg/mL compared to the MeOH extract and other fractions. The hexane fraction with IC50 of 153.854 μg/mL showed the lowest radical scavenging ability. This radical scavenging ability describes the ability of the fractions to act as antioxidants. From the data in Table 2, it can be seen that although all fractions showed oxidative properties under ascorbic acid as a positive control (IC50 11.529 μg/mL), the EtOAc fraction was a potent antioxidant (IC50< 30 μg/mL), while MeOH extract, aqueous fraction, and BuOH fraction are included as moderate antioxidant (IC50< 100 μg/mL).
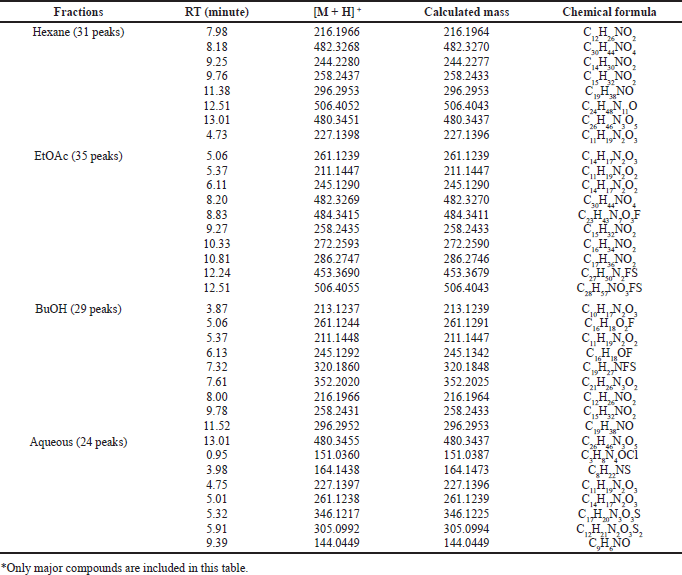 | Table 1. Analysis of LC-HRMS from each fraction of C. affinis (*). [Click here to view] |
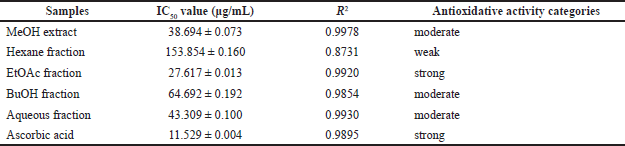 | Table 2. Antioxidative activity and IC50 value of extracts and fractions of sponge C. affinis [Click here to view] |
Antioxidative properties are closely related to preventing the growth of harmful tissues triggered by free radical reaction mechanisms. The formation of free radical substances in the body triggers the growth and development of cancer cells. Therefore, the antioxidant ability of the C. affinis fractions also illustrates its ability to inhibit the formation of cancer cells. Constituent compounds support this activity in each fraction, acting as free radical scavenging. In this case, the compounds that make up the EtOAc fraction are more effective than the MeOH extract as the parent solution as an antioxidant and can inhibit the formation of cancer cells.
Brine shrimp lethality test result
Brine shrimp LC50 values for the MeOH extract and four fractions of sponge C. affinis evaluated in this study are reported in Table 3. All tested fractions and extracts were dose-dependent and highly toxic to A. salina with LC50 values below 100 μg/mL, according to Clarkson et al.’s (2004) toxicity criterion. The EtOAc fraction showed the strongest and highest toxicity among all samples, with an LC50 value of 26.652 μg/mL. MeOH extract with an LC50 value of 27.451 μg/mL was relatively more toxic than other fractions except for the EtOAc fraction. Although the LC50 values of all samples did not show much difference, the MeOH extract and aqueous fraction, which were polar, tended to show a higher level of toxicity than the non-polar and semi-polar hexane and BuOH fractions. These results show a direct relationship with the fractional extraction performed. MeOH was the first solvent used for total extraction, while other solvents were used for fractionation, which showed different toxicity according to the distribution of polar and non-polar toxicants.
The EtOAc fraction had a higher level of toxicity than the extract and other fractions. This high toxicity can be attributed to the presence of fluorinated organic halides with non-polar properties (bound to long hydrocarbon chains) in the EtOAc fraction, which is thought to contribute to the cytotoxicity effect on A. salina.
MTT assay
The toxicity effects of the extract and sponge fractions on HeLa and MCF-7 cells were determined using the MTT assay. The toxicity effect can also be interpreted as the antiproliferative ability of the extract and fractions against HeLa and MCF-7 cells. The toxicity level of the extract and all sponge fractions can be seen in Figure 1, indicated by the decrease in the number of living cells as the concentration of the extract/fractions increases. The EtOAc and hexane fractions showed higher antiproliferative properties than the MeOH extract and other fractions. The EtOAc fraction was more toxic to HeLa and MCF-7 cells than extracts and other fractions with IC50 values of 94.934 ± 0.700 and 97.804 ± 0.24 μg/mL, which are presented in Table 4. Non-polar fractions such as hexane also showed high toxicity even though it was still below the EtOAc fraction. In general, the compound constituents in the fraction with lower polarity showed better antiproliferative activity than the more polar fraction.
