INTRODUCTION
Tuberculosis (TB) is a communicable disease caused by Mycobacterium tuberculosis, one major cause of ill health (World Health Organization report, 2020). It was reported in 2019 before the COVID-19 pandemic to be a devastating global health problem and top infectious killer worldwide (Global tuberculosis report, 2019). It is among the top 10 causes of death worldwide and the number one cause of death from a single infectious agent after COVID-19 (World Health Organization report, 2020).
A report in 2017 estimated that out of the 10 million new cases of TB recorded worldwide with 1.8% decline rate, about 1.57 million people died as a result of TB, representing a 3.9% decline compared to the 2016 report. High TB cases were recorded in Africa and Southeast Asia regions (Ghebreyesus, 2019).
In spite of the effort to end TB, most anti-TB drugs have been confronted with the issue of resistance that prolongs TB management and treatment. Several cases of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB have been reported (Ajay et al., 2018). The rate of eliminating TB globally appealed when the annual statistics from TB incidence were compared, including mortalities in recent years (Macneil et al., 2020). This has intensified the efforts by scientists to improve the diagnosis, treatment, and prevention of TB in order to meet the “end TB” target. The insurgence of MDR/XDR-TB is a major problem in the treatment and management of TB (Seung et al., 2015) and has been a challenge in our healthcare system. World Health Organization (WHO) 2020 global TB report estimated 500,000 cases of MDR-TB, of which 186,772 MDR-TB deaths were confirmed (Tiberi et al., 2021). In the past four decades, there has been a surge in multidrug and XDR-TB, while on the other hand, there is a decline in the number of anti TB drugs developed within the same time, leaving options for fewer drugs to treat and manage TB (Espindola et al., 2017; Tiberi et al., 2018). This trend is alarming, and there is a need to discover and develop lead agents that could be used to treat TB and to identify some mechanisms to tackle antimicrobial resistance.
Nature has provided some important sources of new antimicrobials because of their amazing chemical diversity and their validation over the years (Bednarek and Osbourn, 2009; González-Lamothe et al., 2009). Plants, together with some microbes, use their secondary metabolites to fight environmental infections. The antibacterial activities of plants are known based on their ethnomedicinal or folkloric use (Romha et al., 2018; Sisay et al., 2019; Ullah et al., 2020). Crinum asiaticum bulbs are predominant in the southern part of Ghana (Ofori et al., 2021), and the selection of the plant was based on the evidence that it is used traditionally to treat upper respiratory tract infections, skin infections, and severe chest pains (Ofori et al., 2021). The toxicity profile of the chloroformic C. asiaticum bulb extract (CCAE) was recently reported to possess no known toxicity (Ofori et al., 2021). Several scientific validations on the plant have been reported; a few of them are the analgesic and anti-inflammatory effects of C. asiaticum alcoholic leaf extract in animal models reported by Rahman et al. (2013). Antinociceptive and anti-inflammatory effects of C. asiaticum bulb extract were investigated and reported (Ahmed, 2011). Surain reported the anti-Candida potential of C. asiaticum leaf extract against selected oral and vaginal Candida pathogens. There was a study on the anti-inflammatory and antioxidant activity of leaf extract of C. asiaticum (Uddin et al., 2015). Gas chromatography-mass spectrometry analysis conducted on the chloroform extract of C. asiaticum bulb extract reported in the authors’ recent publication revealed the presence of notable compounds (Ofori et al., 2021).
 | Table 1. Phytochemical compounds identified in the chloroform extract of Crinum asiaticum bulb (Ofori et al., 2021). [Click here to view] |
MATERIALS AND METHODS
Materials
Materials include C. asiaticum bulbs, rotary evaporator (Buchi Labotechnik Rotavap R-210), autoclave (Sano clav), centrifuge (Joshansen), Middle Brook 7H10 agar (Difco), Middle brook 7H9 broth (Difco), 96-well half skirted polymerized chain reaction plate, 96-well microtiter plate (Star lab, UK), aerosol/exposure chamber (Aerosol Products, Colchester Ltd.).
Chemicals and reagents
The list of chemicals and reagents include chloroform (BDH Prolabo), rifampicin (RIF) (Ernest Chemist, Ghana), isoniazid (Entrance Pharmaceuticals), Interleukin-6 (IL-6) ELISA KIT (R and D Systems, UK), tumor necrosis factor-alpha (TNF-α) ELISA KIT (R and D Systems, UK), 10% (V/V) Oleic acid, albumin, dextrose and catalase, 0.5% (V/V) glycerol, and 0.2% (V/V) Tween 80.
Bacterial strain
Mycobacterium smegmatis was obtained from the cell culture laboratory in the Department of Pharmacology, Kwame Nkrumah University of Science and Technology (KNUST).
Plant materials collection and preparation
The bulbs of C. asiaticum were harvested from farmyard belonging to the Department of Horticulture, KNUST, with GPS code 6.6790397, −1.5660286. Dr. Henry Sam in the Department of Herbal Medicine, KNUST, authenticated it, and a herbarium sample with identification number KNUST/HM2020/B004 was kept in the herbarium, KNUST. The bulbs of C. asiaticum were washed in clean water, chopped, and blended fresh with chloroform. Cold maceration was conducted for 72 hours with constant stirring and then after the mixture was filtered. The filtrate containing the extracted bioactive compounds was concentrated using a rotary evaporator (Buchi Labotechnik Rotavap R-210). The concentrated extract was dried well, stored in containers, sealed, and refrigerated.
Laboratory animals
Both male and female albino mice between the ages of 8–10 weeks with their weight ranging from 15 to 20 g were purchased from Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, and were housed in the Animal House of the Department of Pharmacology (KNUST), Kumasi, with conditions prescribed in the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (Guide for the Care and Use of Laboratory Animals, 2011).
