SASPI Ltd.
Biofilm in Cochlear Implant: A Surgeon’s Diagnosis
Shriya Bhattarai, Sushovit Sharma Luitel, Akhilesh Chandra Yadav, Kajal Mahto, Amber Prasad, Ravi Hari Phulware, Nilotpal Chowdhury, Amit Kumar Tyagi*
JASPI March 2024/ Volume 2/Issue 1
Bhattarai S, Luitel SS, Tyagi AK et al.Biofilm in Cochlear Implant: A Surgeon’s Diagnosis. JASPI. 2024;2(1):29-35
ABSTRACT
Biofilms in a cochlear implant can lead to severe intracranial complications like meningitis, and it can cause implant failure, thus requiring an explant, leading to financial and social loss. Meticulous sterility in the intraoperative period and proper hygiene in the postoperative period need to be emphasized in a cochlear implant Patient. We report a case of a 4-year-old girl child who presented with recurrent episodes of wound dehiscence through the cochlear implant site following the asymptomatic period after a course of antibiotics, and finally treated with debridement and flap reconstruction under general anaesthesia. We sought to use novel agents with antimicrobial activity in addition to debridement, vascularized soft tissue cover, and oral rifampicin after surgery. The child underwent serial surgeries, and finally, the use of medical grade Manuka honey and rifampicin demonstrated a successful response to biofilm up to a two-month follow-up period. This case revealed that biofilm is primarily a surgeon’s diagnosis.
KEYWORDS: Biofilm, Implant, Manuka honey, rifampicin, wound dehiscence
INTRODUCTION
Biofilms can be a severe hazard as they are challenging to diagnose microbiologically or pathologically. Biofilms in cochlear implants are rare, with an incidence of up to 1.7-4.1%.1-2 Very few patients with cochlear implants might face the problem and present with recurrent wound site infection, which is challenging to diagnose and thus necessitating exploration of the wound, serial debridement and may require some agents other than conventional antibiotics to combat with the nuisance caused by biofilms. The need for an explant can be accountable to despair in a child’s life and a huge amount of economic burden to a family and country.
Biofilm in an implant is one of the differentials in an implanted recipient when the patient presents with wound dehiscence through the implant site, exposing it with minimal signs of inflammation and fever episodes. Biofilms are constantly evolving and diverse communities of organisms. The protective matrix that the bacteria create and excrete securely binds the biofilm to a living surface. Compared to planktonic cells, biofilm communities have additional resistance mechanisms, which hinder treatment options and promote the spread of infections. Quorum sensing, adverse environmental circumstances, nutritional availability, hydrodynamic conditions, cell-to-cell communication, signaling cascades, and secondary messengers are some of the factors that cause and control the process of biofilm formation.3 We report a case who presented with biofilm in a cochlear implant.
CASE PRESENTATION
A 4-year-old child presented to the Department of Otorhinolaryngology with bilateral prelingual deafness with no significant prenatal, perinatal, or postnatal history. The syndromic evaluation did not reveal any other associated medical condition, and there is no history of prelingual deafness in up to the child’s 3rd-degree relatives. She did not receive any formal education. Her social and motor milestones were age-appropriate. However, the child’s hygiene condition was poor. Pneumococcal and meningococcal vaccination was completed before surgery, apart from the child’s routine immunization. She underwent right cochlear implantation in May 2023. Post-surgery, there were signs of a lack of hygiene at the wound site. She was conservatively managed (oral antibiotics) only to subside symptoms till July 2023, when the child again presented with wound dehiscence exposing the receiver-stimulator component of a cochlear implant (Figure 1A)
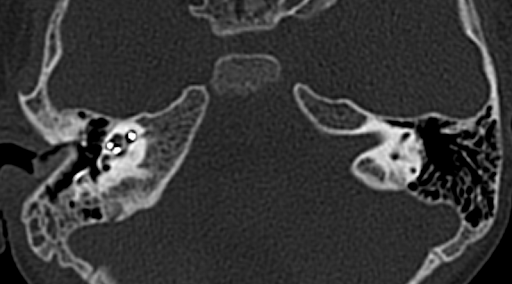
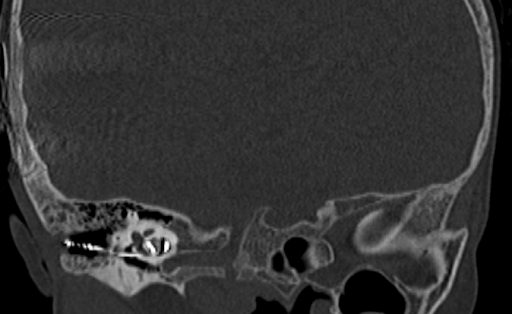
Figure 1: Skin and radiological images of the patient. 1.5 x 2 cm2 epithelial abrasion in right postauricular region exposing the implant in July, 2023 (A), wound discharge with wound dehiscence in December, 2023 (B); High resolution computed tomography of temporal bone revealing axial showing electrodes in middle ear going to right cochlea with reduced mastoid air cells on right side (C, D).