The increase in antiproliferative ability is partly due to electronic interactions such as electron-withdrawing groups attached to the aromatic ring, electron density in the double bond structure in both alkene and aromatic structures, and the presence of heterocyclic rings (Shu et al., 2021). Table 1 shows that the EtOAc fraction contains compounds with molecular formulas indicating the presence of aromatic and heterocyclic rings. Most alkaloids with heterocyclic rings can inhibit CDK-Cyclin Complex activity (Zhang et al., 2021).
Flow cytometry assay
The ability of extract and fractions of C. affinis to exert apoptotic effects on HeLa and MCF-7 cells was determined by flow cytometry assay. Apoptosis is a form of planned necrosis that is defined by morphological and biochemical changes. It is crucial for the development and maintenance of a healthy body since it eliminates old, unneeded, and diseased cells (Raja et al., 2020). Apoptosis can be triggered by factors external to the cell. In this experiment, the EtOAc fraction showed a higher apoptotic effect than the MeOH extract and other fractions with IC50 values of 143.380 ± 0.010 μg/mL for HeLa cells and 131.646 ± 0.011 μg/mL for MCF-7 cells, as shown in Table 5. The proapoptotic ability of the fractions other than EtOAc was still lower than the MeOH extract.
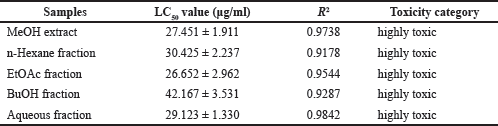 | Table 3. LC50 values based on brine shrimp activity of MeOH extract and fractions from C. affinis. [Click here to view] |
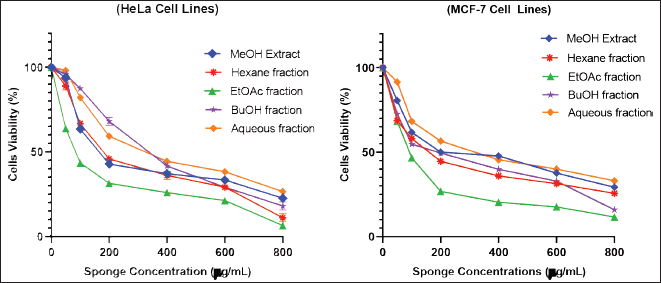 | Figure 1. Cytotoxicity of various concentrations of C. affinis extract and fractions on HeLa and MCF-7 cells. The experiment was done three times, and the results were presented as the mean standard deviation (SD) with p < 0.05. [Click here to view] |
Figure 2 shows the level of apoptosis of MeOH extract cells and various fractions of C. affinis at a concentration of 200 μg/mL. Some samples had entered 50% of cell apoptosis. The process of apoptosis in the two types of cells showed different conditions in terms of cells undergoing early apoptosis (AnnexinV/PI +/−) and late apoptosis (AnnexinV/PI +/+). In HeLa cells, the EtOAc fraction was more potent in inducing early apoptosis. In contrast, MCF-7 cells exhibited late apoptotic cells. Meanwhile, the fraction other than EtOAc showed that most of the apoptotic cells were in late apoptosis. Early apoptotic cells attract phagocytes by releasing specific signals without enhancing inflammation, while late apoptotic cells release additional pro-inflammatory danger signals (Khazaei et al., 2017). In general, extracts and fractions can trigger cells to repair themselves through the process of apoptosis against unwanted cells, such as cancer cells.
 | Table 4. The level of toxicity of extracts and fractions of C. affinis was determined by the IC50value. [Click here to view] |
 | Table 5. IC50 values of apoptosis of extracts and fractions of C. affinis. [Click here to view] |
CONCLUSION
Callyspongia affinis is one of the marine natural products in Kangean Island, which is abundant and has good potential and opportunity to be developed as a new medicinal ingredient which was first investigated and reported in this study. This study presents the content of compounds in C. affinis as a new candidate for cancer treatment of selected marine sponge species that act on different targets in different cancer cells. The chemical content of C. affinis extract and fractions can be developed as a promising anticancer agent. The EtOAc fraction of sponge C. affinis showed the most promising anticancer potential among the investigated fractions and had potential antioxidant, toxic, antiproliferative effects, and apoptotic inducers. Nevertheless, isolating the pure compound from the EtOAc fraction from the C. affinis sponge remains challenging. Thus, it can also be explored as an anticancer agent in various types of cancer.