METHOD
Aerosol infection
The aerosol infection model (Diane and Ian, 2011; Gaonkar et al., 2010; Schwebach et al., 2002) was employed with some modifications using the aerosol chamber (exposure chamber) and fast-growing, nonpathogenic M. smegmatis and a surrogate model of M. tuberculosis. Mycobacterium smegmatis was cultured overnight in MB7H9 broth supplemented with 10% ADC, followed by the addition of 20% Tween 80. Pipette was used to aseptically dispense 1 ml of the Mycobacterium culture into a falcon tube containing 4 ml of sterile water to produce 5 ml of diluted culture suspension (2.0 × 106 colony-forming unit (CFU)/ml) which was used in the inhalation exposure chamber. The standard challenge dose in most TB experiments in mice has CFU of 50–100 and was determined before the aerosol infection (Diane and Ian, 2011; Karaman, 2013). The final suspension (5 ml) was aerosolized in a nebulizer for 30 minutes. Before nebulizing the bacterial suspension, a maximum of 25 mice were placed in the exposure chamber, and the chamber was covered firmly. Glass nebulizer venturi was fixed firmly beside the outer chamber, and the system was run for 30 minutes. The amount of mycobacterial load inhaled during exposure was determined as CFU on a plate culture.
CFU = CFU/ml × dilution factor × volume delivered
Dilution factor = Dilution factor used to prepare the aerosol suspension,
Volume delivered = Total bacteria suspension in nebulizer before the experiment – Amount left in the nebulizer after the experiment.
To authenticate the aerosol-induced TB work, after 24 hours of postexposure, mice were sacrificed, lungs were homogenized and centrifuged, supernatant-containing bacilli was cultured, and the CFU was determined. This was carried out to show how well the lungs were exposed to the bacterium.
In vivo dose-response studies
After 48 hours of postexposure, the mice were administered by oral gavage with the CCAE and RIF as standard drug once daily for 28 days. Eight groups with seven mice in each group were used for the experiment. The first three groups were orally treated with CCAE with doses of 100, 500, and 1,000 mg/kg, respectively. Groups 4, 5, and 6 were treated orally with 30, 90, and 270 mg/kg of RIF. Group seven was the negative control (the untreated group). Group eight was the naïve group (without TB infection) and was administered with 1 ml/kg of normal saline. Treatment lasted for 28 days after aerosol-induced TB.
The weight of animals was recorded every week and the final weight on the 29th day of treatment. Clinical observations were conducted throughout the treatment days, and mortality was recorded. On the day after the final treatment of the mice, blood samples were collected from each group into ethylenediaminetetraacetic acid-containing tubes for hematological studies. Mice were sacrificed, and lungs were aseptically harvested. The left lobe was placed in 10% formalin for histological analysis, and the right lobe was homogenized and processed for CFU count. The cytokines level for IL-6 and TNF-α was determined since they are crucial in assessing the severity of TB. Assessing the levels of these cytokines is one of the hallmarks of TB management, and it is a contributory factor in TB therapy.
STATISTICAL ANALYSIS
GraphPad Prism 8.0 software was used to carry out statistical analysis using a one-way analysis of variance (ANOVA). Results were quantified as mean ± SEM. Statistical differences between mean values were carried out using Dunnett’s multiple comparison test at p < 0.05.
RESULTS
Effect on the body weight of mice
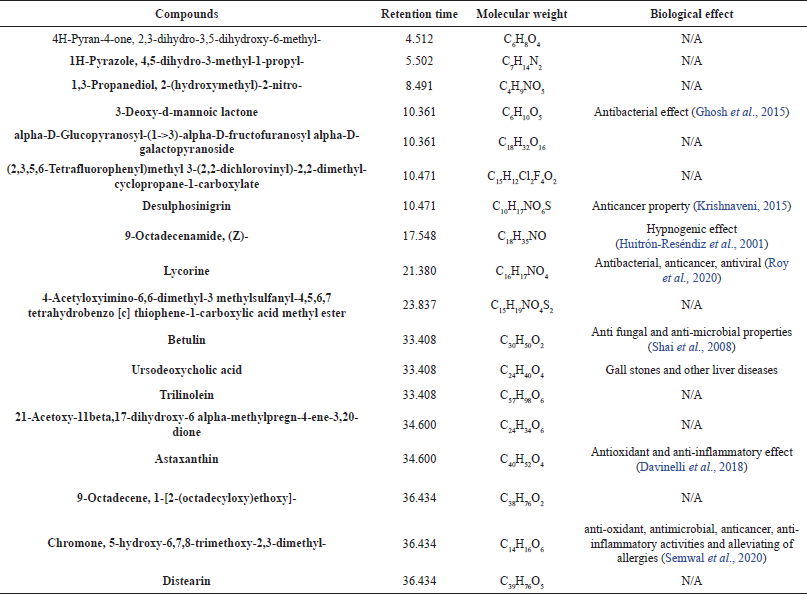
In assessing the changes in mean body weight of mice, there was a gradual increase in the individual weight of mice in the naïve control group. Animals treated with 500 and 1,000 mg/kg of CCAE had a significant increase in their body weight throughout the experiment compared to the initial body weight. Doses of RIF also did not affect the body weight of mice negatively with an increase in the mean body weight of the mice.
