Introduction
Nowadays, more than 3% of infants born in developed countries are conceived through assisted reproductive technology (ART) [1]. It has been suggested that ART pregnancies are related to poorer pregnancy outcomes compared with natural conceptions [2,3]. Recent studies have shown that singleton pregnancies after in vitro fertilization (IVF) treatment have an increased risk of adverse maternal outcomes, such as preeclampsia, placental abruption, placenta previa, placenta accreta and postpartum hemorrhage, and also adverse neonatal outcomes, such as preterm birth, low birth weight and small for gestational age [3-5]. Why IVF has been found to increase the risks of obstetric morbidity is still debated.
Most of these adverse outcomes are related to abnormal placentation [6,7]. It is hypothesized that different aspects related to ART could negatively influence the placentation process. There are increasing concerns over adverse effects of controlled ovarian stimulation (COS) on the endometrial and uterine environment, as well as its consequences on endometrial receptivity [8], particularly after fresh embryo transfer (ET). Elective frozen-thawed embryo transfer (FET) has emerged as an alternative to fresh ET for selected IVF treatments; FET avoids the deleterious effects of hyperstimulation, as the transfer can be performed in a more physiological uterine environment in a later cycle [9,10]. It has thus been suggested that performing FET may improve the maternal and perinatal outcomes of IVF treatment.
Early systematic reviews and meta-analyses reported better perinatal outcomes in children conceived following FET as opposed to fresh ET, with these children showing lower risks of preterm birth and low birthweight [11-14]. However, subsequent systematic reviews and meta-analyses of observational studies reported a higher rate of hypertensive disorders of pregnancy (HDP) following FET [15,16]. Moreover, a recently published meta-analysis of randomized controlled trials (RCTs) found the risk of preeclampsia to be increased after FET [17].
Hence, the association between FET and HDP warrants further investigation, and suggests the need for a conservative attitude towards the use of FET. In addition, since additional RCT data on obstetric outcomes are available [18-22], better-quality evidence from meta-analyses is now required.
HDP, which occur in 10% of pregnancies [23], are among the leading causes of prematurity, and of maternal and neonatal morbidity and mortality [24,25]. The prevalence of these disorders has increased over the last 30 years, consistent with increases in the risk factors of obesity, maternal age and ART [26]. Therefore, studies focused exclusively on this outcome are warranted.
The purpose of the present study was to evaluate the available literature (RCTs) and provide up-to-date and comprehensive evidence to address the question of whether FET increases the risk of HDP compared with fresh ET. We also examined the effects of cryopreservation and subsequent FET on preeclampsia and gestational hypertension separately.
Material and methods
Protocol and registration
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27]. The study protocol is accessible at https://inplasy.com/ (registration number INPLASY202050113). This study did not require institutional review board approval, as it was a meta-analysis.
Search strategy
An electronic search strategy was developed and approved by all authors. The PubMed, Embase and Cochrane databases were searched for RCTs, specifically studies that assessed HDP and adverse perinatal outcomes after FET, published in English from 1978 to December 2019. The following combined search terms were used: (Fresh Embryos) OR (Frozen Embryos OR Cryopreserved Embryos OR Cryopreservation of Embryos OR Frozen Thawed Embryos OR Cryopreserved-thawed Embryos) OR (Embryo Transfer OR Embryo Transfers OR Tubal Embryo Transfer OR Tubal Embryo Stage Transfer) OR (Vitrification OR Slow Freeze OR Slow Frozen OR Slow Freezing) with (In Vitro Fertilizations/IVF OR Fertilization in Vitro) OR (ICSI OR Injections, Sperm, Intracytoplasmic OR Injections, Intracytoplasmic Sperm OR Intracytoplasmic Sperm Injection OR Sperm Injection, Intracytoplasmic OR Intracytoplasmic Sperm Injections) AND (Pregnancy induced hypertension OR Preeclampsia). We also searched the references of the relevant articles.
Eligibility criteria and data extraction
The review included RCTs that reported perinatal outcomes in pregnancies after IVF and compared FET to fresh ET cycles. Data were also obtained on secondary outcomes of RCTs in which the primary outcomes were live birth or ongoing pregnancy. Studies including only frozen and donor oocytes were excluded. In a first screening, two authors (J.M, P.S) independently assessed all of the abstracts retrieved from the search, and then obtained the full manuscripts of citations that met the inclusion criteria. They judged study eligibility and quality, and extracted data. Any discrepancies were resolved by agreement, and if needed, by reaching a consensus with a third author (M.C). The summarized results were critically appraised; the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to evaluate the quality of the evidence for each outcome [28]. We contacted the authors of the primary studies for additional information, but were able to obtain further data only from some of the studies.