ACKNOWLEDGMENTS
The authors acknowledge The Central Laboratory of Life Sciences, Brawijaya University, Malang, Indonesia, for providing all the necessary facilities to carry out the present work and The Department of Chemistry, Brawijaya University, for any support given. Brawijaya University Internal Grant funded this article.
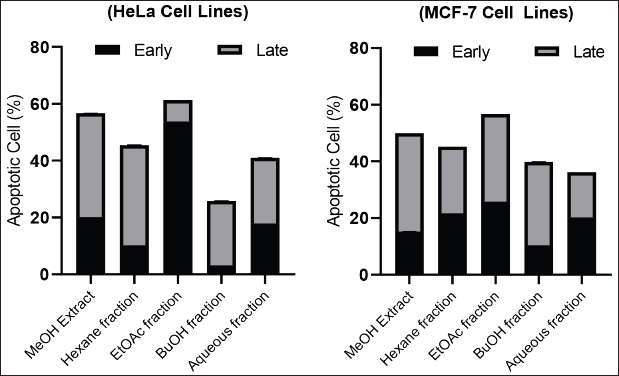 | Figure 2. Effects of C. affinis extract and various fractions on the apoptotic of HeLa and MCF-7 cells at 200 mg/mL. [Click here to view] |
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdillah S, Nurhayati APD, Nurhatika S, Setiawan E, Heffen WL. Cytotoxic and antioxidant activities of marine sponge diversity at Pecaron Bay Pasir Putih Situbondo East Java, Indonesia. J Pharm Res, 2013; 6(7):685–9; doi: 10.1016/j.jopr.2013.07.001.
Arunachalam K, Amirtham Jacob Appadorai R. Antioxidant potential and biochemical evaluation of metabolites from the marine bacteria Virgibacillus sp. associated with the sponge Callyspongia diffusa. Free Radic Antioxid, 2013; 3(1):47–51; doi: 10.1016/j.fra.2013.04.004.
Calcabrini C, Catanzaro E, Bishayee A, Turrini E, Fimognari C. Marine sponge natural products with anticancer potential: an updated review. Mar Drugs, 2017; 15(10):310; doi: 10.3390/md15100310.
Chakraborty K, Francis P. Callypyrones from marine Callyspongiidae sponge Callyspongia diffusa: antihypertensive bis-γ-pyrone polypropionates attenuate angiotensin-converting enzyme. Nat Prod Res, 2021; 35(24):5801–12; doi: 10.1080/14786419.2020.1837819.
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol, 2004; 92(2–3):177–191; doi: 10.1016/j.jep.2004.02.011.
El-Hawary SS, Sayed AM, Mohammed R, Hassan HM, Rateb ME, Amin E, Mohammed TA, El-Mesery M, Muhsinah AB, Alsayari A, Wajant H, Anany MA, Abdelmohsen UR. Bioactive brominated oxindole alkaloids from the red sea sponge Callyspongia siphonella. Mar Drugs, 2019; 17(8):1–13; doi: 10.3390/md17080465.
Erpenbeck D, Galitz A, Ekins M, Cook S de C, van Soest RWM, Hooper JNA, Wörheide G. Soft sponges with tricky tree: on the phylogeny of dictyoceratid sponges. J Zool Syst Evol Res, 2020; 58(1):27–40; doi: 10.1111/jzs.12351.
Hadisaputri YE, Andhika R, Sopyan I, Zuhrotun A, Maharani R, Rachmat R, Abdullah, R. Caspase cascade activation during apoptotic cell death of human lung carcinoma cells A549 induced by marine sponge Callyspongia aerizusa. Drug Des Devel Ther, 2021; 15:1357–68; doi: 10.2147/DDDT.S282913.
Ibrahim HAH, El-Naggar HA, El-Damhougy KA, Bashar MAE, Abou Senna FM. Callyspongia crassa and C. siphonella (Porifera, Callyspongiidae) as a potential source for medical bioactive substances, Aqaba Gulf, Red Sea, Egypt. J Basic Appl Zool, 2017; 78(1):1-10; doi: 10.1186/ s41936-017-0011-5.
Ibrahim SRM, Min CC, Teuscher F, Ebel R, Kakoschke C, Lin W, Wray V, Edrada-Ebel R, Proksch P. Callyaerins A-F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg Med Chem, 2010; 18(14):4947–56; doi: 10.1016/j.bmc.2010.06.012.
Kapojos MM, Abdjul DB, Yamazaki H, Ohshiro T, Rotinsulu H, Wewengkang DS, Sumilat DA, Tomoda H, Namikoshi M, Uchida R. Callyspongiamides A and B, sterol O-acyltransferase inhibitors, from the Indonesian marine sponge Callyspongia sp. Bioorg Med Chem Lett, 2018; 28(10):1911–14; doi: 10.1016/j.bmcl.2018.03.077.