Figure 1(A) Figure 1(B)
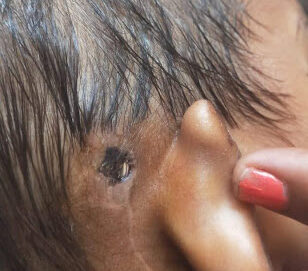
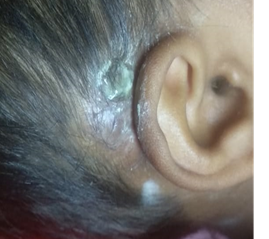
Figure 1(C) Figure 1(D)
After unsuccessful attempts to debride and close the wound with secondary suturing (July 2023) and local flap (December 2023), she again presented with wound dehiscence through the implant site (Figure 1B) along with fever episodes in January 2024, for which another flap was required along with debridement in January 2024. The timeline showing a series of chief complaints at different periods is mentioned in Figure 2.
Figure 2: Timeline showing a series of chief complaints of the patient at different periods of time

Routine blood investigations revealed only an elevated total leucocyte count (up to 21,000/cmm). Multiple culture & sensitivity (C/S) specimens from the discharge as well as the intraoperative tissue at the wound bed did not reveal any organism except one in December showing Methicillin-sensitive Staphylococcus aureus sensitive to clindamycin, erythromycin, and vancomycin which also coincided with raised leucocyte count (19,000/cmm).
C-reactive protein and procalcitonin were also mildly elevated. When the patient had fever spikes in January 2024, contrast-enhanced computed tomography of the head and high-resolution computed tomography (HRCT) of the temporal bone were made to rule out meningitis and see the position of electrodes of the implant, which revealed normal findings (Figure 1C-D).
Histopathological findings of wound bed tissue showed features of chronic inflammation, but no organism was detected in various stains (Figure 3A-B). The initial differential diagnosis in June 2023, when the patient presented with serous discharge from the wound site with intermittent, low-grade fever episodes one-month post cochlear implantation, was surgical site infection (SSI). Skin dehiscence secondary to foreign body reaction (suture, gauze piece, the implant itself) was also considered due to recurrent wound gaping with serous discharge and a history of fever, but there was no erythema, tenderness, or redness.
As there were intermittent healing periods after the antibiotic course and no typical yellowish pus discharge/sinus tracts, tubercular wound infection and SSI due to actinomycosis were ruled out on clinical grounds. Temporal bone bacterial or fungal osteomyelitis was considered due to the involvement of subperiosteal bone below the implant without cervical lymphadenopathy but excluded due to the lack of characteristic pain and the localized nature rather than extensive involvement. Skin dehiscence due to biofilm formation at the implant site was our intraoperative diagnosis after exploration to find a thick, slimy layer beneath and above the implant receiver-stimulator.