Outcome measures
The primary outcome measure was HDP. According to the International Society for the Study of Hypertension in Pregnancy, HDP include chronic hypertension, white-coat hypertension, masked hypertension, gestational hypertension and preeclampsia [29]. The articles included in the current study refer to gestational hypertension and preeclampsia. Gestational hypertension is defined as hypertension arising de novo after 20 weeks’ gestation in the absence of proteinuria and without biochemical or hematological abnormalities. Preeclampsia is diagnosed by the presence of de novo hypertension after 20 weeks’ gestation accompanied by proteinuria and/or evidence of maternal acute kidney injury, liver dysfunction, neurological features, hemolysis or thrombocytopenia, and/or fetal growth restriction [29].
Risk of bias assessment
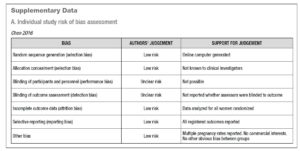
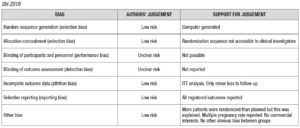
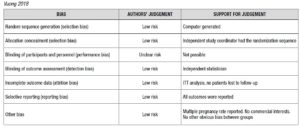
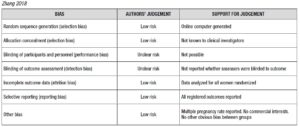
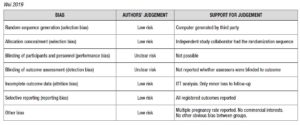
Risk of bias for RCTs was evaluated according to the Cochrane Handbook recommendations [30]. The quality of the studies was assessed by two investigators independently in reference to five categories: adequate sequence generation; allocation concealment; blinding of the outcome assessors; handling of missing data (intention-to-treat or per-protocol analysis); selective outcome reporting (Supplementary Data - A).
Analysis
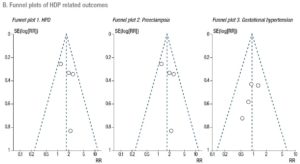
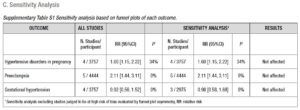
A Mantel-Haenszel model was used to determine the pooled effect of each variable. Estimates of effect for dichotomous data accompanied by the relative 95% confidence intervals (CIs) were calculated with a fixed effects model and expressed as risk ratios (RRs). Statistical significance was set at p<.05. The degree of variation across studies attributable to heterogeneity was evaluated with the I2 statistic. The random-effects model was applied when the heterogeneity was greater than 50% (I2 >50%) [31]. Sub-analyses were performed to assess the effect of FET on the outcomes, stratifying PCOS patients and hyper-responder patients for comparison with non-PCOS/normo-responders. At the time of the search, there were no published RCTs that focused on poor responders. Finally, publication bias was evaluated using funnel plots (Supplementary Data - B). Sensitivity analysis was performed to investigate the effect of a single study on the results by omitting one study at a time (Supplementary Data - C) [31]. We conducted a meta-analysis using Review Manager Software 5.3.
Results
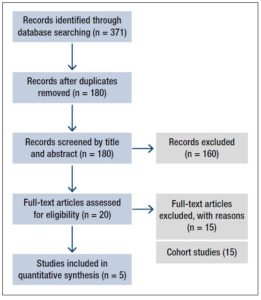
A total of 371 records were initially identified according to the search strategy. Of these, 191 were excluded owing to duplication; a further 160 were then excluded after screening of the titles and abstracts showed that they were irrelevant to our study. The remaining 20 studies underwent full-text review. On the basis of the inclusion and exclusion criteria, 15 studies were removed, because they were cohort studies. The other five RCTs assessing HDP in pregnancies after FET versus fresh ET met the inclusion criteria and were thus deemed eligible. Figure 1 is a flow diagram detailing the study selection process.
Synthesis of results
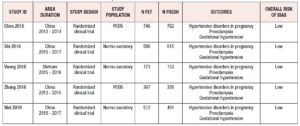
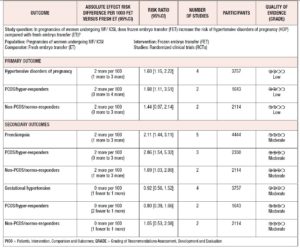
The characteristics of the five studies are summarized in Table 1. A summary of their findings is given in Table 2.