Effect on hematological parameters during infection
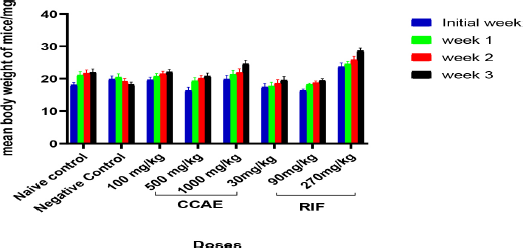
There was a hematological assessment of some key parameters which are affected negatively during M. tuberculosis infection; such parameters are red blood cells (RBC), hemoglobin (HGB), and white blood cells (WBC) count. In assessing the hematological parameters after the experiment, there was an elevation of WBC in the negative control groups with a mean value of 9.466 103/µl ± 0.404. There were significant decreases in WBC levels in the CCAE-treated groups with doses of 500 and 1,000 mg/kg (3.637 103/µl ± 0.562 and 2.627 103/µl ± 0.401, respectively) compared to the negative control group and the naïve group as two-way ANOVA.
Doses of RIF (30, 90, and 270 mg/kg) also produced a significant reduction in WBC level compared to the negative control. The RBC and HGB levels appreciably increased in the treatment groups of CCAE compared to the negative control. Mean RBC levels for CCAE treated groups for 100, 500, and 1,000 mg/kg were 9.110 103/µl ± 0.301, 10.433 103/µl ± 0.531, and 10.740 103/µl ± 0.408, respectively, which increased significantly compared to the negative control with mean RBC level of 8.053 103/µl ± 0.650. The HGB levels in CCAE treatment groups (100, 500, and 1,000 mg/kg) appreciably increased with 500 and 1,000 mg/kg increasing significantly compared with the negative control group.
Effect of the CCAE on M. smegmatis CFU count in aerosol-induced TB in mice
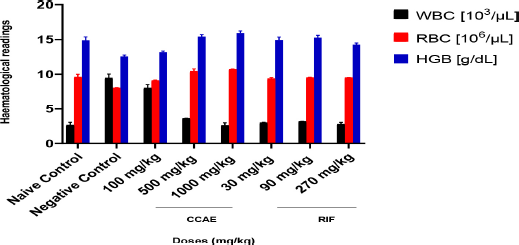
There was a significant decrease in the bacteria CFU/ml with C. asiaticum extract in the treatment doses of 100, 500, and 1,000 mg/kg, which produced mean CFU/ml of 6.20 × 109 ± 0.02, 3.71 × 109 ± 0.06 and 2.83 × 109 ± 0.02, respectively, compared to the negative control with 9.24 × 109 ± 0.080 CFU/ml. RIF, a standard drug, showed a significant decrease in the bacteria CFU/ml assessed on infected lungs of mice during aerosol-induced TB in mice.
IL-6 concentration
The CCAE showed a significant dose-dependent decrease in IL-6 concentration within the dose range of 100, 500, and 1,000 mg/kg compared to the negative control group when their levels were assessed in lung tissue homogenate.
TNF-ALPHA CONCENTRATION
The CCAE produced a significant dose-dependent decrease in TNF-α concentration within the dose range of 100, 500, and 1,000 mg/kg compared to the negative control group when their levels were assessed in lung tissue homogenate.
HISTOLOGICAL FINDINGS
The histological findings on the lungs of mice after treatment had a strong association with the bacterial load in terms of the CFU count. The histologic examination of the lungs in the negative control group presented with extensive necrotizing lung parenchyma, multinucleated giant cells, scattered lymphocytes, and large areas of collapsing alveoli, and also there was massive nonspecific granulomatous lesion (Fig. 6). These indicators suggest how the lungs were infected by M. smegmatis to a greater extent.
The doses of CCAE treatment groups with the exception of 100 mg/kg produced a significant improvement in relation to the histology of the lungs. Doses (500 and 1,000 mg/kg) produced marked effects on the lung tissues, presented with large areas of viable alveolar spaces, few foci points of necrosis, terminal lymph nodes were normal, and evidence of little blood vessel (BV) congestion (Fig. 6f–h).
RIF as a standard antitubercular agent with doses of 90 and 270 mg/kg produced a high antitubercular effect in regard to lung histology (Fig. 6i–k).
DISCUSSION
Management of TB is threatened with the insurgence of MDR/XDR-TB in medicine and public health becoming one major threat to control around the globe (Angelina et al., 2021). Current anti-TB agents are not functioning effectively due to the emergence of the issue of multidrug and XDR-TB in the healthcare systems (Gygli et al., 2017; Nainu et al., 2021; Prestinaci et al., 2015); therefore, there is a need to discover and develop new compounds with novel mechanisms of action effective in the management of TB.
Natural products such as medicinal plants offer a great platform to meet the needs of new anti TB regimens (Fauziyah et al., 2017; Ramadwa et al., 2019). Several plant species have been investigated for their effects against M. tuberculosis (Kumar et al., 2017). More recently, natural products investigations have reported the antimycobacterial properties of native Indonesian plants that are used traditionally for respiratory diseases (Fauziyah et al., 2017).
In the disease progression of TB, there is suppression of appetite due to decreased plasma leptin concentrations and therefore the need for nutritional support (Kim et al., 2010). It has been reported that the serum leptin level expressed in pulmonary TB may be implicated independently by inflammation and weight loss (Ye and Bian, 2018). In clinical research, it has been found that serum leptin level becomes very low in pulmonary TB due to the loss of body weight and that confirms that prolonged production of inflammatory cytokines may further suppress leptin production (Herlina et al., 2011; Kim et al., 2010).
In this study, the albino mice body weight was determined, and there were gradual increases in the weight of mice throughout the experiment for the CCAE treatment groups, the RIF treatment groups, and the naïve control group (Fig. 1) compared to the negative control group showing a decrease in the mean body weight. This suggested that, in the CCAE administration, the disease did not get to a stage that would suppress appetite and that could have caused loss of weight in the various treatment groups compared with the negative control. This confirms an investigation by (Kim et al., 2010) which reported that there is production of proinflammatory cytokines in TB that suppress plasma leptin level and that would obviously lead to wasting (Buyukoglan et al., 2007; D’Attilio et al., 2018; Kim et al., 2010; Wieland et al., 2005).