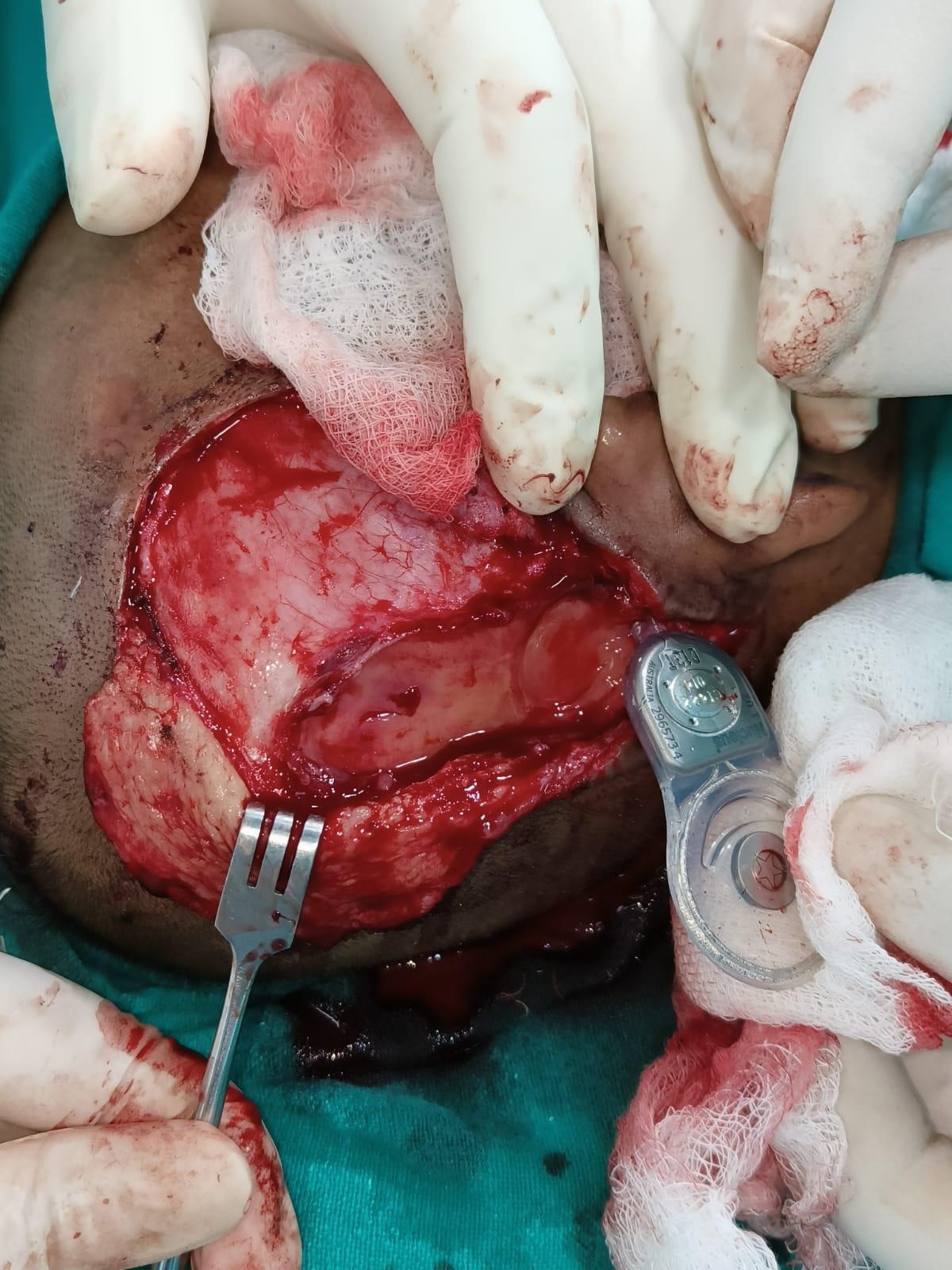
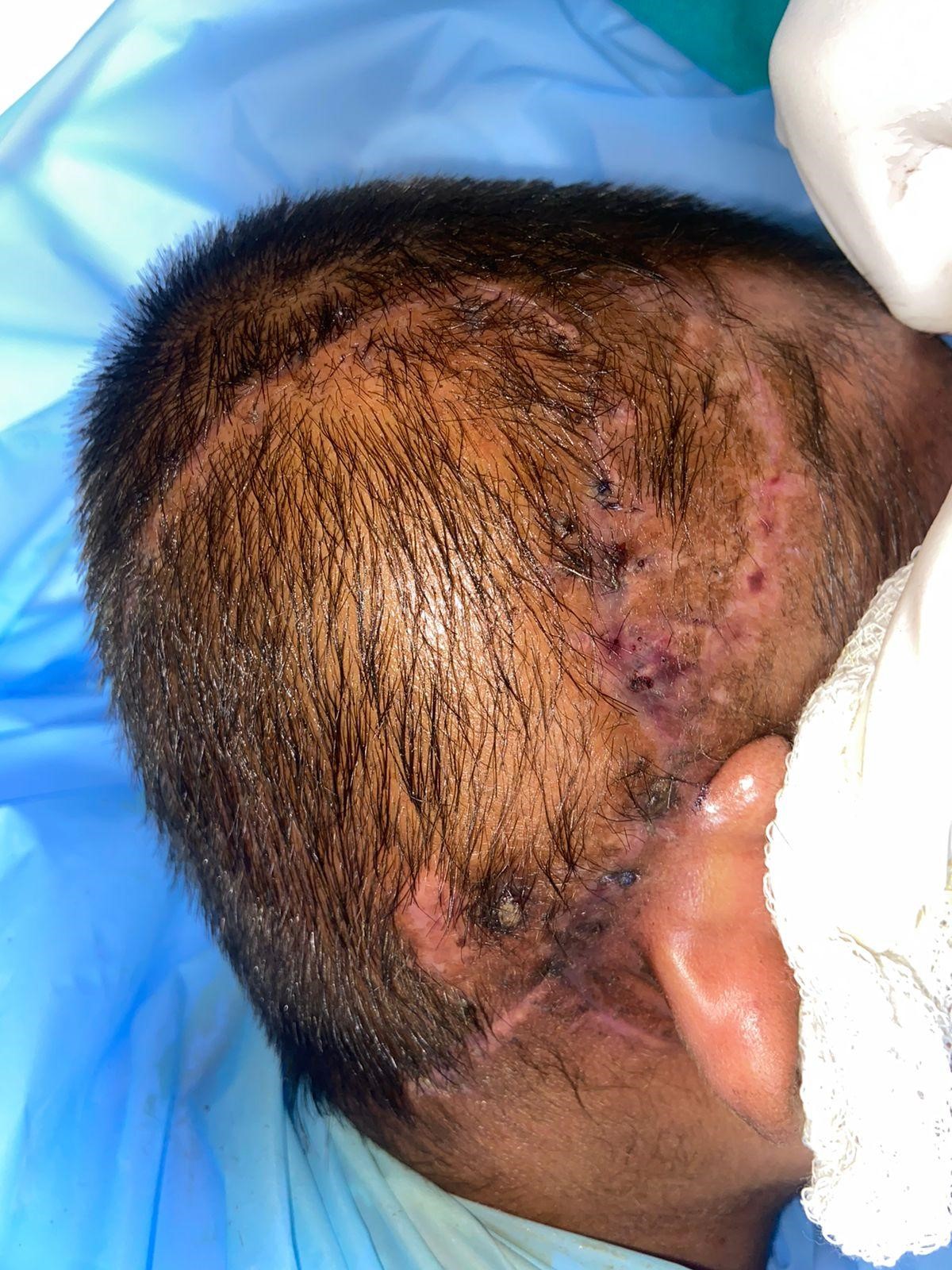
In July 2023, the child underwent debridement with secondary suturing of the wound site under general anaesthesia. She was asymptomatic for four months and again presented with discharge, a wound gaping with the exposed implant, in December 2023. This time, debridement and anteriorly based temporoparietal flap (blood supply being superficial temporal artery) were done. Intra-operatively, a thick, slimy biofilm layer was found above and below the implant receiver-stimulator (Figure 3C). This was removed and sent for C/S and HPE. The receiver-stimulator of the implant was also cleaned with 10% betadine solution and normal saline solution for 30 minutes. Drilling the bone beneath and creating a new well posteriorly for the receiver-stimulator of the implant was done. The child was now started on injection vancomycin as one C/S was positive for Staphylococcus aureus. Empirically, rifampicin (10 mg/kg/day) was also started simultaneously, as the child had previously been kept on multiple conventional antimicrobials, which did not completely resolve the infection. One month later, the wound again dehisced and was taken up for another surgery. This time, along with debridement and another posteriorly based occipital flap (blood supply being occipital artery) was done. The receiver-stimulator was washed with betadine and normal Saline and then painted with medihoney. The patient was continued with rifampicin with a plan to stop after a total duration of 6 months. Intraoperative Neural Response Telemetry showed functioning electrodes. The patient is presently asymptomatic with a healthy wound site until two months of follow-up (Figure 3D). There are no fever episodes; the child is playful and can do routine activities. She is attending her postoperative speech and language rehabilitation courses. Detailed follow-up is further required to ensure this case is a success.