Primary outcome
Hypertensive disorders of pregnancy
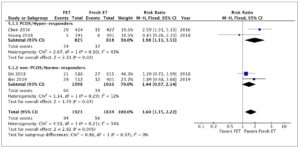
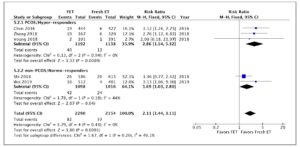
Four studies, including 3,757 patients, were pooled in this analysis. Overall, they evaluated 1,705 deliveries after FET and 1,596 after fresh ET. The risk of HDP was significantly higher in pregnancies resulting from FET than in those resulting from fresh ET cycles (RR 1.60; 95% CI 1.15-2.22; I2=34%; Fig. 2). A subgroup analysis, dividing patients by ovarian response, indicated that in PCOS/hyper-responder patients (two studies; n = 1,643 patients) FET led to an increase in HDP, with an RR of 1.98 (95% CI: 1.11–3.51; I2 = 63%; low quality of evidence; Fig. 2). However, in non-PCOS/normo-responders (two studies; n = 2,114 patients) there was no significant difference in HDP between the treatment groups (RR = 1.44; 95% CI: 0.97–2.14; I2 = 12%; low quality of evidence; Fig. 2).
Secondary outcomes
Preeclampsia
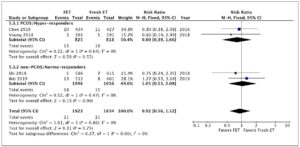
Five studies were included in this analysis. Overall, they evaluated 2,290 deliveries after FET and 2,154 after fresh ET. The fixed effects analysis showed an RR of 2.11 (95% CI 1.44–3.11; I2=0%) when comparing pregnancies after FET versus fresh ET (Fig. 3). We again performed a sub-analysis according to ovarian response. Compared with fresh ET, FET was associated with an increase in preeclampsia both in PCOS/hyper-responder (three studies; n = 2,330 patients; RR = 2.86; 95% CI: 1.54–5.32; I2 = 0%; moderate quality of evidence; Fig. 3) and in non-PCOS/normo-responder patients (two studies; n = 2,114 patients; RR = 1.69; 95% CI: 1.03–2.80; I2 = 44%; moderate quality of evidence; Fig. 3).
Gestational hypertension
Four studies were pooled in this analysis. The fixed effects analysis revealed no significant differences between the groups with regard to gestational hypertension (RR 0.92, 95% CI 0.56-1.52; I2=0%; Fig. 4). The ovarian response did not affect the risk of gestational hypertension
among patients undergoing FET (PCOS/hyper-responder: RR = 0.80; 95% CI: 0.39–1.66, I2 = 0%; non-PCOS/normo-responders: RR = 1.05; 95% CI: 0.53–2.08, I2 = 0%, moderate quality of evidence; Fig. 4).
Sensitivity analyses
Sensitivity analyses were performed to examine the influence of between-study variance on overall risk estimates. No significant impact was noted on the pooled effect size (Supplementary Data).
Discussion
Main findings
This systematic review and meta-analysis showed that pregnancies after FET are associated with an increased risk of HDP and preeclampsia. However, subgroup analyses from RCTs investigating PCOS/hyper-responders and non-PCOS/normo-responders indicated that FET is associated with a significantly higher HDP risk than fresh ET in the PCOS/hyper-responder group only. By contrast, no effect was noted in the non-PCOS/normo-responders group. The GRADE quality of evidence was low, mainly due to the substantial interstudy heterogeneity, which was presumed to be caused by differences in the study populations, multiple pregnancies, and the use of different types of luteal phase support in the FET cycles.
Regarding preeclampsia, moderate quality evidence indicated that FET is associated with an increased risk of preeclampsia both in PCOS/hyper-responder patients and in non-PCOS/normo-responders. Lastly, moderate quality evidence also indicated that there are no differences in the risk of gestational hypertension linked to the use of FET in preference to fresh ET in the population undergoing IVF.
Strengths
This study is, to our knowledge, the most up-to-date review on this subject, and the largest meta-analysis of RCTs comparing HDP between pregnancies after FET and fresh ET. In addition, the study shows narrow confidence levels and low I2 values for preeclampsia and gestational hypertension, suggesting that the precision of the meta-analysis is good and that the estimated value is relatively stable for these variables. Moreover, this systematic review and meta-analysis study was performed according to the PRISMA statement, thereby ensuring high methodological quality. Finally, the strength of the evidence was rated with reference to GRADE. These factors significantly increase the validity of our findings.
Limitations
The most important source of bias of this study is that there was no stratification by natural or medicated cycle, including different estradiol schemes for endometrial proliferation and different progesterone regimens for luteal phase support; this was mainly because the studies included in the analysis did not provide subset analyses of outcomes based on these characteristics of FET. A recently published study [32], which showed no increased risk of HDP and preeclampsia in FET using natural cycles, confirmed the bias deriving from not separating natural cycles from artificial ones in FET. In addition, there was no stratification by singleton and multiple pregnancies, as not all the authors provided additional information when contacted. Hence, we cannot exclude that the pooled effect estimates reported in this meta-analysis were confounded by multiple pregnancies or different estradiol or progesterone regimens. For this reason, our findings should be interpreted with caution. A further limitation was that only English language articles were allowed.
Comparison with other studies
Some recent reviews showed that pregnancies following FET had significantly higher odds of HDP [15-17], but only one performed a specific analysis of preeclampsia separately [17], finding FET to be associated with an elevated risk for this outcome compared with fresh ET (RR 1.79; 95% CI: 1.03–3.09). Our study includes a larger study population and we performed subgroup stratification by ovarian response. Lastly, no previous studies have assessed the risk of gestational hypertension comparing fresh ET and FET.
Interpretation of the results
Different mechanisms could explain the origin of adverse obstetric outcomes, such as HDP in ART patients, including infertility per se, or aspects related to the IVF treatment [5].
The etiology of HDP and preeclampsia is commonly associated with abnormal placentation and evidence of a maternal inflammatory response [33,34]. Utero-placental ischemia, probably as a consequence of partial myometrial spiral artery remodeling, is considered a marker of abnormal placentation [6,7]. This is supported by Doppler velocimetry alterations of uterine and umbilical arteries [35-37], biochemical factors associated with angiogenesis [38-43], and placental histological findings [44,45].
Different studies have supported the idea that COS may impact decidualization and placentation, contributing to the development of placental insufficiency, and consequently increasing the risk of adverse outcomes related to ischemic placental disease [46,47], although they did not specifically evaluate differences between fresh and frozen cycles. Subsequent studies have confirmed different placental alterations in patients after both FET and fresh ET [48,49].
In fresh ET, the supraphysiological hormone levels achieved during COS may be associated with alterations in endometrial receptivity [50-52] and endometrial gene expression [53] that could affect remodeling and angiogenesis, leading to impaired extravillous trophoblast invasion of spiral arteries and finally abnormal placentation [54,55]. However, the mechanisms underlying the increased risk of preeclampsia in pregnancies after FET are not clearly understood. Some cryoprotectants or the vitrification and thawing process per se could lead to certain metabolic or epigenetic changes related to alterations in methylation of regulatory genes involved in implantation [56]. Differences in gene expression, mostly in the trophectoderm, may lead to abnormal placentation and eventually to preeclampsia [57,58]. Other current research links pleiotrophin, a heparin-binding protein expressed in trophoblasts that has a role in angiogenesis, with preeclampsia in ART. The knockdown of pleiotrophin increases the risk of preeclampsia following vitrified-thawed ET [59,60].
Another etiological theory assigns more weight to impaired development of the placenta due to prolonged exposure to hormone replacement in protocols used for endometrial priming for the reception of embryos, and not necessarily to characteristics and manipulations of the embryo [61,62]. In programmed FET cycles, the estradiol supplementation used for endometrial priming suppresses the pituitary-ovarian axis, resulting in the absence of a corpus luteum, which has a key function as a major source of reproductive hormones. Although estradiol and progesterone are replaced during these artificial FET protocols, other products of the corpus luteum are not administered in the first trimester. Some recent studies have reported a crucial role of hormones such as relaxin, mainly in maternal cardiovascular adaptation to pregnancy [63]. Early gestation after FET was found to be linked to an increased incidence of deficient circulatory adaptations related to adverse pregnancy outcomes, including preeclampsia [64,65]. Probably, other unknown factors may be related to the risk of developing a future placental dysfunction in the absence of a corpus luteum. A natural cycle before FET allows more physiological development of the corpus luteum, as suggested in a retrospective study that reported higher rates of preeclampsia in artificial FET cycles compared with modified natural FET cycles [63]. A recent RCT found no increased risk of preeclampsia in pregnancies after FET compared with pregnancies following fresh ET, but it is important to note that most of the FET cycles were performed in natural cycles [20]. Further investigations are needed to compare maternal and perinatal outcomes of stimulated cycles versus natural modified cycles in FET in order to clarify this matter.
