Published online May 21, 2019. doi: 10.5495/wjcid.v9.i1.1
Peer-review started: January 3, 2019
First decision: March 15, 2019
Revised: March 29, 2019
Accepted: April 8, 2019
Article in press: April 9, 2019
Published online: May 21, 2019
Methicillin-resistant Staphylococcus aureus (MRSA) has remained a major threat to healthcare; in both hospital and community settings over the past five decades. With the current use of antibiotics for a variety of infections, including MRSA, emerging resistance is a major concern. Currently available treatments have restrictions limiting their use. These issues include, but are not limited to, side effects, cross-resistance, lack of understanding of pharmacokinetics and clinical pharmacodynamics, gradual increment in minimal inhibitory concentration over the period (MIC creep) and ineffectiveness in dealing with bacterial biofilms. Despite availability of various therapeutic options for MRSA, the clinical cure rates remain low with high morbidity and mortality. Given these challenges with existing treatments, there is a need for development of novel agents for MRSA. Along with prompt infection control strategies and strict implementation of antibiotic stewardship, cautious use of newer anti-MRSA agents will be of utmost importance. This article reviews the treatments and limitations of MRSA management and highlights the future path.
Core tip: Methicillin-resistant S. aureus (MRSA) remains a major threat despite availability of multiple treatments. Limitations of the current anti-MRSA treatments demand more careful use of these agents. Using antibiotics in combination for MRSA treatment needs further evaluation. Multiple strategies including research and development of new antibiotics and antibiotic stewardship are necessary to contain the MRSA.
- Citation: Kashyap R, Shah A, Dutt T, Wieruszewski PM, Ahdal J, Jain R. Treatments and limitations for methicillin-resistant Staphylococcus aureus: A review of current literature. World J Clin Infect Dis 2019; 9(1): 1-10
- URL: https://www.wjgnet.com/2220-3176/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v9.i1.1
Staphylococci have been involved in human disease for centuries and were identified first as the cause of incurable boils. Sir Alexander Ogsto and Friedrich J Rosenbach identified, classified, and contributed to the nomenclature of Staphylococci[1]. S. aureus has since evolved as a major infectious pathogen being severely detrimental to the health of millions of patients. S. aureus possesses resistance mechanisms to standard agents. The first incidence of penicillin resistance was reported in 1942 which was identified to be due to inducible beta-lactamase. After introduction of methicillin in 1959, methicillin-resistant S. aureus (MRSA) was reported in 1961[2].
Burden of MRSA is high in middle-income countries like India. Amongst all S. aureus isolates, Indian Network for Surveillance of Antimicrobial Resistance group reported methicillin resistance in 41% of their isolates[3]. This high burden of MRSA in India is the cause of significant morbidity and mortality. Additionally, formation of biofilms in MRSA isolates is associated with increased virulence, pose a challenge in clinical management, and may also contribute to the development of resistance[4,5].
Current treatment strategies have limitations and improper source control may add to that, especially in severe MRSA infections. Thus, we aim to review the current treatment strategies, their limitations, and a way forward for effective management of MRSA infections.
MRSA infections involve a wide disease spectrum. Common sites include skin/soft tissue, bone/joint, vascular line, native valve/prosthetic valve endocarditis, central nervous system shunt infections and meningitis/brain abscesses. The Infectious Disease Society of America (IDSA) provides treatment recommendations for MRSA infections[6] (Table 1).