Figure 3: Histopathological, Intra-operative, and post-operative skin findings. H&E stained tissue preparation showing inflammatory cells – eosinophils, plasma cells, lymphocytes but no granuloma, malignancy seen (A); Gram staining showed no bacteria (B); Intra-operative finding of slimy layer (Blue arrow) of biofilm beneath the receiver-stimulator (Yellow arrow) part of cochlear implant (C); Image captured on post-operative day 50 following the last surgery with healthy post-operative wound site and no signs of inflammation.
Figure 3 (A) Figure 3 (B)
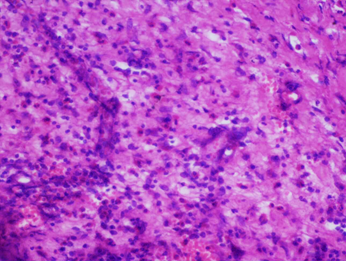
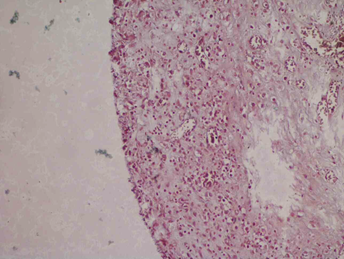
Figure 3 (C) Figure 3 (D)
DISCUSSION
This case was a young girl with repeated head & neck surgeries with wound dehiscence. Biofilm in the cochlear implant was later proved as the cause. Biofilm infections can be challenging to diagnose routinely, and to date, no standard diagnostic protocol is confirmatory to detect biofilm. Studies have shown biofilms using histochemistry and immunohistochemistry together.4 Hematoxylin-eosin staining of surgical specimens is considered a method for detecting bacterial biofilm in chronic infectious disease.5 In resource-constraint settings, sophisticated tests might not be available to detect biofilms microbiologically and histopathologically. However, knowing its presence is important for clinicians to start appropriate antibiotic therapy.
Conservative management efforts would be ineffective unless biofilm is removed from the implant surface. Powerful combination therapy to quickly and efficiently lower biofilm levels in wounds, which in turn lowers inflammation, reactive oxygen species, and protease activity, includes debridement in concert with the most effective anti biofilm treatment.6 Prophylactic perioperative antibiotics should be given to patients, and in cases where there are underlying medical comorbidities, long-term antibiotics should be taken into consideration.7 Despite adequate peri and postoperative antibiotics, in the present case, the patient failed to show complete recovery.
In pediatric cochlear implants, longer operating times and younger ages raise the risk of SSI substantially.8 A foreign body or hypersensitivity reaction in the form of giant cells and lymphocytic cell infiltration is common after cochlear implantation and may be one possible cause of failure.9 In a case of biofilm associated cochlear implant, following debridement, injection of piperacillin and amoxicillin-clavulanate for ten days, followed by oral rifampicin (10 mg/kg/day) for six months was given to salvage cochlear implant following which no indications of infection a year following the procedure was observed except 1 out of 5 patients for whom cochlear explant was required.10 Biofilm research can benefit from identifying genes involved in biofilm formation and evaluating gene expression due to drugs’ antibiofilm and anti-bacterial activity.11
Pseudomonas aeruginosa and Staphylococcus aureus have different capacities for biofilm formation on different cochlear implant materials depending on whether bioactive glass (BAG) is present. Biofilm morphology is significantly altered by applying BAG, as evident from scanning electron microscopy studies.12
In a cochlear implant child, when an antibiotic treatment seems to be working, recurrent infections should prompt the doctor to check for an underlying immunodeficiency.13 However, in the present case, the child had no known underlying immunodeficient condition. Vancomycin, clindamycin, cephalothin, teicoplanin, and ofloxacin generally showed less killing power in biofilms than fosfomycin, erythromycin, rifampicin, and tetracycline.14 The polysulfobetaine methacrylate/polydopamine (Copper) / PSB/PDA(Cu) coating with remarkable biocompatibility in both in vitro and in vivo studies on cochlear implants having anti-inflammatory, potent anti-bacterial, and anti-biofilm characteristics was proven to be a particular anti-bacterial tactic to improve the performance of cochlear implants.15 Previously, the biofilm by methicillin-sensitive Staphylococcus aureus isolates formed in the cochlear implant was found to be susceptible to hydrogen peroxide, terpinen-4-ol, and tea tree oil even after a brief contact.16 Biofilms can be eliminated with readily accessible polyhexanide and betadine surfactants.10
