Abstract
Objective:
Methicillin-resistant Staphylococcus epidermidis (MRSE) remains one of the most prevalent drug-resistant bacteria causing health care infections. Limited data are available about how the frequency of MRSE changed in Iran over the past years. The current study aimed at determining the frequency of MRSE in different cities of Iran.Methods:
Databases including Web of Sciences, Scopus, Embase, Medline, and Iranian databases were searched to find studies addressing the frequency of MRSE in Iran published from Mar 2006 to Jan 2016. The data were analyzed using comprehensive meta-analysis version 2.2 (Biostat). Of the 139 records identified in the databases, 15 studies met the inclusion criteria.Results:
The analyses showed that the frequency of MRSE infections was 73.9% [95% confidence interval (95% CI) 61.4 - 83.4] among culture-positive cases of S. epidermidis in different parts of Iran. The frequency of MRSE was higher in the studies conducted from 2011 to 2015, based on further stratified analyses.Conclusions:
The regular surveillance on antimicrobial susceptibility pattern and formulation of definite antibiotic policy may control high rate of MRSE associated infections in Iran. Moreover, rapid and reliable diagnosis of MRSE isolates and regular screening of the personnel and surfaces of hospitals in terms of MRSE are indispensable.Keywords
Methicillin-Resistant Staphylococcus epidermidis (MRSE) Meta-Analysis Iran
1. Context
Health care complication due to coagulase negative staphylococci (CoNS) are increased worldwide (1, 2). CoNS were known as major human skin resident organisms and nowadays they are considered as one of the most important agents of frequent nosocomial infections (3-5). Based on recent studies, in patients that should be hospitalized and use indwelling medical devices, Staphylococcus epidermidis (S. epidermidis) is characterized as one of the main opportunistic pathogens and the number of life threatening cases of biomaterial related infections are intensified in many communities (6-9). The number of methicillin-resistant S. epidermidis (MRSE) infections is dramatically increased and is a serious problem in terms of nosocomial infections worldwide (10). In the past 10 years, an alarming increase in the prevalence of MRSE is observed (11, 12). Previously, S. epidermidis was described as a commensal human skin and mucosa micro flora (13, 14). Its appearance in laboratory samples was interpreted as a confusing accidental contamination, but now with the development of implanted devices in modern medicine, researcher gradually observed a relationship between chronic infections and the use of these prosthetic tools (15, 16). The vast majority of studies insisted on the role of S. epidermidis in catheter-associated infections that relies on bacterial capacity to colonize on such devices and protect itself from host immunity or antibiotic therapy (17-20). Emergence of S. epidermidis strains resistant to strong antibiotics increased recently and made momentous severity to manage the disease and health care control (21, 22). Moreover, MRSE is one of the main post surgery infectious elements (23). The close scrutiny of the rising number of MRSE isolates divulges concern for diagnosis, control, and treatment (24). To the best authors knowledge, there was no comprehensive analysis of MRSE infection prevalence in Iran. Furthermore, few studies reported the prevalence of MRSE infections. The current study mainly aimed at assessing the prevalence of MRSE infection in Iran.
2. Methods
2.1. Search Strategies
From 2006 to June 2016, all studies discussing MRSE infections in Iran were collected from databases of Web of Science, PubMed, Scopus, Embase, Cochrane Library, Iranmedex, Google Scholar, and Scientific Information Database (SID). The search was restricted to original research articles published in English or Persian and presenting the frequency or incidence of MRSE infections in Iran. The following keywords containing Medical Subject Headings (MeSh) or keywords in titles or abstracts were used with Boolean operators (“and” or “or”): ‘‘Staphylococcus epidermidis”, ‘‘S. epidermidis”, ‘‘coagulase negative staphylococcus”, “CoNS”, ‘‘MRSE”, ‘‘methicillin-resistant Staphylococcus epidermidis”, and ‘‘Iran”. Meanwhile, bibliographies from retrieved papers were revised for any additional studies. In addition to English papers, all relevant articles in Iranian databases such as Magiran (www.magiran.com), Iranmedex (www.iranmedex.com), Scientific Information Database (www.sid.ir), Iranian National Library (www.nlai.ir), and Irandoc (www.irandoc.ac.ir) were searched with similar strategy and the related Persian keywords. Furthermore, no contact was made with the authors regarding the previous experiences (25-28).