| Infections | Antibiotic Treatment | |
| Skin and soft tissue infections (SSTIs) | ||
| Uncomplicated SSTIs | Clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), a tetracycline (doxycycline or minocycline) (A-II), linezolid | |
| Complicated SSTIs | IV Vancomycin, Linezolid (oral or IV 600 mg twice daily), Daptomycin (4 mg/kg/dose IV once daily), Telavancin (10 mg/kg/dose IV once daily), Clindamycin (600 mg IV or PO 3 times a day) | |
| Recurrent SSTIs | Nasal decolonization - mupirocin twice daily +/- topical body decolonization - skin antiseptic solution (e.g. chlorhexidine) or dilute bleach baths. | |
| Bacteraemia and infective endocarditis | ||
| Native valve endocarditis | Vancomycin; Daptomycin (6 mg/kg/dose IV once daily) | |
| Prosthetic valve endocarditis | Vancomycin + Rifampin (300 mg PO/IV every 8 hour) followed by Gentamicin (1 mg/kg/dose IV every 8 hour) | |
| Pneumonia | ||
| Community acquired, or healthcare associated | IV vancomycin or linezolid (600 mg PO/IV twice daily) or clindamycin (600 mg PO/IV 3 times daily) | |
| Bone and joint infections | ||
| Osteomyelitis or Septic arthritis | Vancomycin; Daptomycin (6 mg/kg/dose IV once daily); TMP-SMX [4 mg/kg/dose (TMP component) twice daily] + Rifampin (600 mg once daily) | |
| Device-related osteo-articular infections (early onset < 2 mo - prosthetic joint infections) | Vancomycin or Daptomycin (6 mg/kg/dose IV once daily) + Rifampin (600 mg once daily) followed by; Rifampin + fluoroquinolone / TMP- SMX / tetracycline / clindamycin | |
| Device-related osteo-articular infections (early onset < 2 mo - spinal implant infections) | Initial parenteral therapy + Rifampin followed by prolonged oral therapy | |
| CNS infections | ||
| Meningitis, Brain abscess, subdural empyema, spinal epidural abscess, Septic Thrombosis of Cavernous or Dural Venous Sinus | IV Vancomycin +/- Rifampin; OR; Linezolid 600 mg PO/IV twice daily or TMP-SMX 5 mg/kg/dose IV every 8-12 hour | |
Vancomycin is one of the mainstays of therapy for MRSA infections. In adults, IV vancomycin at a dose of 15-20 mg/kg/dose (max 2 g/dose) every 8-12 h based on renal function is recommended with a loading dose of 25-30 mg/kg in seriously ill patients[7]. Therapeutic drug monitoring (TDM) is recommended to ensure adequacy of dosing, with most infections necessitating trough concentrations of 10-20 μg/mL, with concentrations at the higher end of this range (i.e., 15-20 μg/mL) reserved for difficult to penetrate sites such as pulmonary and central nervous system. However, in skin and skin structure infections (SSTIs), trough monitoring may not be necessary and vancomycin in a dose of 1 mg every 12 h may be adequate[6].
An ideal anti-MRSA agent does not exist; desirable properties in anti-MRSA antibi-otics include rapid bactericidal action, excellent penetration in tissue, consistent and predictable pharmacokinetics to support reliable dosing, low probability of resistance development, lower risk of side effects, and good microbiological and clinical cure rates. Biofilm formation with S. aureus is known and contributes to antibacterial tolerance by promoting bacterial persistence in biofilms.
Thus, identifying an ideal antibiotic which will also be active against biofilms can be a challenge. Table 2 enumerates some of the limitations of major existing anti-MRSA treatments.
| Treatment | Limitations |
| Vancomycin | Higher MBC: MIC ratio |
| Polymorphisms or changes in gene function (e.g. agr pathway) | |
| MIC creep | |
| Development of hetero-resistance (hVISA) | |
| Variable tissue penetration | |
| AUC: MIC ratio | |
| Nephrotoxicity | |
| Red man syndrome | |
| Teicoplanin | Therapeutic drug monitoring may be necessary |
| Need to generate evidence on pharmacokinetics and clinical pharmacodynamics | |
| Daptomycin | Resistance development |
| Possible cross-resistance in hVISA | |
| Inactivation by alveolar surfactant | |
| Linezolid | Serious adverse drug reactions e.g., thrombocytopenia, optic neuropathy, peripheral neuropathy, lactic acidosis, monoamine oxidase inhibition |
| MIC creep | |
| Limited efficacy in bacteraemia or endocarditis | |
| TMP/SMX | High degree of resistance |