In evidence-based medicine specific to cochlear implants, Manuka-type honey has shown to be an effective topical treatment for chronic wound infections because it may be utilized to destroy Staphylococcus aureus and Pseudomonas aeruginosa. Manuka-type honey can destroy this organism’s biofilms because it includes one or more substances besides sugar and methylglyoxal, like low pH, hydrogen peroxide, phenolics, and other unidentified substances.17 In our institute, a pilot study was conducted from November 2017 to September 2018 on 20 patients of otitis externa where the patient’s ear was packed with umbilical tape impregnated with around 3–4 ml of Manuka honey gel (Medihoney gel), following which pain score and canal edema were found to decrease significantly.18 Various Indian articles supported the use of Manuka honey in biofilms as a promising material for chronically discharging wounds, but no such article specific to cochlear implants has been found.19-21 Soft tissue reaction may arise from implant, surgeon, or patient-related factors. The thickness and form of the skin flap have been identified as key risk factors for this. A tiny skin flap is required since a thick one cannot hold an external device in place via magnetic force. Most implant manufacturers recommend that this thickness should not exceed 6 mm. One of the leading causes of the wound’s eventual disintegration is the excessive thinning of this flap.22 A crucial component of cochlear implant surgery is maintaining complete sterilization; any failure to do so may result in infection, which may subsequently progress to skin necrosis and eventual explantation. There is published research on extrusion caused by silicone and platinum allergies and foreign body response.23,24 Failure to maintain proper hygiene at the postoperative wound site and excessive sweating may have led to biofilm formation in our case.
Given the high cost and probable side effects of cochlear implant replacement, including discomfort and social isolation, more research should be done to prevent and treat biofilm-mediated cochlear implant infections. Financial challenges prevent us from getting new implant devices in our Indian setup, which is concerning when patients have auditory loss. Simultaneous application of ultrasound and increased oxygen tension have also been advocated to eradicate biofilms.25,26 In the era of state-of-the-art technology where multiple implant and prosthetic surgeries are being done, more research on the relationship of hygiene with implant biofilm and a diagnostic protocol for biofilms is needed.
CONCLUSION
In a cochlear implanted child, counselling regarding maintenance of proper hygiene at the wound site should be done; otherwise, it can lead to surgical site infection and biofilm formation, causing serious complications like meningitis and explantation of the implant. Once a biofilm has been suspected, the use of novel agents like Manuka honey, tea tree oil, and surgical debridement should be considered. Biofilm is primarily a surgeon’s diagnosis; a slimy gel-like layer in the implant bed should lead the surgeon to communicate inter-departmentally to detect organisms causing biofilm to start targeted antibacterial therapy.
INFORMED CONSENT
The father provided consent to publish the case report. Confidentiality of the patient was maintained in the article.
The authors declare no conflict of interest.
SOURCE OF FUNDING
None
AUTHORS’ CONTRIBUTIONS
SB: Conceptualization; Data curation; Analysis; Writing the draft
SSL: Analysis, Review & Editing
ACY: Supervision; Validation; Review & Editing
KM: Data collection; Analysis, Review & Editing
AP: Investigations, Review & Editing
RHP: Investigations, Review & Editing
NC: Investigations, Review & Editing
AKT: Supervision; Validation; Review & Editing
REFERENCES
1. Cunningham CD 3rd, Slattery WH 3rd, Luxford WM. Postoperative infection in cochlear implant patients. Otolaryngol Head Neck Surg. 2004;131(1):109-14.
2. Cohen NL, Hoffman RA. Complications of cochlear implant surgery in adults and children. Ann Otol Rhinol Laryngol. 1991;100(9 Pt 1):708-11.
3. Rather MA, Gupta K, Mandal M. Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz J Microbiol. 2021;52(4):1701-18.
4. Jensen LK, Henriksen NL, Bjarnsholt T, Kragh KN, Jensen HE. Combined Staining Techniques for Demonstration of Staphylococcus aureus Biofilm in Routine Histopathology. J Bone Jt Infect. 2018;3(1):27-36.