Khazaei S, Esa NM, Ramachandran V, Hamid RA, Pandurangan AK, Etemad A, Ismail P. In vitro antiproliferative and apoptosis inducing effect of allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front Pharmacol, 2017; 8:1–16; doi: 10.3389/fphar.2017.00005.
Ki DW, El-Desoky AH, Kodama T, Wong CP, Ghani MA, El-Beih AA, Mizuguchi M, Morita H. New cytotoxic polyacetylene amides from the Egyptian marine sponge Siphonochalina siphonella. Fitoterapia, 2020; 142(January):104511; doi: 10.1016/j.fitote.2020.104511.
Kumar MS, Adki KM. Marine natural products for multi-targeted cancer treatment: a future insight. Biomed Pharmacother, 2018; 105(February):233–45; doi: 10.1016/j.biopha.2018.05.142.
Latifah LA, Soekamto NH, Tahir A. New antibacterial activities of brominated c18 and c20 fatty acids isolated from marine sponge Xestospongia testudinaria against shrimp pathogenic bacteria. Rasayan J Chem, 2021; 14(1):460–5; doi: 10.31788/RJC.2021.1415999.
Lim HK, Bae W, Lee H-S, Jung J. Anticancer activity of marine sponge Hyrtios sp. extract in human colorectal carcinoma RKO cells with different p53 status. Biomed Res Int, 2014:5:1-5; doi: 10.1155/2014/413575.
Lorig-Roach N, Hamkins-Indik F, Johnson TA, Tenney K, Valeriote FA, Crews P. The potential of achiral sponge-derived and synthetic bromoindoles as selective cytotoxins against PANC-1 tumor cells. Tetrahedron, 2018; 74(2):217–23; doi: 10.1016/j.tet.2017.11.029.
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Plant Med, 1982; 45(1):31–4; doi: 10.1055/s-2007-971236.
Oogarah PN, Ramanjooloo A, Rovisham J, Doorga S, Meyepa C, Wilhelmus R, Soest M Van, Edgard D, Marie P. Assessing antioxidant activity and phenolic content of marine sponges from mauritius waters. Int J Pharmacogn Phytochem Res, 2020; 12(3):123–31; doi: 10.25258/phyto.12.3.1.
Pawlik JR, McMurray SE. The emerging ecological and biogeochemical importance of sponges on coral reefs. Annu Rev Mar Sci, 2020; 12:315–37; doi: 10.1146/annurev-marine-010419-010807.
Rady I, Bashar AEM. Novel extracts from Callyspongia siphonella and Negombata magnifica sponges from the Red Sea, induced antiproliferative and proapoptotic activity in HepG-2, MCF-7, and Caco-2 cancer cell lines. Egypt J Aquat Biol Fish, 2020; 24(7):319–47; doi: 10.21608/EJABF.2020.121064.
Raja K, Martin LC, Bose L, Sahayanathan GJ, Padmanaban D, Chinnasamy A. Anti-proliferative and apoptotic effects of by-product (skin extract) from marine catfish Tachysurus dussumieri. Biocatal Agric Biotechnol, 2020; 29(October):101816; doi: 10.1016/j.bcab.2020.101816.
Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol, 2001; 1(4):364–9; doi: 10.1016/S1471-4892(01)00063-7.
Shaala LA, Youssef DTA, Ibrahim SRM, Mohamed GA. Callyptide A, a new cytotoxic peptide from the Red Sea marine sponge Callyspongia species. Nat Prod Res, 2016; 30(24):2783–90; doi: 10.1080/14786419.2016.1155577.
Shu P, Yu M, Li Y, Luo Y, Liu H, Zhu H, Zhang J, Zhang L, Wei X, Xiao F. Synthesis of cinnamoyl glucoside derivatives and their antiproliferation activities against murine melanoma B16-F10 cell line. Carbohydr Res, 2021; 504(April):108332; doi: 10.1016/j.carres.2021.108332.
Umeyama A, Matsuoka N, Mine R, Nakata A, Arimoto E, Matsui M, Shoji N, Arihara S, Takei M, Hashimoto T. Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J Nat Med, 2010; 64(1):93–7; doi: 10.1007/s11418-009-0363-3.
Wao YP, Priska M. Daya Hambat Ekstrak Callyspongia sp. terhadap Bakteri dari Eucheuma Cottoni berpenyakit Ice-Ice. EduMatSains, 2021; 6(1):111–22.
Youssef DTA, Van Soest RWM, Fusetani N. Callyspongamide A, a new cytotoxic polyacetylenic amide from the Red Sea sponge Callyspongia fistularis. J Nat Prod, 2003; 66(6):861–2; doi: 10.1021/np0205809.
Zhang M, Zhang L, Hei R, Li X, Cai H, Wu X, Zheng Q, Cai C. CDK inhibitors in cancer therapy, an overview of recent development. Am J Cancer Res, 2021; 11(5):1913–35.