| Limited efficacy in bacteraemia | |
| Thymidine salvage in presence of pus | |
| Clindamycin | High rates of inducible and constitutive resistance |
| Risk of Clostridium difficile infection | |
| Tetracyclines | Limited utility in severe invasive infections |
| Tigecycline | Low serum levels with limited efficacy in bacteraemia |
| Poor tissue penetration and AUC: MIC ratio | |
| Black box warning from the USFDA for all-cause mortality, Mortality Imbalance and Lower Cure Rates in VAP and pancreatitis | |
| Quinupristin/ Dalfopristin | Limiting side effects like infusion-site inflammation, pain, and oedema, thrombophlebitis, arthralgia, myalgia, nausea, diarrhoea, vomiting, and rash |
| Drug interactions with CYP3A4 inhibitors | |
| Ceftaroline | Risk of agranulocytosis |
| Telavancin | Risk of nephrotoxicity |
| Oritavancin and Dalbavancin | Long half-life - delayed hypersensitivity if occurs may persist for weeks |
| Clinical failure may get unnoticed if there is lack of daily follow-up evaluations | |
| Effectiveness in bacteraemia, pneumonia, bone and joint infections, and prosthetic infections has not been established | |
| Higher occurrence of osteomyelitis reported in clinical studies with oritavancin |
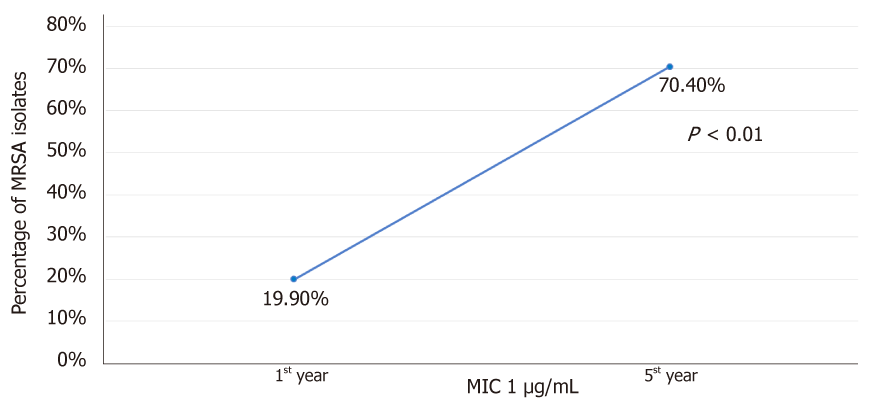
Over the years of vancomycin use, resistance is now beginning to emerge in MRSA isolates[8]. Vancomycin has several limitations. First is the ratio of minimum bactericidal to inhibitory concentration (MBC: MIC ratio). A study from Sader et al[9] demonstrated that 20.1% of tested MRSA strains (n = 900) were vancomycin tolerant defined by MBC: MIC ratio of ≥ 32. This varied from 10.0% to 43.0% among different centres evaluated[9]. Secondly, the accessory gene regulator pathway is associated with regulation of quorum sensing and endotoxin production[10]. Development of polymorphisms or loss of function of accessory gene regulator (agr) pathway is associated with failure of vancomycin therapy[11]. Thirdly, the “MIC creep” phenomenon wherein there is a gradual reduction in susceptibility of S. aureus to vancomycin despite concentrations in the susceptible range (≤ 2 mg/L) can develop with continued use of vancomycin[8]. A study from California by Wang et al[12], demonstrated a gradual shift of MIC from ≤ 0.5 to 1.0 µg/mL over 5 years to vancomycin in MRSA strains (n = 6002). The proportion of isolates with MIC 1 µg/mL increased from 19.9% to 70.4% over study duration (Figure 1). Fourth concern is development of hetero-resistance to vancomycin (hVISA). In this phenomenon, from among the isolated MRSA, a subpopulation demonstrates intermediate level of vancomycin resistance, but the colony as a whole remains susceptible. The mechanisms for this remains unclear but may involve thickening of cell wall avoiding penetration of vancomycin, and alteration in agr pathway[10]. A study from Sader et al[9] involving nine hospitals in the United States showed hVISA prevalence of 13.4%. The development of hVISA was more common (45.6%) in MRSA isolates with MIC ≥ 1 mg/L. Fifth, the extensive protein binding of vancomycin leads to variable tissue penetration which can further be different in comorbidities like diabetes, meningitis, etc[10]. Sixth, the pharmacodynamics of vancomycin has been considered to be an important aspect in determining efficacy. The area under the curve (AUC) and MIC ratio of 400 or more is believed to provide therapeutic effectiveness for which vancomycin trough concentration should reach 15-20 mg/L especially in severe MRSA infections[7]. For achieving AUC: MIC ratio of 400 or more at MIC of 1 mg/L, dose of 3-4 mg/d is necessary. For MIC of 2 mg/L, achieving target AUC: MIC ratio is not possible even when higher doses are used. This can result in poor clinical and microbiological cure.
Nephrotoxicity is an important adverse effect associated with vancomycin. The reported incidence varies from nearly 14% in children to 35% in adults. In adults, trough concentration beyond 15 µg/mL is associated with increased risk of renal injury. Attaining AUC: MIC ratio of ≥ 400 is therefore harmful especially wherein the isolate MIC is > 2 mg/L. In such cases, use of alternative agents is advised[13].