5. Hochstim CJ, Choi JY, Lowe D, Masood R, Rice DH. Biofilm detection with hematoxylin-eosin staining. Arch Otolaryngol Head Neck Surg. 2010;136(5):453-6.
6. Silva NBS, Marques LA, Röder DDB. Diagnosis of biofilm infections: current methods used, challenges and perspectives for the future. J Appl Microbiol. 2021;131(5):2148-60.
7. Basavaraj S, Najaraj S, Shanks M, Wardrop P, Allen AA. Short-term versus long-term antibiotic prophylaxis in cochlear implant surgery. Otol Neurotol. 2004;25(5):720-722.
8. Quimby AE, Grose E, Reddy D, Webster R, Malic C, Vaccani JP. Predictors of Surgical Site Infection in Pediatric Cochlear Implantation. Otolaryngol Head Neck Surg. 2023;168(3):484-90.
9. Nadol JB Jr, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Otol Neurotol. 2008;29(8):1076-84.
10. Suri N, Yadav C, Sandilya S, Bhalodia N. Salvaging Cochlear Implant After Suspected Biofilm Infection: Our Experience. Indian J Otolaryngol Head Neck Surg. 2021;73(4):499-503.
11. Kırmusaoglu S. The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents. In: Kırmusaoglu S, eds. Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods. IntechOpen; 2019.
12. Kirchhoff L, Arweiler-Harbeck D, Arnolds J, et al. Imaging studies of bacterial biofilms on cochlear implants-Bioactive glass (BAG) inhibits mature biofilm. PLoS One. 2020;15(2):e0229198.
13. Rubin LG, Papsin B; Committee on Infectious Diseases and Section on Otolaryngology-Head and Neck Surgery. Cochlear implants in children: surgical site infections and prevention and treatment of acute otitis media and meningitis. Pediatrics. 2010;126(2):381-91.
14. Monzón M, Oteiza C, Leiva J, Lamata M, Amorena B. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn Microbiol Infect Dis. 2002;44(4):319-24.
15. Chen A, Wang Z, Chen H, et al. Zwitterion modified cochlear implants resist postoperative infection and inflammation. Mater Today Bio. 2023;23:100856.
16. Brady AJ, Farnan TB, Toner JG, Gilpin DF, Tunney MM. Treatment of a cochlear implant biofilm infection: a potential role for alternative antimicrobial agents. J Laryngol Otol. 2010;124(7):729-38.
17. Lu J, Turnbull L, Burke CM, et al. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ. 2014;2:e326.
18. Kumar A, Mittal S, Tyagi AK, Romesh H, Varshney S, Malhotra M. Efficacy of Medical Grade Manuka Honey in Acute Otitis Externa: A Pilot Study. Indian Journal of Otology. 2020;26(3):151-4.
19. Kapoor N, Yadav R. Manuka honey: A promising wound dressing material for the chronic nonhealing discharging wounds: A retrospective study. Natl J Maxillofac Surg. 2021;12(2):233-7.
20. Kumar ND, Kalluru RS, Ahmed S, et al. Comparison of the Anti-bacterial Efficacy of Manuka Honey Against E.faecalis and E.coli – An In vitro Study. J Clin Diagn Res. 2014;8(8):ZC29-ZC31.
21. Goswami AG, Basu S, Banerjee T, Shukla VK. Biofilm and wound healing: from bench to bedside. Eur J Med Res. 2023;28(1):157.
22. Filipo R, D’Elia C, Covelli E, et al. Haematoma after cochlear implantation: management of a minor complication. Acta Otolaryngol. 2010;130(1):108-13.
23. Lim HJ, Lee ES, Park HY, Park K, Choung YH. Foreign body reaction after cochlear implantation. Int J Pediatr Otorhinolaryngol. 2011;75(11):1455-8.
24. O’Malley JT, Burgess BJ, Galler D, Nadol JB Jr. Foreign Body Response to Silicone in Cochlear Implant Electrodes in the Human. Otol Neurotol. 2017;38(7):970-7.
25. Qian Z, Sagers RD, Pitt WG. The effect of ultrasonic frequency upon enhanced killing of P. aeruginosa biofilms. Ann Biomed Eng. 1997;25(1):69-76.
26. Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111(12):2083-94.
Submit a Manuscript:
©The Author(s) 2024. Published by Society of Antimicrobial Stewardship practIces (SASPI) in India. All rights reserved.