Abnormalities in hematological parameters are common in pulmonary tuberculosis patients, which is one of the major public health problems globally (Abay et al., 2018). TB effect on hematological parameters has been reported to cause a decrease in HGB (Iqbal et al., 2015), RBC count, and altered WBC count (Kulkarni and Jaju, 2017). This spells out one major reason why most TB patients are diagnosed with iron deficiency anemia (Chu et al., 2019; Gunda et al., 2016; Isanaka et al., 2012), and it is as a result of hematopoietic cells destruction (Iqbal et al., 2015). The WBC count elevation in (Fig. 2) was due to the existing infection. The negative control group showed a decrease in HGB and RBC (Fig. 2), but there was an increase in the WBC (Fig. 2), while on the other hand, the CCAE treated groups showed a dose-dependent increase in RBC and HGB levels and a decrease in WBC compared with the negative control (Fig. 2). The effect of CCAE on hematological parameters showed how effective the extract was in elevating the levels of parameters such as RBC, HGB, and WBC during TB infection (Fig. 2). In TB, some cardinal signs are investigated as evidence of existing TB in an individual (WHO Report, 2013), which include the presence of viable M. tuberculosis and pathological markers such as granulomatous lesion, caseous necrosis, and multinucleated giant cells (Kumar et al., 2013; Shah et al., 2017).
In this study, the number of viable M. smegmatis on the lungs was determined as their CFU/ml. The doses of CCAE significantly (p ? 0.005) decreased the CFU count on the lungs in the infected mice, suggesting how well the CCAE was able to inhibit the mycobacterial (Fig. 3). Mycobacterium smegmatis presence within the alveolar spaces induces the activation of macrophages which modulate the immune response by upregulating the presence of inflammatory cytokines such as IL-1β, IL-6, and TNF-α (Arora et al., 2020; Li et al., 2014; Yamawaki et al., 2016).
 | Figure 1. Effect of CCAE on body weight of mice (CCAE: chloroformic C. asiaticum bulb extract; RIF: rifampicin). [Click here to view] |
 | Figure 2. Assessment of hematological parameters during TB management with CCAE (CCAE: chloroformic C. asiaticum bulb extract, RIF: rifampicin, WBC: white blood cells, RBC: red blood cells, and HGB: hemoglobin). [Click here to view] |
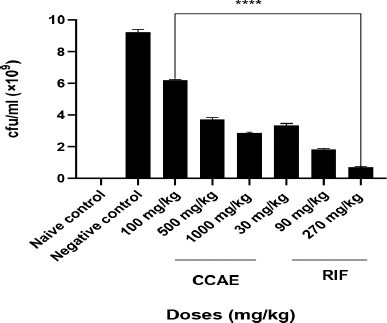 | Figure 3. Effect of CCAE on M. smegmatis CFU/ml count. One-way ANOVA. ****p-value ? 0.0001 of treatments versus negative control (CCAE: chloroformic C. asiaticum bulb extract; RIF: rifampicin). [Click here to view] |
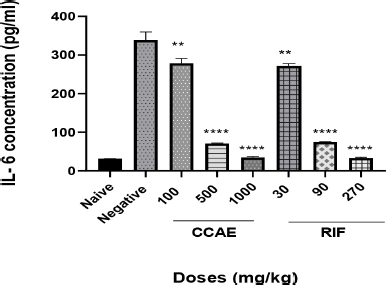 | Figure 4. Effect of CAE, negative control, naïve, and RIF on IL-6 expression. Data are expressed as mean ± SEM. n = 5, one-way ANOVA followed by Dunnett’s multiple comparison test, ****p < 0.0001 of negative control versus 500 and 1,000 mg/kg CCAE (CCAE: chloroformic C. asiaticum bulb extract; RIF: rifampicin). [Click here to view] |
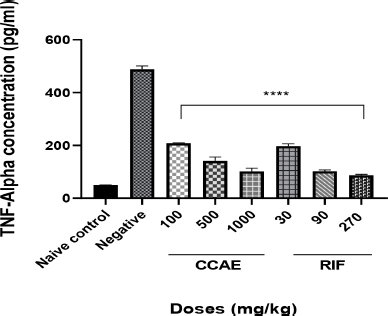 | Figure 5. Effect of CAE, negative control, naïve, and RIF on TNF-α expression. Data are expressed as mean ± SEM. n = 5, one-way ANOVA followed by Dunnett’s multiple comparison test, ****p < 0.0001 of negative control versus 100, 500, and 1,000 mg/kg CCAE. [Click here to view] |
Assessing the levels of cytokines such as TNF-α and IL-6 is one of the hallmarks in TB management (Basaraba, 2008), and it is a contributory factor in TB therapy. Low levels of TNF-α and IL-6 combined with other markers depict the effectiveness of antitubercular agents (Joshi et al., 2015; Mesquita et al., 2016). It was reported that the levels of cytokines such as TNF-α and IL-6 levels are elevated in TB -infected mice (Domingo-gonzalez et al., 2017; Kramnik et al., 2016). The elevation is attributed to the aggregation of macrophages and other immune cells that are produced when there is infection. CCAE markedly decreased the level of IL-6 (Fig. 4) and TNF-α (Fig. 5), and that suggested how the extract was to control the infection and how effective the extract was in managing TB.