Daptomycin, a branched cyclic anionic lipopeptide exerts bactericidal action via calcium-dependent modification in membrane potential causing leaking of intracellular ions and cell death[14]. It has shown similar efficacy to vancomycin in MRSA bacteraemia, endocarditis, complicated SSTIs, but not in pneumonia due to inactivation by alveolar surfactant[10]. However, point mutation in MprF gene (L431F substitution) identified in clinical isolates was associated with reduced negative cell membrane charge, thicker cell wall, and longer doubling time. This was found to confer increased resistance to daptomycin and vancomycin[15]. Daptomycin-non-susceptible (DAP-NS) phenotype has also been reported in MRSA infections. Among 2.4% DAP-NS strains (n = 208), one was sequence type 72 (ST72) and other four were ST5. Three of these strains were also found to be hVISA. The resistance mechanism in ST72 was charge repulsion, ST5 showed charge independent mechanisms. Changes in cell wall thickness were not found in any of the DAP-NS strains[16]. DAP-NS isolates were not sensitive to high-dose of daptomycin[17]. Increased MIC of daptomycin was found to be associated with increased mortality in patients with MRSA bacteraemia[18]. Finally, daptomycin has been associated with elevated creatine kinase and rhabdomyolysis, which is problematic in critically ill patients already at risk of such increases and sequalae thereof, such as renal injury[19].
Linezolid, a synthetic antibiotic, binds to ribosomal RNA on both 30S and 50S subunits and thereby inhibits protein synthesis. Additionally, it inhibits formation of initiation complex and reduce the rate of translation process[20]. Occurrence of serious adverse drug reactions like thrombocytopenia, optic neuropathy, peripheral neuropathy, lactic acidosis, and potential serotonin syndrome through monoamine oxidase inhibition have important therapeutic limitations resulting in poor adherence to therapy[21]. MIC creep with linezolid similar to that of vancomycin has also been reported[22]. Being a bacteriostatic agent, its first line use in severe invasive infections especially bacteraemia and endocarditis is avoided[10].
In persistent MRSA bacteraemia (> 7 d) despite therapy with glycopeptides like vancomycin or teicoplanin, shifting to linezolid failed to show superiority in microbiologic response, treatment success, and mortality compared to the patients who continued glycopeptides[23].
Teicoplanin: TDM may be necessary in ascertaining the teicoplanin concentrations as daily dosages of 4 mg/kg have been reported to result in treatment failure compared to a 6 mg/kg dose. Also, trough concentrations of > 10, > 20, and > 30 mg/L have been reported to be necessary for successful treatment of S. aureus septicemia, MRSA endocarditis, and MRSA osteomyelitis, respectively[24]. Also, given its important role in MRSA management, there is more need to generate evidence on pharmacokinetics and clinical pharmacodynamics[24].
Trimethoprim-sulfamethoxazole (TMP-SMX): In MRSA infections, its utility is limited by development of resistance and poor efficacy[25,26]. Therefore, TMP-SMX is mainly confined to treatment of uncomplicated skin and skin structure infections from an MRSA standpoint[27].
Clindamycin: Clindamycin has bacteriostatic activity and high rates of inducible and constitutive resistance, limiting its utility for MRSA infections[28,29]. Further, risk of Clostridium difficile infection (CDI) might deter use of clindamycin as sole agent for MRSA as duration of exposure has been identified as an important determinant of CDI[30].
Tetracyclines: Tetracyclines such as doxycycline and minocycline are limited to uncomplicated SSTIs by community-acquired MRSA. Bacteriostatic activity and limited spectrum limits utility in severe invasive MRSA infections[10].
Fucidin (fusidic acid): Fusidic acid inhibits bacterial protein synthesis via action on RNA. As a topical agent, it has been used for treatment of skin infection, though there has been recent interest in rectifying its use in combination with rifampicin for infected joint prostheses. This however has been limited by significant drug-drug interactions resulting in ineffective fusidic acid exposure[31].
Tigecycline: Tigecycline has shown promise in MRSA infections equivalent to vancomycin[32]. It is effective in SSTIs and complicated intraabdominal infections[33]. However, high protein binding can result in low serum levels thereby limiting effectiveness in MRSA bacteraemia. Black box warning issued from the US Food and Drug Administration for all-cause mortality, mortality imbalance and lower cure rates in VAP and pancreatitis is a concern with tigecycline[34].