2.2. Inclusion and Exclusion Criteria
All original articles presenting cross sectional studies on the frequency of MRSE in Iran were considered. The selection of articles for review was completed based on three stages: titles, abstracts, and full texts. The inclusion criteria were application of standard methods for drug susceptibility testing (DST) of S. epidermidis against beta-lactam antibiotics (penicillin, methicillin, oxacillin and cefoxitin) and presenting data on the number of S. epidermidis and MRSE isolates. Standard DST methods include the oxacillin screening plate, broth microdilution (BMD), Epsilon test (E test), disk diffusion method (DDM) for cefoxitin, and polymerase chain reaction (PCR) technique for mecA detection. Studies were excluded from analysis for any of the following reasons: considering only specific subpopulation, irrelevancy of the title, studies on non-human samples, not reporting S. epidermidis numbers, application of non-standard methods for MRSE detection, and not reporting MRSE numbers. Review articles, meta-analyses or systematic reviews, duplicate publications of the same study and articles available only in abstract form were also excluded from the current study.
2.3. Data Extraction and Definitions
For all studies, the following data were extracted: the first author’s name, year of publication, year of study, study setting, number of cases investigated, method of studies, source of samples, sample size, frequency of S. epidermidis and MRSE, and if existed, other information. All the included studies were extracted independently by two investigators and results were reviewed by a third investigator and finally inconsistencies were discussed to achieve consensus. PRISMA guidelines were used for the current study (29).
2.4. Quality Assessment
Included studies were appraised for quality, using a quality assessment checklist designed by the Joanna Briggs Institute (29).
2.5. Meta-Analysis
Comprehensive meta-analysis version 2.0 (Biostat) was used for analysis. The point estimate of effect size, the frequency of MRSE, and its 95% confidence interval (95% CI) were approximated for each survey. Random-effects models were utilized. Cochran Q-statistic was used to consider the possibility of heterogeneity between the studies. To assess possible publication bias, Egger weighted regression methods were applied. P < 0.05 was considered indicative of statistically significant publication bias.
3. Results
3.1. Characteristics of the Reviewed Articles
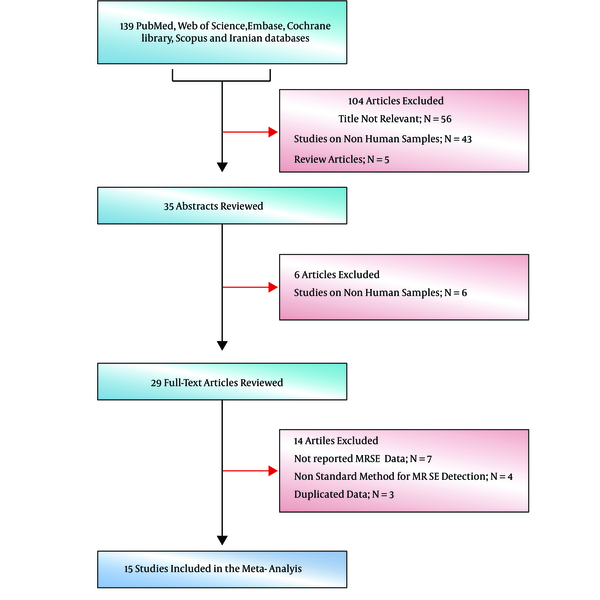
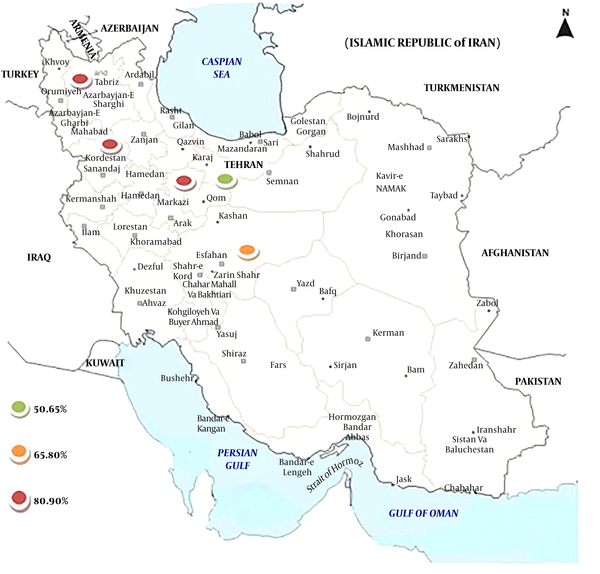
Primarily, 139 studies were selected and in the next screening, 26 of them were included based on title and abstract screening (Figure 1). In the third phase, 20 of the 26 article were selected upon a full text search and finally 15 eligible articles were selected (Figure 1). Table 1 shows the details of the reviewed studies (30-44). Geographical location of studies mostly covered center and west of Iran. Moreover, MRSE isolates were isolated from different clinical samples, especially blood.