The histological examination on the lungs of mice helps to investigate how extensive the disease destroys the cells of the lungs (Fukushi et al., 2011; Ravimohan et al., 2018). In TB, there are inflammatory cells infiltrations and macrophages adherence, which affect the lung parenchyma, and therefore the formation of granulomatous lesions and necrosis, which may cause alveolar collapsed (Guirado and Schlesinger, 2013; Kradin and Mark, 2018; Rosen, 2020). The histology of the lungs of infected mice showed dose-dependent improvement in lung function following the administration of CCAE (Fig. 6). In the negative control group (Fig. 6b and c), there were a large focal area of caseous necrosis, several alveolar spaces collapsed, bronchi infiltrations with inflammatory cells, and many congested BV compared to the naïve control group (Fig. 6a), where the lung architecture was maintained devoid of any inflammatory cells infiltrations. The treatment groups (Fig. 6d, e, f g, and h) showed dose-dependent improved lung functions, and the biggest improvement was seen in the 1,000 mg/kg CCAE (Fig. 6h). Comparatively, the level of lung tissues maintained in the CCAE treatment groups (Fig. 6d, e, f, g, and h) was similar to the RIF treatment groups (Fig. 6i, j, and k). The histological improvement of the lungs of mice after TB induction using M. smegmatis as a surrogate model for screening drugs against M. tuberculosis (Gupta and Bhakta, 2012; Namouchi et al., 2017) suggested the anti-Mycobacterium activity of CCAE.
CONCLUSION
This study demonstrated that C. asiaticum bulbs extract has an anti TB effect, was achieved in the inhibition of M. smegmatis in vivo, and improved weight loss and hematological parameters as well as reducing some notable inflammatory cytokines and the maintenance of lung architecture in the histological analysis. This would serve as a lead in the drug discovery process in developing novel compounds that could exhibit diverse mechanisms to treat TB and tackle associated drug-resistant TB.
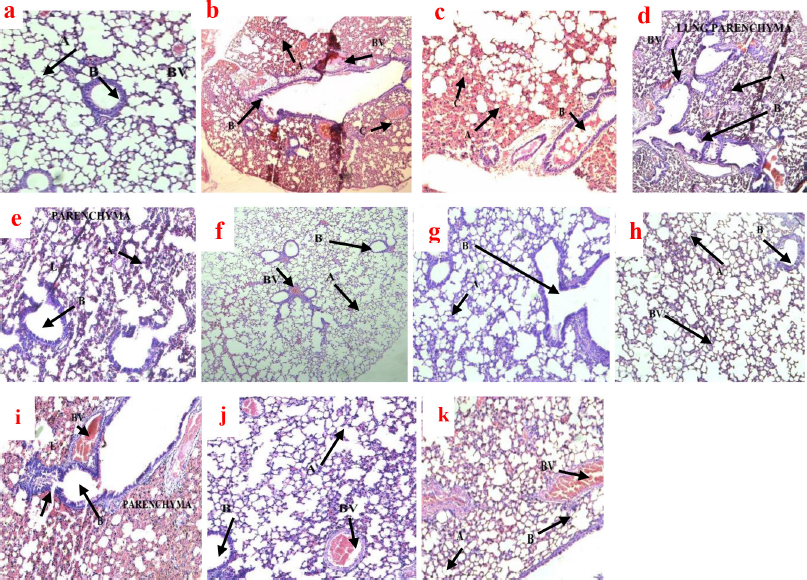 | Figure 6. Photomicrograph of the histology of mice lungs after TB treatment with CCAE in H and E stains. (a) Magnification ×10 is naïve control presented with intact terminal bronchiole (B), BV not congested, and well-defined alveolar spaces (A). (b and c) are the negative control groups (×4 and ×10, respectively) and showed extensive caseous necrosis (C), giant cells, granulomatous lesion, extensive collapse of alveoli (A), and numerous BV congestion. (d and e) represented 100 mg/kg CCAE (×4 and ×10, respectively) and showed collapsed alveolar spaces (A), a large field of caseous necrosis (C), and granulomatous lesion and numerous BV congestion. (f and g) (×4 and ×10, respectively) were 500 mg/kg CCAE-treated groups presented with few collapsed alveolar spaces (A), small foci of necrosis, little BV congestion, and little terminal bronchiole congestion (B). Micrograph (h) is 1,000 mg/kg CCAE (×10) which showed small foci of caseous necrosis, normal terminal bronchioles (B), and a large area of normal alveolar space (A). (i) represents 30 mg/kg of RIF (×10) and showed a large area of collapsed alveolar spaces (A), wider areas of granulomatous lesion and necrosis, and presence of residual alveolar (A). (j) was 90 mg/kg of RIF (×10) and showed few collapsed alveolar spaces (A), little BV congestion, and little terminal bronchiole congestion (B), and (k) represents 270 mg/kg of RIF (×10) with small foci of necrosis and granulomatous lesion, and terminal bronchiole bronchus appeared normal (B). [Click here to view] |
ACKNOWLEDGMENTS
The authors would like to thank the technical staff at the animal house and anti-infective drug discovery laboratory in the Department of Pharmacology, KNUST, for their immense contribution towards this work.
LIST OF ABBREVIATIONS
CCAE: chloroformic Crinum asiaticum bulb extract; CFU: colony-forming unit; HGB: hemoglobin; MDR: multiple drug resistance; RBC: red blood cell; WBC: white blood cells; XDR: extensively drug-resistant.
AUTHORS’ CONTRIBUTIONS
MO and CAD conceived and designed the study and were involved with data analysis and interpretation. MO and INN carried out the experiments, generated the data, and wrote the first draft of the manuscript. TN, PPSO, and PD contributed equally by providing the plant material, processing, and extraction. RG conducted the biochemical aspect of this work. All authors contributed to revising the final manuscript
FUNDING
There is no funding for this work.
CONFLICT OF INTERESTS
This is to certify that the authors have no conflict of interest.