Quinupristin/Dalfopristin: Quinupristin/Dalfopristin is considered among the effective agents in Staphylococcal infections and may be effective in MRSA bacteremia[35]. However, occurrence of side effects like infusion-site inflammation, pain, and edema, thrombophlebitis, arthralgia, myalgia, nausea, diarrhoea, vomiting, and rash limit its use. Also, inhibition of cytochrome P450 3A4 with qui-nupristin/dalfopristin warrants caution with use of drugs metabolized through this enzymatic pathway[36]. Interference with other drugs metabolism may result in QTc prolongation with use of quinupristin/dalfopristin.
Ceftaroline: Ceftaroline is an effective agent for severe MRSA infections and provides clinical cure in nearly 74% cases. The major concern with this agent is development of agranulocytosis. Prolonged therapy (≥ 21 d) increases risk of leukopenia and therefore treatment with ceftaroline should be closely monitored in these situations[37].
Telavancin: It is another effective agent in MRSA with resistance to vancomycin, linezolid and daptomycin. However, nephrotoxicity is an important limitation. An increased mortality has been observed in hospital or ventilator associated pneumonia[38].
Oritavancin and Dalbavancin: These lipoglycopeptides have ultra-long half-life upwards of 346 h making them attractive as single-dose antibiotics. This and the inability to remove via dialysis, however also raises a concern as injury resulting from delayed hypersensitivity (if occurs) or other adverse effects may persist for weeks. It’s effectiveness has not been established in bacteraemia, pneumonia, bone and joint infections, or prosthetic infections[39]. While these agents have potential for ambulatory infectious diseases management, particularly in areas of poor clinic access for frequent intravenous infusions, their utility in acute and critical care remains to be proven.
With development of resistance and limitations of individual agents discussed above, combination therapy is suggested for most severe and invasive MRSA infections. The objectives are to broaden the coverage, prevent or reduce development of resistance, improve the effectiveness of individual agents, enhance capacity to penetrate biofilms, and to reduce toxin production[40].
Rifampicin is bactericidal to S. aureus, achieves high intracellular concentration, and penetrates biofilms. A systematic review in 2008 reported that in-vitro findings identified with rifampicin combination did not relate to in-vivo findings[41]. Another review in 2013 reported limited evidence to support adjunctive use of rifampicin in MRSA infections. The increased risk of drug interactions, adverse effects with rifampicin and development of rifampicin resistance are possibilities with use of rifampicin in combination[42]. Latter is especially important in Indian context where the rifampicin is the primary drug against tuberculous infection and burden of tuberculosis is enormous. Currently, IDSA guidelines recommend use of rifampicin in combination only in prosthetic valve endocarditis and in osteoarticular infections associated with prostheses[6]. Rifampicin should not be used as monotherapy for the treatment of MRSA infections.
In vitro studies have demonstrated increased bactericidal activity of vancomycin and animal studies have shown to shorten the duration of bacteraemia. Nephrotoxicity associated with gentamycin can add to the nephrotoxic potential of vancomycin[40].
Laboratory analyses have shown synergism with this combination[10]. However, clinical evidence is restricted to case reports.
Similar to other combination treatments, the evidence from in-vitro studies shows synergistic activity with this combination as well[43,44]. However, clinical evidence is restricted to case reports[45-47]. In time-kill study, addition of gentamicin rather than rifampicin has been shown to provide synergism with daptomycin[48].
With beta-lactams active against MRSA (e.g. ceftaroline), daptomycin has shown synergistic activity[49]. In MRSA strains from endocarditis, ceftaroline in addition to daptomycin also cleared daptomycin non-susceptible strains. Daptomycin at 6 mg/kg every 48 h was and ceftaroline at 200 mg every 12 h enhanced bacterial killing[50]. The finding from this single study demands further careful determination of optimal dosing regimen for effective utilization of active agents like ceftaroline. Another study reported rapid clearance of bacteraemia with addition of high dose nafcillin or oxacillin (2 mg IV every 4 h) to high-dose daptomycin (8-10 mg/d) in 7 cases of vancomycin and daptomycin resistant MRSA[51]. Though this points to enhanced efficacy of beta-lactams, further evaluation in prospective studies is necessary.
An in-vitro study involving pharmacokinetic/pharmacodynamic model of biofilm for 3 d showed greater activity with combination of daptomycin and linezolid than either agent alone suggesting potential for biofilm associated MRSA infections[52]. However, there is lack of clinical studies to substantiate the findings of in-vitro studies.