Flowdiagram of study
Characteristics of Studies Included in Meta-Analysis
| Study | Time of Study | Publication Data | City | No. of S. epidermidis | No. of MRSE Isolates | Diagnostic Methods for MRSE (CLSI) | Source of Samples |
|---|---|---|---|---|---|---|---|
| Ebrahimzadeh Namvar (36) | 2014 | 2014 | Isfahan | 115 | 96 | PCR/DDM | Blood, catheters |
| Pishva (37) | 2009 | 2013 | Isfahan | 146 | 103 | E test | - |
| Eftekhar (31) | 2007 | 2011 | Tehran | 55 | 50 | DDM | Blood, wound |
| Pourmand (38) | 2008 | 2011 | Tehran | 99 | 56 | DDM | Nasal swab |
| Taheri (43) | 2013 | 2014 | Arak | 54 | 40 | E test/PCR | Surfaces and Staff |
| Soroush (42) | 2012 - 2013 | 2016 | Tehran | 256 | 80 | PCR | Different |
| Havaei (34) | 2014 | 2015 | Isfahan | 70 | 61 | DDM/PCR | Blood, catheters |
| Najar-Peerayeh (35) | 2010 - 2011 | 2014 | Tehran | 64 | 59 | PCR | Different |
| Shoja (41) | 2006 | 2006 | Tabriz | 100 | 89 | DDM/PCR | Blood |
| Fateh Rahimi (32) | 2014 | 2014 | Tehran | 193 | 72 | E test/PCR | Body fluid and wound |
| Raei (39) | 2007 | 2010 | Tehran | 55 | 42 | PCR | Blood |
| Ramazanzadeh (40) | 2011-2012 | 2015 | Sanandaj | 170 | 151 | DDM | Clinical sample, staff |
| Ghotaslou (33) | 2012 - 2013 | 2014 | Tabriz | 57 | 46 | PCR | Blood, tracheal tube |
| Sotoudeh Anvari (30) | 2007 - 2012 | 2015 | Tehran | 20 | 7 | DDM | Pericardial effusions |
| Talebi (44) | 2010 - 2012 | 2015 | Tehran | 90 | 58 | PCR/BMD | - |
3.2. The Frequency of MRSE Infections
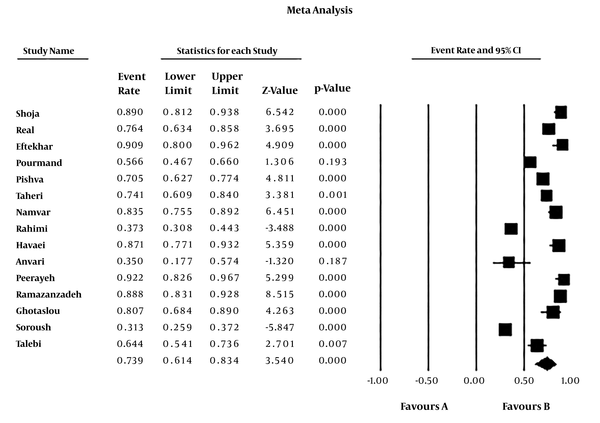
The rate of MRSE infections among culture-positive cases of S. epidermidis was 73.9% (95% CI: 61.4 - 83.4) (Table 2). The heterogeneity evaluation showed heterogeneity among articles (I2 = 95.327, P < 0.001). Forest plot of meta-analysis on the rate of MRSE infections are indicated in Table 2 and Figure 2. According to Table 2 and Figure 3, there was no publication bias in the current study (P > 0.05 for Egger weighted regression analysis). Table 3 demonstrated the stratified analyses based on the geographical locations of the reviewed articles. Distribution of MRSE infections in different parts of Iran is revealed in Figure 4.
Meta-Analysis of the Prevalence of MRSE Infections in Iran
| Subgroup | No. of Study | Prevalence of MRSE (95%CI) | n/N | Heterogeneity Test, I2 (%) | Heterogeneity Test, P Value | Egger Test, t | Egger Test, P Value |
|---|---|---|---|---|---|---|---|
| Overall effects | 15 | 73.9 (61.4 - 83.4) | 1010/1544 | 95.327 | < 0.001 | 3.887 | 0.001 |
| Researches from 2006 to 2010 | 6 | 73.3 (58.0 - 84.5) | 347/475 | 88.596 | < 0.001 | 0.619 | 0.569 |
| Researches from 2011 from 2015 | 9 | 74.3 (55.9 - 86.9) | 663/1069 | 96.627 | < 0.001 | 4.997 | 0.001 |
Forest plot of meta-analysis on the prevalence of MRSE infection
Funnel plot of meta-analysis on the prevalance of MRSE infections
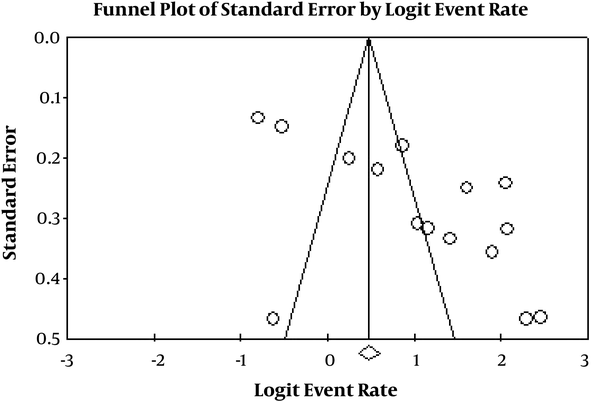
Meta-Analysis of the Prevalence of MRSE Infections in Different Parts of Iran
| City | No. of Study | Prevalence of MRSE (95%CI) | n/N | Heterogeneity Test, I2 (%) | Heterogeneity Test, P Value | Egger Test, t | Egger Test, P Value |
|---|---|---|---|---|---|---|---|
| Overall effects | 15 | 73.9 (61.4 - 83.4) | 1010/1544 | 95.327 | < 0.001 | 3.887 | 0.001 |
| Tehran | 8 | 63.3 (46.6 - 77.4) | 424/832 | 94.221 | < 0.001 | 3.224 | 0.017 |
| Isfahan | 3 | 80.6 (68.5 - 88.8) | 260/331 | 79.842 | < 0.001 | 2.727 | 0.223 |
| Tabriz, Arak, Sanandaj | 4 | 84.3 (76.4 - 89.9) | 326/381 | 66.090 | < 0.001 | 0.944 | 0.444 |
Distribution of MRSE infection in Iran based on the rate of incidence
4. Discussion
The incidence of MRSE is gradually rising since its emergence was reported. Although there were occasional reports of MRSE infections in the past, it is one of the recently established nosocomial pathogens (45). The current systematic review reports the frequency of MRSE infections in Iran. The current study analyses showed that the frequency of MRSE infections was 73.9% (95% CI: 61.4 - 83.4) among culture-positive samples of S. epidermidis in different parts of Iran (Table 2). According to the results of the current study, the MRSE had higher incidence rate in Tabriz, Arak, Sanandaj, and Isfahan than Tehran. Despite the large population in Tehran, capital of Iran, better infection control policies in Tehran may reduce the MRSE incidence than other parts of Iran. Although no article was related to the studies in the east of Iran, data showed that the rate of MRSE infections were higher in the western parts of Iran than the other parts. Also, the obtained results showed that MRSE incidence rates increased from 2011 to 2015 [74.3% (95% CI: 55.9 - 86.9)] in comparison with other studies, from 2006 to 2010. This event had several reasons such as increasing rate of MRSE during the recent years, the increase of knowledge about MRSE recognition, and use of advanced devices and methods such as E test and PCR for MRSE identification, etc.
Hospital-acquired infections, according to centers for disease control (CDC), are the infection that is acquired in a hospital or other health care facilities after 2 days of admission. High population societies, such as Iran, with less attention to hygienic practices and standards in some parts of the country can be regarded as the main factor for hospital-acquired infections (46). There is little information about the different SCCmec types of MRSE in Iran. In agreement with prior information, the rate of SCCmec in types I and II was low, while type IV was comparatively prevalent (47, 48). According to the results of an investigation, SCCmec type IV was the most prevalent type followed by type V and III in MR-CoNS strains, respectively (49). Also, another study indicated that 36% of MRSE isolates had a type IV of SCCmec (50). Based on the recent information, origin of SCCmec is more various in MR-CoNS via novel types of ccr genes (51). Other factors that can raise MRSE incidence are the intensity of disease, transition of pathogenic bacteria among the hospital wards, the disorganized implementation of prophylactic hygiene measures, inadequate staff training, and lack of hospital infection control programs. Thus, insufficient MRSE management leads to the continued spread of MRSE in hospitals of Iran. Moreover, the association of multidrug resistance with MRSE adds to the problem (52). B-lactam antibiotics such as penicillin and cephalexin were not effective against MRSE (53, 54). The maximum impressive antibacterial drugs against S. epidermidis were cefoxitin, ciprofloxacin, and gentamicin. Therapy for patients with staphylococcal infections is made hard by bacterial resistances to beta-lactams and other antibacterial agents such as aminoglycosides and glycopeptides. Lately offered or empirical drugs such as dalfopristin and quinupristin belonging to streptogramin class, and linezolid may be beneficial in the treatment of patients with multi-drug resistant staphylococci infections (30, 55). MRSE can be nosocomial or community-acquired. MRSE can be defined as community-acquired if the positive culture is obtained in less than two days of admission or outside hospital settings (56). Due to the lack of enough information, it is not exactly claimed whether the MRSE were from community or hospital. Another notable point is some limitations to perform this investigation, which should be discussed. First of all, only published studies were accepted for including in the current meta-analysis. Then, biases between publications and systematic reviews were inevitable. Second, heterogeneity was observed among the studies. Third, since there was no study on MRSE infections in many regions of Iran, it cannot fully represent the frequency of MRSE in Iran. Fourth, since it was a prevalence survey, it is not possible to determine the risk factors for MRSE colonization or infection.
In conclusion, high rate of MRSE isolates is a public health problem in Iran that needs further attention by microbiologists, health authorities, and physicians. The regular surveillance on antimicrobial susceptibility patterns and formulation of definite antibiotic policy may control high rates of MRSE associated infections in Iran. Moreover, rapid and reliable diagnosis of MRSE isolates and regular screening of the hospitals surfaces and personnel in terms of MRSE are indispensable.
References
-
1.
Tewhey R, Gu B, Kelesidis T, Charlton C, Bobenchik A, Hindler J, et al. Mechanisms of linezolid resistance among coagulase-negative staphylococci determined by whole-genome sequencing. MBio. 2014;5(3):e00894-14. [PubMed ID: 24915435]. [PubMed Central ID: PMC4030478]. https://doi.org/10.1128/mBio.00894-14.
-
2.
Falcone M, Russo A, Venditti M. Optimizing antibiotic therapy of bacteremia and endocarditis due to staphylococci and enterococci: new insights and evidence from the literature. J Infect Chemother. 2015;21(5):330-9. [PubMed ID: 25813608]. https://doi.org/10.1016/j.jiac.2015.02.012.
-
3.
Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21(12):660-8. [PubMed ID: 24238601]. [PubMed Central ID: PMC4744460]. https://doi.org/10.1016/j.tim.2013.10.001.
-
4.
Rezai MS, Pourmousa R, Dadashzadeh R, Ahangarkani F. Multidrug resistance pattern of bacterial agents isolated from patient with chronic sinusitis. Caspian J Intern Med. 2016;7(2):114-9. [PubMed ID: 27386063]. [PubMed Central ID: PMC4913714].
-
5.
Tajeddin E, Rashidan M, Razaghi M, Javadi SS, Sherafat SJ, Alebouyeh M, et al. The role of the intensive care unit environment and health-care workers in the transmission of bacteria associated with hospital acquired infections. J Infect Public Health. 2016;9(1):13-23. [PubMed ID: 26117707]. https://doi.org/10.1016/j.jiph.2015.05.010.
-
6.
Albertini M, Lopez-Cerero L, O'Sullivan MG, Chereguini CF, Ballesta S, Rios V, et al. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin Oral Implants Res. 2015;26(8):937-41. [PubMed ID: 24720498]. https://doi.org/10.1111/clr.12387.
-
7.
Mishra AK, Dufour H, Roche PH, Lonjon M, Raoult D, Fournier PE. Molecular revolution in the diagnosis of microbial brain abscesses. Eur J Clin Microbiol Infect Dis. 2014;33(12):2083-93. [PubMed ID: 24935615]. https://doi.org/10.1007/s10096-014-2166-z.
-
8.
Lotte R, Lotte L, Degand N, Gaudart A, Gabriel S, Ben H'dech M, et al. Infectious endocarditis caused by Helcococcus kunzii in a vascular patient: a case report and literature review. BMC Infect Dis. 2015;15:238. [PubMed ID: 26099275]. [PubMed Central ID: PMC4477501]. https://doi.org/10.1186/s12879-015-0984-y.
-
9.
Anacarso I, Condo C, Sabia C, Messi P, Niederhausern S, Bondi M, et al. Antimicrobial Resistance and Other Related Virulence Factors in Staphylococcus Spp isolated from Food, Environmental and Humans in Italy. Uni J Microbiol Res. 2013;1(1):1-9. https://doi.org/10.13189/ujmr.2013.010101.
-
10.
Peel TN, Buising KL, Dowsey MM, Aboltins CA, Daffy JR, Stanley PA, et al. Outcome of debridement and retention in prosthetic joint infections by methicillin-resistant staphylococci, with special reference to rifampin and fusidic acid combination therapy. Antimicrob Agents Chemother. 2013;57(1):350-5. [PubMed ID: 23114758]. [PubMed Central ID: PMC3535910]. https://doi.org/10.1128/AAC.02061-12.
-
11.
Linhares I, Raposo T, Rodrigues A, Almeida A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: a ten-year surveillance study (2000-2009). BMC Infect Dis. 2013;13:19. [PubMed ID: 23327474]. [PubMed Central ID: PMC3556060]. https://doi.org/10.1186/1471-2334-13-19.
-
12.
Cherifi S, Byl B, Deplano A, Nonhoff C, Denis O, Hallin M. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J Clin Microbiol. 2013;51(5):1541-7. [PubMed ID: 23486718]. [PubMed Central ID: PMC3647944]. https://doi.org/10.1128/JCM.03378-12.
-
13.
Fernandez L, Langa S, Martin V, Maldonado A, Jimenez E, Martin R, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69(1):1-10. [PubMed ID: 22974824]. https://doi.org/10.1016/j.phrs.2012.09.001.
-
14.
Dongarra ML, Rizzello V, Muccio L, Fries W, Cascio A, Bonaccorsi I, et al. Mucosal immunology and probiotics. Curr Allergy Asthma Rep. 2013;13(1):19-26. [PubMed ID: 23054627]. https://doi.org/10.1007/s11882-012-0313-0.
-
15.
Goes C. Central Venous Catheters: Care and Complications. Support Care Pediatr Oncology. 2015:283-300. https://doi.org/10.1007/978-3-662-44317-0_17.
-
16.
Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60(8):1176-83. [PubMed ID: 25537876]. [PubMed Central ID: PMC4400412]. https://doi.org/10.1093/cid/ciu1154.
-
17.
Hogan S, Stevens NT, Humphreys H, O'Gara JP, O'Neill E. Current and future approaches to the prevention and treatment of staphylococcal medical device-related infections. Curr Pharm Des. 2015;21(1):100-13. [PubMed ID: 25189861].
-
18.
Schaeffer CR, Woods KM, Longo GM, Kiedrowski MR, Paharik AE, Buttner H, et al. Accumulation-associated protein enhances Staphylococcus epidermidis biofilm formation under dynamic conditions and is required for infection in a rat catheter model. Infect Immun. 2015;83(1):214-26. [PubMed ID: 25332125]. [PubMed Central ID: PMC4288872]. https://doi.org/10.1128/IAI.02177-14.
-
19.
Chessa D, Ganau G, Spiga L, Bulla A, Mazzarello V, Campus GV, et al. Staphylococcus aureus and Staphylococcus epidermidis Virulence Strains as Causative Agents of Persistent Infections in Breast Implants. PLoS One. 2016;11(1). e0146668. [PubMed ID: 26811915]. [PubMed Central ID: PMC4727902]. https://doi.org/10.1371/journal.pone.0146668.
-
20.
Rodriguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. 2014;5(6):779-84. [PubMed ID: 25398740]. [PubMed Central ID: PMC4224214]. https://doi.org/10.3945/an.114.007229.
-
21.
Sakoulas G, Okumura CY, Thienphrapa W, Olson J, Nonejuie P, Dam Q, et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl). 2014;92(2):139-49. [PubMed ID: 24297496]. [PubMed Central ID: PMC3926703]. https://doi.org/10.1007/s00109-013-1100-7.
-
22.
Sakoulas G, Moise PA, Casapao AM, Nonejuie P, Olson J, Okumura CY, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther. 2014;36(10):1317-33. [PubMed ID: 25017183]. https://doi.org/10.1016/j.clinthera.2014.05.061.
-
23.
Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-41. [PubMed ID: 24296963]. https://doi.org/10.1177/0363546513510686.
-
24.
Miedzybrodzki R, Fortuna W, Weber-Dabrowska B, Gorski A. Phage therapy of staphylococcal infections (including MRSA) may be less expensive than antibiotic treatment. Postepy Hig Med Dosw (online). 2007;61:461-5. [PubMed ID: 17679835].
-
25.
Pormohammad A, Riahi SM, Nasiri MJ, Fallah F, Aghazadeh M, Doustdar F, et al. Diagnostic test accuracy of adenosine deaminase for tuberculous meningitis: A systematic review and meta-analysis. J Infect. 2017;74(6):545-54. [PubMed ID: 28366687]. https://doi.org/10.1016/j.jinf.2017.02.012.
-
26.
Pormohammad A, Azimi T, Falah F, Faghihloo E. Relationship of human herpes virus 6 and multiple sclerosis: A systematic review and meta-analysis. J Cell Physiol. 2018;233(4):2850-62. [PubMed ID: 28631829]. https://doi.org/10.1002/jcp.26000.
-
27.
Houri H, Pormohammad A, Riahi SM, Nasiri MJ, Fallah F, Dabiri H, et al. Acute bacterial meningitis in Iran: Systematic review and meta-analysis. PLoS One. 2017;12(2). e0169617. [PubMed ID: 28170400]. [PubMed Central ID: PMC5295700]. https://doi.org/10.1371/journal.pone.0169617.
-
28.