ETHICAL APPROVAL
Ethical clearance from the animal ethical committee, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, was issued with ethical code FFPS-AEC/CA05/18, dated May, 2018.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abay F, Yalew A, Shibabaw A, Enawgaw, B. Hematological abnormalities of pulmonary tuberculosis patients with and without HIV at the University of Gondar Hospital, Northwest Ethiopia: a comparative cross-sectional study. Tuberc Res Treat, 2018; 2018:5740951; doi:10.1155/2018/5740951 CrossRef
Ahmed U. Antinociceptive and antiinflammatory effect of Crinum asiaticum bulb extract. Asian J Pharm Clin Res, 2017.
Ajay H, Sahajal D, Inderpaul SS, Agarwal R. Primary cavitary sarcoidosis: a case report, systematic review, and proposal of new diagnostic criteria. Lung India, 2018; 35(1):41–6; doi:10.4103/lungindia.lungindia CrossRef
Angelina A, Id S, Kwarteng A, Twumasi S, Owusu M, Arthur R. A, Mawunyo R, Id D, Adu-amoah L, Addofoh N, Okyere PB, Dzata F, Bonsu F, Adusi-poku Y, Kranzer K, Siroka A., Gemert V, Dean A, Owusu-dabo E. The burden of drug resistance tuberculosis in Ghana?; results of the First National Survey. PLoS One, 2021; 16:1–14; doi:10.1371/journal.pone.0252819 CrossRef
Arora SK, Naqvi N, Alam A, Ahmad J, Alsati BS, Sheikh JA, Kumar P, Mitra DK, Rahman SA, Hasnain SE, Ehtesham NZ. Mycobacterium smegmatis bacteria expressing Mycobacterium tuberculosis -specific Rv1954A induce macrophage activation and modulate the immune response. Front Cell Infect Microbiol, 2020; 10: 1–19. https://doi.org/10.3389/fcimb.2020.564565 CrossRef
Basaraba RJ. The role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Exp Tuberc, 2008; 88:S35–47. CrossRef
Bednarek P, Osbourn A. Plant-microbe interactions: chemical diversity in plant defense. Science, 2009; 324(5928):746–8; doi:10.1126/science.1171661 CrossRef
Buyukoglan H, Gulmez I, Kelestimur F, Kart L, Oymak FS, Demir R, Ozesmi M. Leptin levels in various manifestations of pulmonary tuberculosis. Mediat Inflam, 2007; doi:10.1155/2007/64859 CrossRef
Chu KA, Hsu CH, Lin MC, Chu YH, Hung YM, Wei J CC. Association of iron deficiency anemia with tuberculosis in Taiwan: a nationwide population-based study. PloS One, 2019; 14(8):e0221908; doi:10.1371/journal.pone.0221908 CrossRef
D’Attilio L, Santucci N, Bongiovanni B, Bay ML, Bottasso O. Tuberculosis, the disrupted immune-endocrine response and the potential thymic repercussion as a contributing factor to disease physiopathology. Front Endocrinol, 2018; 9:214; doi:10.3389/fendo.2018.00214 CrossRef
Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients, 2018; 10(4):1–12; doi:10.3390/nu10040522 CrossRef
Diane JO, Ian MO. Animal models of mycobacteria infection. Curr Protoc Immunol, 2011; 94(1):19–5.; doi:10.1038/jid.2014.371 CrossRef
Domingo-gonzalez R, Prince O, Cooper A, Khader S. Cytokines and chemokines in Mycobacterium tuberculosis infection. Microbiol Spectr, 2017; 4(5):1–58; doi:10.1128/microbiolspec.TBTB2-0018-2016 CrossRef
Espindola AL, Varughese M, Laskowski M, Shoukat A, Heffernan JM, Moghadas SM. Strategies for halting the rise of multidrug resistant TB epidemics: assessing the effect of early case detection and isolation. Int Health, 2017; 9(2):80–90; doi:10.1093/inthealth/ihw059 CrossRef
Fauziyah PN, Sukandar EY, Ayuningtyas DK. Combination effect of antituberculosis drugs and ethanolic extract of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Sci Pharm, 2017; 85:1–9; doi:10.3390/scipharm85010014 CrossRef
Fukushi M, Ito T, Oka T, Kitazawa T, Miyoshi-Akiyama T, Kirikae T, Yamashita M, Kudo K. Serial histopathological examination of the lungs of mice infected with influenza A virus PR8 strain. PloS One, 2011; 6(6):e21207; doi:10.1371/journal.pone.0021207 CrossRef
Gaonkar S, Bharath S, Kumar N, Balasubramanian V, Shandil RK. Aerosol infection model of tuberculosis in wistar rats. Int J Microbiol, 2010; 2010:426035; doi:10.1155/2010/426035 CrossRef
Ghebreyesus T. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland, pp 1–297, vol. 92, 2019. Available via apps.who.int/bookorders
Ghosh G, Panda P, Rath M, Pal A, Sharma T, Das D. GC-MS analysis of bioactive compounds in the methanol extract of clerodendrum viscosum leaves. Pharmacogn Res, 2015; 7(1):110–3; doi.org.10.4103/0974-8490.147223 CrossRef
Global tuberculosis report. WHO, Geneva, Switzerland, pp 251–8, 2019. Available via www.who.int/tb/data CrossRef
González-Lamothe R, Mitchell G, Gattuso M, Diarra M S, Malouin F, Bouarab K. Plant antimicrobial agents and their effects on plant and human pathogens. Int J Mol Sci, 2009; 10(8):3400–19; doi:10.3390/ijms10083400
Guide for the care and use of laboratory animals. Guide for the care and use of laboratory animals. 8th edition, National Academies press, Washington, DC, 2011. CrossRef