In combination with rifampicin, time kill studies of linezolid did not show synergism or antagonism but linezolid prevented emergence of mutant resistance in rifampicin[53]. One major issue with this combination is that rifampicin can reduce the linezolid concentration which can be well below the MIC90 for Staphylococci and effect may persist longer than 3 wk even after withdrawal of rifampicin[54,55]. With tedizolid and rifampicin combination, activity is increased but synergy observed was not found to be universal[56].
Poor efficacy, development of resistance and side effects and drug interactions as mentioned above in their individual discussion, render this regimen redundant.
The evidence is very limited for effectiveness and utility of triple drug combinations including beta-lactams, aminoglycosides, and vancomycin, barring isolated case reports[57].
Despite availability of multiple treatment options for MRSA, burden of MRSA remains substantial. While choosing an effective therapeutic strategy, multiple factors play a vital role in antibiotic selection. Development of resistance with anti-MRSA antibiotics has led to the use of antibiotics in combinations. There is no concrete evidence as to decide on specific combination neither there are any comparative data with different combinations. Success of new molecules like ceftaroline, tedizolid, and plazomicin should stimulate further research and development of new anti-MRSA therapies.
A number of anti-MRSA molecules are in different phases of development. But, to identify truly novel anti-MRSA agent that will act on new targets in the pathogen, there is need to invest further.
Current therapeutic management of MRSA is mainly focused on vancomycin and it still remains an effective therapy either alone or in combination. However, development of intermediate level of resistance, MIC creep, adverse effects, and vigilant TDM have been path-blockers for the sole use of vancomycin in MRSA. At present, selecting an individual agent that can provide the best synergy and minimal adverse effects remains the frontline therapeutic option against MRSA. Stimulating and supporting new and ongoing research for development of effective anti-MRSA therapies and implementation of infection control strategies are of urgent necessity. A collaborative action from policy makers, prescribers, and consumers is essential to safeguard the judicious use of newer agents in the management of MRSA infections.
We are thankful to Dr. Vijay M. Katekhaye for his assistance in drafting and re-viewing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: García-Elorriaga G, Liu L S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
| 1. | Thomer L, Schneewind O, Missiakas D. Pathogenesis of Staphylococcus aureus Bloodstream Infections. Annu Rev Pathol. 2016;11:343-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 2. | Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res. 2013;137:363-369. [PubMed] [Cited in This Article: ] |
| 4. | Jimi S, Miyazaki M, Takata T, Ohjimi H, Akita S, Hara S. Increased drug resistance of meticillin-resistant Staphylococcus aureus biofilms formed on a mouse dermal chip model. J Med Microbiol. 2017;66:542-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Mottola C, Matias CS, Mendes JJ, Melo-Cristino J, Tavares L, Cavaco-Silva P, Oliveira M. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016;16:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF; Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1692] [Cited by in F6Publishing: 1843] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 7. | Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1253] [Cited by in F6Publishing: 1284] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 8. | Deresinski S. Counterpoint: Vancomycin and Staphylococcus aureus--an antibiotic enters obsolescence. Clin Infect Dis. 2007;44:1543-1548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother. 2009;64:1024-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Nguyen HM, Graber CJ. Limitations of antibiotic options for invasive infections caused by methicillin-resistant Staphylococcus aureus: is combination therapy the answer? J Antimicrob Chemother. 2010;65:24-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Fowler VG, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190:1140-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883-3886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Patel K, Crumby AS, Maples HD. Balancing vancomycin efficacy and nephrotoxicity: should we be aiming for trough or AUC/MIC? Paediatr Drugs. 2015;17:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Vilhena C, Bettencourt A. Daptomycin: a review of properties, clinical use, drug delivery and resistance. Mini Rev Med Chem. 2012;12:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Chen FJ, Lauderdale TL, Lee CH, Hsu YC, Huang IW, Hsu PC, Yang CS. Effect of a Point Mutation in mprF on Susceptibility to Daptomycin, Vancomycin, and Oxacillin in an MRSA Clinical Strain. Front Microbiol. 