Nasiri MJ, Imani Fooladi AA, Dabiri H, Pormohammad A, Salimi Chirani A, Dadashi M, et al. Primary ethambutol resistance among Iranian pulmonary tuberculosis patients: a systematic review. Ther Adv Infect Dis. 2016;3(5):133-8. [PubMed ID: 28149517]. [PubMed Central ID: PMC5256191]. https://doi.org/10.1177/2049936116661962.
-
29.
Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123-8. [PubMed ID: 25197676]. [PubMed Central ID: PMC4154549]. https://doi.org/10.15171/ijhpm.2014.71.
-
30.
Sotoudeh Anvari M, Kianinejad R, Boroumand MA, Arzhan S, Jalali A. Bacterial pericarditis and antimicrobial resistance at the Tehran Heart Center, Iran. J Infect Dev Ctries. 2015;9(7):780-4. [PubMed ID: 26230130]. https://doi.org/10.3855/jidc.6027.
-
31.
Eftekhar F, Raei F. Correlation of Minimum Inhibitory Concentration Breakpoints and Methicillin Resistance Gene Carriage in Clinical Isolates of Staphylococcus epidermidis. Iran J Med Sci. 2011;36(3):213-6. [PubMed ID: 23359643]. [PubMed Central ID: PMC3556765].
-
32.
Fateh Rahimi MA, Karimi S. Antibiotic resistance pattern of methicillin resistance s. epidermidis isolates in clinical samples in Tehran. Jundishapur J Microbiol. 2014;7.
-
33.
Ghotaslou R, Aghazadeh M, Ahangarzadeh Rezaee M, Moshafi MH, Forootanfar H, Hojabri Z, et al. The prevalence of aminoglycoside-modifying enzymes among coagulase negative staphylococci in Iranian pediatric patients. J Infect Chemother. 2014;20(9):569-73. [PubMed ID: 25023717]. https://doi.org/10.1016/j.jiac.2014.05.004.
-
34.
Havaei SA, Ebrahimzadeh Namvar A, Moghim S, Lari AR. Evaluation of various staphylococcal cassette chromosome mec (SCCmec) types in Staphylococcus epidermidis invasive strains from hospitalised patients in Iran. Infez Med. 2015;23(1):18-22. [PubMed ID: 25819046].
-
35.
Najar-Peerayeh S, Jazayeri Moghadas A, Behmanesh M. Antibiotic Susceptibility and mecA Frequency in Staphylococcus epidermidis, Isolated From Intensive Care Unit Patients. Jundishapur J Microbiol. 2014;7(8). e11188. [PubMed ID: 25485050]. [PubMed Central ID: PMC4255212]. https://doi.org/10.5812/jjm.11188.
-
36.
Ebrahimzadeh Namvar A, Havaei SA, Moghim S, Rastegar Lari AR. Characterization of Staphylococcus epidermidis isolates from hospitalized patients in Isfahan and Tehran teaching hospitals. Molecular Genet, Microbiol Virol. 2015;29(4):216-9. https://doi.org/10.3103/s0891416814040065.
-
37.
Pishva E, Havaei SA, Arsalani F, Narimani T, Azimian A, Akbari M. Detection of methicillin-resistance gene in Staphylococcus epidermidis strains isolated from patients in Al-Zahra Hospital using polymerase chain reaction and minimum inhibitory concentration methods. Adv Biomed Res. 2013;2:23. [PubMed ID: 23977651]. [PubMed Central ID: PMC3748670]. https://doi.org/10.4103/2277-9175.108008.
-
38.
Pourmand MR, Abdossamadi Z, Salari MH, Hosseini M. Slime layer formation and the prevalence of mecA and aap genes in Staphylococcus epidermidis isolates. J Infect Dev Ctries. 2011;5(1):34-40. [PubMed ID: 21330738].
-
39.
Raei F, Eftekhar F. [Comparison of Methicillin Resistance Phenotype with Meca Gene Carriage in Clinical Isolates of Staphylococcus Epidermidis]. Iran J Biol. 2010;23(1):108-14. Persian.
-
40.
Ramazanzadeh R, Salimizand H, Shahbazi B, Narenji H. Prevalence of mecA Gene of Methicillin Resistant Staphylococcus spp. Isolated from Nosocomial Infections and Environmental Specimens in Sanandaj Hospitals, Kurdistan, Iran. Res Mol Med. 2015;3(3):38-42. https://doi.org/10.7508/rmm.2015.03.008.
-
41.
Shoja S, Nahaei MR, Nahaei M, Farajnia S, Ahangarzadeh RM, Nikvash S. [Study of methicillin-resistance by oxacillin disc diffusion and pcr methods in Staphylococcus epidermidis isalates collected from blood cultures and their antibiotic susceptibility]. Majalleh Tabriz U Med Sci. 2009;31:39-44. Persian.
-
42.
Soroush S, Jabalameli F, Taherikalani M, Amirmozafari N, Fooladi AA, Asadollahi K, et al. Investigation of biofilm formation ability, antimicrobial resistance and the staphylococcal cassette chromosome mec patterns of methicillin resistant Staphylococcus epidermidis with different sequence types isolated from children. Microb Pathog. 2016;93:126-30. [PubMed ID: 26821355]. https://doi.org/10.1016/j.micpath.2016.01.018.
-
43.
Taheri N, Abtahi H, Amozande-Nobaveh A, Zarinfar N, Ghaznavi-Rad E. The Antibiotic Resistant Determinant of Pathogenic Bacteria Isolated from Medical Equipment and Hospital Environment in Valiasr Hospital, Arak, 2013. J Mazandaran U Med Sci. 2014;24(114):60-73.
-
44.
Talebi M, Shafiee M, Sadeghi J, Moghadam NA, Saifi M, Pourshafie MR. Genotypic Diversity of Methicillin-Resistant Coagulase-Negative Staphylococci Isolated from Inpatients and Outpatients. Microb Drug Resist. 2016;22(2):147-54. [PubMed ID: 26248114]. https://doi.org/10.1089/mdr.2014.0195.
-
45.
Maseda E, Gimenez MJ, Gilsanz F, Aguilar L. Basis for selecting optimum antibiotic regimens for secondary peritonitis. Expert Rev Anti Infect Ther. 2016;14(1):109-24. [PubMed ID: 26568097]. https://doi.org/10.1586/14787210.2016.1120669.
-
46.
Dadashi M, Nasiri MJ, Fallah F, Owlia P, Hajikhani B, Emaneini M, et al. Methicillin-Resistant Staphylococcus aureus (MRSA) in Iran: A Systematic Review and Meta-analysis. J Global Antimicrob Resistance. 2017;12:96-103.
-
47.
Wisplinghoff H, Rosato AE, Enright MC, Noto M, Craig W, Archer GL. Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrob Agents Chemother. 2003;47(11):3574-9. [PubMed ID: 14576120]. [PubMed Central ID: PMC253785].
-
48.
Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends in microbiol. 2001;9(10):486-93.
-
49.
Iravani Mohammad Abadi M, Moniri R, Khorshidi A, Piroozmand A, Mousavi SG, Dastehgoli K, et al. Molecular Characteristics of Nasal Carriage Methicillin-Resistant Coagulase Negative Staphylococci in School Students. Jundishapur J Microbiol. 2015;8(6). e18591. [PubMed ID: 26301061]. [PubMed Central ID: PMC4541167]. https://doi.org/10.5812/jjm.18591v2.
-
50.
Miragaia M, Couto I, de Lencastre H. Genetic diversity among methicillin-resistant Staphylococcus epidermidis (MRSE). Microb Drug Resist. 2005;11(2):83-93. [PubMed ID: 15910220]. https://doi.org/10.1089/mdr.2005.11.83.
-
51.
Ibrahem S, Salmenlinna S, Virolainen A, Kerttula AM, Lyytikainen O, Jagerroos H, et al. Carriage of methicillin-resistant Staphylococci and their SCCmec types in a long-term-care facility. J Clin Microbiol. 2009;47(1):32-7. [PubMed ID: 18971358]. [PubMed Central ID: PMC2620858]. https://doi.org/10.1128/JCM.01085-08.
-
52.
Trang VT, Takeuchi H, Kudo H, Katsuno S, Shimamura T, Kashiwagi T, et al. In vitro antimicrobial activity of aminoreductone against the pathogenic bacteria methicillin-resistant Staphylococcus aureus (MRSA). J Agric Food Chem. 2011;59(16):8953-60. [PubMed ID: 21739996]. https://doi.org/10.1021/jf201928f.
-
53.
Streit JM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001). Int J Antimicrob Agents. 2004;24(2):111-8. [PubMed ID: 15288308]. https://doi.org/10.1016/j.ijantimicag.2003.12.019.
-
54.
Weinstein RA. Controlling antimicrobial resistance in hospitals: infection control and use of antibiotics. Emerg Infect Dis. 2001;7(2):188-92. [PubMed ID: 11294703]. [PubMed Central ID: PMC2631704]. https://doi.org/10.3201/eid0702.700188.
-
55.
Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134(1-2):45-54. [PubMed ID: 18986783]. https://doi.org/10.1016/j.vetmic.2008.09.009.
-
56.
Skov R, Christiansen K, Dancer SJ, Daum RS, Dryden M, Huang YC, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents. 2012;39(3):193-200. [PubMed ID: 22226649]. https://doi.org/10.1016/j.ijantimicag.2011.09.029.