Guirado E, Schlesinger LS. Modeling the Mycobacterium tuberculosis Granuloma - the critical battlefield in host immunity and disease. Front Immunol, 2013; 4:98; doi:10.3389/fimmu.2013.00098 CrossRef
Gunda DW, Kilonzo SB, Bulegesi SM, Mpondo BC, Shao ER. Risk factors for mortality among tuberculosis patients on treatment at Bugando Medical Centre in north-western Tanzania?: a retrospective cross- sectional study. Tanzan J Health Res, 2016; 18(4):1–9. CrossRef
Gupta A, Bhakta S. An integrated surrogate model for screening of drugs against Mycobacterium tuberculosis. J Antimicrob Chemother, 2012; 67(6):1380–91; doi:10.1093/jac/dks056 CrossRef
Gygli SM, Borrell S, Trauner A, Gagneux S. Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS Microbiol Rev, 2017; 41:354–73; doi:10.1093/femsre/fux011
Herlina M, Nataprawira HM. D, Garna H. Association of serum C-reactive protein and leptin levels with wasting in childhood tuberculosis. Singapore Med J, 2011; 52(6):446–50. CrossRef
Huitrón-Reséndiz S, Gombart L, Cravatt BF, Henriksen SJ. Effect of oleamide on sleep and its relationship to blood pressure, body temperature, and locomotor activity in rats. Exp Neurol, 2001; 172(1):235–43; doi:10.1006/exnr.2001.7792
Iqbal S, Ahmed U, Khan MA. Haematological parameters altered in tuberculosis. Pak J Physiol, 2015; 11(1):13–6. CrossRef
Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, Spiegelman D, Duggan C, Fawzi WW. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr, 2012; 142(2):350–7; doi:10.3945/jn.111.144287 CrossRef
Joshi L, Ponnana M, Sivangala R, Chelluri LK, Nallari P, Penmetsa S, Valluri V, Gaddam S. Evaluation of TNF-α, IL-10 and IL-6 cytokine production and their correlation with genotype variants amongst tuberculosis patients and their household contacts. PLoS One, 2015; 10(9):e0137727; doi:10.1371/journal.pone.0137727 CrossRef
Karaman M. Mouse models of experimental tuberculosis in ABSL-3 conditions and assessment of animal welfare. Mycobact Dis, 2013; 4(1):10–2; doi:10.4172/2161-1068.1000137 CrossRef
Kim JH, Lee CT, Yoon H, Song J, Shin WG, Lee JH. Relation of ghrelin, leptin and inflammatory markers to nutritional status in active pulmonary tuberculosis. Clin Nutr (Edinburgh, Scotland), 2010; 29(4):512–8; doi:10.1016/j.clnu.2010.01.008 CrossRef
Kradin RL, Mark EJ. Pathology of pulmonary infection. Diagn Pathol Infect Dis, 2018; 143–206; doi:10.1016/B978-0-323-44585-6.00008-4 CrossRef
Kramnik I, Beamer G. Mouse models of human TB pathology?: roles in the analysis of necrosis and the development of host-directed therapies. Semin Immunopathol, 2016; 38:221–37; doi:10.1007/s00281-015-0538-9
Krishnaveni M. Docking, simulation studies of desulphosinigrin – cyclin dependent kinase 2, an anticancer drug target. Int J Pharm Sci Rev Res, 2015; 30(2):115–8.
Kulkarni NS, Jaju S. Study of hematological and biochemical parameters in pulmonary tuberculosis. Int J Sci Res, 2017; 6(9):2015–7. CrossRef
Kumar SN, Prasad TS, Narayan PA, Muruganandhan J. Granuloma with langhans giant cells: an overview. J Oral Maxillofacial Pathol?: JOMFP, 2013; 17(3):420–3; doi:10.4103/0973-029X.125211 CrossRef
Kumar V, Kumar MM, Bisht D, Kaushik A. Plants in our combating strategies against Mycobacterium tuberculosis?: progress made and obstacles met. Pharma Biol, 2017; 55(1):1536–44; doi:10.1080/13880209.2017.1309440 CrossRef
Li W, Zhao Q, Deng W, Chen T, Liu M, Xie J. Mycobacterium tuberculosis Rv3402c enhances mycobacterial survival within macrophages and modulates the host pro-inflammatory cytokines production via NF-kappa B / ERK / p38 signaling. PLoS One, 2014; 9(4):1–10; doi:10.1371/journal.pone.0094418 CrossRef
Macneil A, Glaziou P, Sismanidis C, Date A, Maloney S, Floyd K. Global epidemiology of tuberculosis and progress toward meeting global targets—worldwide, 2018. Morbid Mortal Wkly Rep, 2020; 69(11):281 . CrossRef
Mesquita EDD, Gil-santana L, Ramalho D, Tonomura E, Silva EC, Oliveira MM, Andrade BB, Kritski A, Study R. Associations between systemic inflammation , mycobacterial loads in sputum and radiological improvement after treatment initiation in pulmonary TB patients from Brazil?: a prospective cohort study. BMC Infect Dis, 2016; 16:368; doi:10.1186/s12879-016-1736-3 CrossRef
Nainu F, Permana AD, Juniarti N, Djide N, Anjani QK, Utami RN, Rumata NR, Zhang J, Emran TB, Simal-gandara J. Pharmaceutical approaches on antimicrobial resistance?: prospects and challenges. Antibiotics (Basel), 2021; 10(8):981. CrossRef
Namouchi A, Cimino M, Favre-Rochex S, Charles P, Gicquel B. Phenotypic and genomic comparison of Mycobacterium aurum and surrogate model species to Mycobacterium tuberculosis: implications for drug discovery. BMC Genomics, 2017; 18(1):25–8; doi:10.1186/s12864-017-3924-y CrossRef