2018;9:1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Nam EY, Yang SJ, Kim ES, Cho JE, Park KH, Jung SI, Yoon N, Kim DM, Lee CS, Jang HC, Park Y, Lee KS, Kwak YG, Lee JH, Park SY, Hwang JH, Kim M, Song KH, Kim HB. Emergence of Daptomycin-Nonsusceptible Methicillin-Resistant Staphylococcus aureus Clinical Isolates Among Daptomycin-Naive Patients in Korea. Microb Drug Resist. 2018;24:534-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Rose WE, Leonard SN, Rybak MJ. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2008;52:3061-3067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Ruiz J, Ramirez P, Concha P, Salavert Lletí M, Villarreal E, Gordon M, Frasquet J, Castellanos Ortega Á. Vancomycin and daptomycin minimum inhibitory concentrations as a predictor of outcome of methicillin-resistant Staphylococcus aureus bacteraemia. J Glob Antimicrob Resist. 2018;14:141-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Papadopoulos S, Ball AM, Liewer SE, Martin CA, Winstead PS, Murphy BS. Rhabdomyolysis during therapy with daptomycin. Clin Infect Dis. 2006;42:e108-e110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 21. | Kishor K, Dhasmana N, Kamble SS, Sahu RK. Linezolid Induced Adverse Drug Reactions - An Update. Curr Drug Metab. 2015;16:553-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Miyazaki M, Nagata N, Miyazaki H, Matsuo K, Takata T, Tanihara S, Kamimura H. Linezolid minimum inhibitory concentration (MIC) creep in methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates at a single Japanese center. Biol Pharm Bull. 2014;37:679-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Holland TL, Arnold C, Fowler VG. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312:1330-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 302] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 24. | Kim SW. Is therapeutic drug monitoring of teicoplanin useful? Infect Chemother. 2014;46:64-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Sharma NK, Garg R, Baliga S, Bhat K G. Nosocomial Infections and Drug Susceptibility Patterns in Methicillin Sensitive and Methicillin Resistant Staphylococcus aureus. J Clin Diagn Res. 2013;7:2178-2180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Nurjadi D, Olalekan AO, Layer F, Shittu AO, Alabi A, Ghebremedhin B, Schaumburg F, Hofmann-Eifler J, Van Genderen PJ, Caumes E, Fleck R, Mockenhaupt FP, Herrmann M, Kern WV, Abdulla S, Grobusch MP, Kremsner PG, Wolz C, Zanger P. Emergence of trimethoprim resistance gene dfrG in Staphylococcus aureus causing human infection and colonization in sub-Saharan Africa and its import to Europe. J Antimicrob Chemother. 2014;69:2361-2368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Talan DA, Lovecchio F, Abrahamian FM, Karras DJ, Steele MT, Rothman RE, Krishnadasan A, Mower WR, Hoagland R, Moran GJ. A Randomized Trial of Clindamycin Versus Trimethoprim-sulfamethoxazole for Uncomplicated Wound Infection. Clin Infect Dis. 2016;62:1505-1513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Tekin A, Dal T, Deveci O, Tekin R, Atmaca S, Dayan S. Assessment of methicillin and clindamycin resistance patterns in Staphylococcus aureus isolated from a tertiary hospital in Turkey. Infez Med. 2013;21:111-116. [PubMed] [Cited in This Article: ] |
| 29. | Shoji K, Shinjoh M, Horikoshi Y, Tang J, Watanabe Y, Sugita K, Tame T, Iwata S, Miyairi I, Saitoh A. High rate of inducible clindamycin resistance in Staphylococcus aureus isolates--a multicenter study in Tokyo, Japan. J Infect Chemother. 2015;21:81-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Carnahan RM, Kuntz JL, Wang SV, Fuller C, Gagne JJ, Leonard CE, Hennessy S, Meyer T, Archdeacon P, Chen CY, Panozzo CA, Toh S, Katcoff H, Woodworth T, Iyer A, Axtman S, Chrischilles EA. Evaluation of the US Food and Drug Administration sentinel analysis tools in confirming previously observed drug-outcome associations: The case of clindamycin and Clostridium difficile infection. Pharmacoepidemiol Drug Saf. 2018;27:731-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Pushkin R, Iglesias-Ussel MD, Keedy K, MacLauchlin C, Mould DR, Berkowitz R, Kreuzer S, Darouiche R, Oldach D, Fernandes P. A Randomized Study Evaluating Oral Fusidic Acid (CEM-102) in Combination With Oral Rifampin Compared With Standard-of-Care Antibiotics for Treatment of Prosthetic Joint Infections: A Newly Identified Drug-Drug Interaction. Clin Infect Dis. 2016;63:1599-1604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Florescu I, Beuran M, Dimov R, Razbadauskas A, Bochan M, Fichev G, Dukart G, Babinchak T, Cooper CA, Ellis-Grosse EJ, Dartois N, Gandjini H; 307 Study Group. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a Phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother. 2008;62 Suppl 1:i17-i28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Rose WE, Rybak MJ. Tigecycline: first of a new class of antimicrobial agents. Pharmacotherapy. 2006;26:1099-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Highlights of prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205645lbl.pdf. [Cited in This Article: ] |