On the other hand, the findings of the current study showed that PCOS/hyper-responder patients have an increased risk of developing HDP compared with non-PCOS/normo-responder patients. A large retrospective population-based study reported that HDP have a higher prevalence among PCOS than non-PCOS women (16.1% vs 7.45%) [66]. The underlying relationship between PCOS and HDP is thought to be related to the negative impact on placental function of two clinical hallmarks of PCOS: androgen excess and insulin resistance [67,68]. Epigenetic alterations such as DNA methylation and miRNA expression may occur in certain endocrine and metabolic tissues of women with PCOS, including the ovaries and placenta [69,70]. These epigenetic modifications are thought to have an important role in the development of HDP and preeclampsia [67,71], but an improved understanding of placental development and function in the pregnancies of PCOS women is needed. At present, there are significant methodological challenges in investigating placental dysfunction in PCOS, mainly due to variability in PCOS phenotypic expression, the use of ART, and confounding comorbidities, including obesity, diabetes and chronic hypertension [67].
Although gestational hypertension is usually associated with good outcomes, the notion that gestational hypertension is intrinsically less concerning than preeclampsia is incorrect. Gestational hypertension is associated with adverse pregnancy outcomes [72] and may not represent a separate entity from preeclampsia [25]. Up to 50% of women with gestational hypertension will eventually develop preeclampsia, and this progression is more likely when hypertension is diagnosed before 32 weeks of gestation [73]. In the current study, the risk of gestational hypertension was not increased with FET. The reason for these findings is unknown, but probably the cryopreservation process does not influence the placentation mechanisms that trigger gestational hypertension in the same way as it influences other mechanisms that contribute to the development of preeclampsia.
Clinical considerations and future research
Our findings represent a detailed assessment of the risk of HDP after fresh ET and FET leading to live births, and it contributes to the ongoing discussion on which transfer type is safer for each patient circumstance. The increased risk of HDP after FET observed in this study emphasizes that the freeze-all policy should be implemented weighing up the overall benefits and risks for mothers and their children.
It is imperative to clarify in future research whether the increase in HDP is due to the cryopreservation process or to the artificial preparation of the endometrium. Since recently published data revealed that the increased risk of HDP and preeclampsia was not observed in FET using natural cycles [32,65], future RCTs or individual patient data meta-analysis should further explore pregnancy outcomes between autologous FET natural cycles and artificial cycles. Furthermore, studying the influence of cryopreservation on metabolic and epigenetic changes associated with abnormal decidualization, implantation and placentation could be revealing.
An improved understanding of the association between dysregulated decidualization and preeclampsia in pregnancies following FET and fresh ET is also warranted, in order to design prophylactic or therapeutic interventions that optimize decidualization before and during early pregnancy.
The prediction of pregnancy-related complications after IVF is a crucial topic. From the data obtained in our meta-analysis, practitioners can increase the safety of their interventions, identifying those pregnant women who potentially require additional care [74]. Finally, a panel of biomarkers reflecting endometrial dysfunction (e.g., IGFBP-1 or glycodelin) might be helpful in identifying women at increased risk [75].
Conclusions
The present study found that pregnancies conceived after FET have a higher risk of HDP compared with pregnancies after fresh ET in PCOS/hyper-responders. Both non-PCOS/normo-responders and PCOS/hyper-responders have an increased risk of preeclampsia after FET. The growing concerns about the safety of cryopreservation and subsequent FET, particularly as this procedure has become increasingly used, must be weighed up against the decreased risks of other important conditions in pregnancy, such as low birth weight and preterm delivery. Meanwhile, IVF specialists and obstetricians must be aware of this clinically relevant risk in patients undergoing IVF-FET and implement adequate monitoring strategies during the prenatal care. The development of gestational hypertension in IVF patients seems not to be influenced by FET. Future research focused on the pathophysiology underlying these differences and the study of possible strategies to reduce these risks in IVF pregnancies are warranted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.