Ofori M, Danquah CA, Ossei PPS, Rahamani G, Asamoah WA, Ativui S, Doe P. Acute and sub-acute toxicity studies of the chloroform extract of Crinum asiaticum bulbs in mice. South Afr J Bot, 2021; 143:133–40; doi:10.1016/j.sajb.2021.07.047 CrossRef
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Patho Global Health, 2015; 109(7):309–18; doi:10.1179/2047773215Y.0000000030 CrossRef
Rahman MA, Hossain SA, Ahmed NU, Islam MS. Analgesic and anti-inflammatory effects of Crinum asiaticum leaf alcoholic extract in animal models. Afr J Biotechnol, 2013; 12(2):212–8; doi:10.5897/ajb12.1431 CrossRef
Ramadwa TE, Awouafack MD, Sonopo MS, Eloff JN. Antibacterial and antimycobacterial activity of crude extracts, fractions and isolated compounds from leaves of sneezewood, Ptaeroxylon obliquum (Rutaceae). Nat Prod Commun, 2019; 14(11); doi:10.1177/1934578X19872927 CrossRef
Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage?: from epidemiology to pathophysiology. Eur Resp Rev 2018; 27(147); doi:10.1183/16000617.0077-2017 CrossRef
Romha G, Admasu B, Hiwot Gebrekidan T, Aleme H, Gebru G. Antibacterial activities of five medicinal plants in Ethiopia against some human and animal pathogens. Evid-Based Complement Alternat Med, 2018, 2950758; doi:10.1155/2018/2950758 CrossRef
Rosen Y. Pathology of granulomatous pulmonary diseases. Arch Pathol Lab Med, 2020; doi:10.5858/arpa.2020-0543-RA CrossRef
Roy M, Liang L, Xiao X, Feng P, Ye M, Liu J.Lycorine: a prospective natural lead for anticancer drug discovery. Biomed Pharmacother, 2018; 107:615–24. CrossRef
Schwebach JR, Chen B, Glatman-Freedman A, Casadevall A, McKinney JD, Harb JL, McGuire PJ, Barkley WE, Bloom BR, Jacobs WR. Infection of mice with aerosolized Mycobacterium tuberculosis: Use of a nose-only apparatus for delivery of low doses of inocula and design of an ultrasafe facility. Appl Environ Microbiol, 2002; 68(9):4646–9;doi:10.1128/AEM.68.9.4646-4649.2002
Semwal RB, Semwal DK Combrinck S, Viljoen A. Health benefits of chromones: common ingredients of our daily diet. Phytochem Rev, 2020; 19(4):761–85. https://doi.org/10.1007/s11101-020-09681-w CrossRef
Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb Perspect Med, 2015; 5(9):a017863; doi:10.1101/cshperspect.a017863 CrossRef
Shah KK, Pritt BS, Alexander M. P. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis, 2017; 7:1–12; doi:10.1016/j.jctube.2017.02.001 CrossRef
Shai LJ, McGaw LJ, Aderogba MA, Mdee LK, Eloff JN. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm.f) C.A. Sm. leaves. J Ethnopharmacol, 2008; 119(2):238–44; doi:10.1016/j.jep.2008.06.036 CrossRef
Sisay M, Bussa N, Gashaw T, Mengistu G. Investigating in vitro antibacterial activities of medicinal plants having folkloric repute in ethiopian traditional medicine. J Evid-Based Integr Med, 2019; 24:1–9; doi:10.1177/2515690X19886276 CrossRef
Tiberi S, Muñoz-Torrico M, Duarte R, Dalcolmo M, D’Ambrosio L, Migliori, GB. New drugs and perspectives for new anti-tuberculosis regimens. Pulmonology, 2018; 24(2):86–98; doi:10.1016/j.rppnen.2017.10.009 CrossRef
Tiberi S, Vjecha MJ, Zumla A, Galvin J, Migliori GB, Zumla A. Accelerating development of new shorter TB treatment regimens in anticipation of a resurgence of multi-drug resistant TB due to the COVID-19 pandemic. Int J Infect Dis, 2021; 2–5; doi:10.1016/j.ijid.2021.02.067 CrossRef
Uddin Z, Bin T, Kumar A, Jenny A, Dutta M, Morshed M, Kawsar H. Anti-inflammatory and antioxidant activity of leaf extract of Crinum asiaticum anti-inflammatory and antioxidant activity of leaf extract of Crinum asiaticum. J Pharm Res, 2015; 5(12):5553–6.
Ullah R, Alqahtani AS, Noman OM, Alqahtani AM, Ibenmoussa S, Bourhia M. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi J Biol Sci, 2020; 27(10):2706–18: doi:10.1016/j.sjbs.2020.06.020 CrossRef
WHO report. Systematic screening for active tuberculosis. WHO, Geneva, Switzerland, 2013.
Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, Fantuzzi G, Poll T Van Der. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob / ob mice. Int Immunol, 2005; 17(11):1399–408; doi:10.1093/intimm/dxh317 CrossRef
World Health Organization report. Global tuberculosis report. J Chem Inform Model, 2020; 53(9):1689–99.
Yamawaki T, Ito E, Mukai, A, Ueno M, Yamada J, Sotozono C, Kinoshita S, Hamuro J. 2016. The ingenious interactions between macrophages and functionally plastic retinal pigment epithelium cells. Invest Ophthalmol Vis Sci, 2016; 57(14):5945–53; doi:10.1167/iovs.16-20604 CrossRef
Ye M, Bian LF. Association of serum leptin levels and pulmonary tuberculosis: a meta-analysis. J Thora Dis, 2018; 10(2):1027–36; doi:10.21037/jtd.2018.01.70 CrossRef