| 35. | Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21:211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 323] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 36. | Allington DR, Rivey MP. Quinupristin/dalfopristin: a therapeutic review. Clin Ther. 2001;23:24-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Cosimi RA, Beik N, Kubiak DW, Johnson JA. Ceftaroline for Severe Methicillin-Resistant Staphylococcus aureus Infections: A Systematic Review. Open Forum Infect Dis. 2017;4:ofx084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Holubar M, Meng L, Deresinski S. Bacteremia due to Methicillin-Resistant Staphylococcus aureus: New Therapeutic Approaches. Infect Dis Clin North Am. 2016;30:491-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Saravolatz LD, Stein GE. Oritavancin: A Long-Half-Life Lipoglycopeptide. Clin Infect Dis. 2015;61:627-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2009;49:1072-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med. 2008;168:805-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Tremblay S, Lau TT, Ensom MH. Addition of rifampin to vancomycin for methicillin-resistant Staphylococcus aureus infections: what is the evidence? Ann Pharmacother. 2013;47:1045-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Garrigós C, Murillo O, Euba G, Verdaguer R, Tubau F, Cabellos C, Cabo J, Ariza J. Efficacy of usual and high doses of daptomycin in combination with rifampin versus alternative therapies in experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:5251-5256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Rose WE, Berti AD, Hatch JB, Maki DG. Relationship of in vitro synergy and treatment outcome with daptomycin plus rifampin in patients with invasive methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 2013;57:3450-3452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Hagiya H, Terasaka T, Kimura K, Satou A, Asano K, Waseda K, Hanayama Y, Otsuka F. Successful treatment of persistent MRSA bacteremia using high-dose daptomycin combined with rifampicin. Intern Med. 2014;53:2159-2163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Kelesidis T, Humphries R, Ward K, Lewinski MA, Yang OO. Combination therapy with daptomycin, linezolid, and rifampin as treatment option for MRSA meningitis and bacteremia. Diagn Microbiol Infect Dis. 2011;71:286-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Yazaki M, Oami T, Nakanishi K, Hase R, Watanabe H. A successful salvage therapy with daptomycin and linezolid for right-sided infective endocarditis and septic pulmonary embolism caused by methicillin-resistant Staphylococcus aureus. J Infect Chemother. 2018;24:845-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Credito K, Lin G, Appelbaum PC. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob Agents Chemother. 2007;51:1504-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Dhand A, Sakoulas G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin Ther. 2014;36:1303-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Rose WE, Schulz LT, Andes D, Striker R, Berti AD, Hutson PR, Shukla SK. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother. 2012;56:5296-5302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53:158-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 52. | Parra-Ruiz J, Bravo-Molina A, Peña-Monje A, Hernández-Quero J. Activity of linezolid and high-dose daptomycin, alone or in combination, in an in vitro model of Staphylococcus aureus biofilm. J Antimicrob Chemother. 2012;67:2682-2685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Yehia H, El Said M, Azmy M, Badawy M, Mansy S, Gohar H, Madany N. Effect of linezolid alone and in combination with other antibiotics, on methicillin-resistant staphylococcus aureus. J Egypt Soc Parasitol. 2016;46:57-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Gervasoni C, Simonetti FR, Resnati C, Charbe N, Clementi E, Cattaneo D. Prolonged inductive effect of rifampicin on linezolid exposure. Eur J Clin Pharmacol. 2015;71:643-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Hoyo I, Martínez-Pastor J, Garcia-Ramiro S, Climent C, Brunet M, Cuesta M, Mensa J, Soriano A. Decreased serum linezolid concentrations in two patients receiving linezolid and rifampicin due to bone infections. Scand J Infect Dis. 2012;44:548-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Werth BJ. Exploring the pharmacodynamic interactions between tedizolid and other orally bioavailable antimicrobials against Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 2017;72:1410-1414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Fujino T, Amari Y, Mohri M, Noma M, Yamamoto H. MRSA tricuspid valve infective endocarditis with multiple embolic lung abscesses treated by combination therapy of vancomycin, rifampicin, and sulfamethoxazole/trimethoprim. J Cardiol. 2009;53:146-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |