Published online Dec 24, 2023. doi: 10.5306/wjco.v14.i12.620
Peer-review started: September 5, 2023
First decision: November 14, 2023
Revised: November 22, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: December 24, 2023
Intraductal tubulopapillary neoplasm (ITPN) is a rare disease accounting for approximately 3% of all intraductal pancreatic tumors, with intraductal papillary mucinous neoplasm (IPMN) being one of the most common differential diagnoses. Both ITPN and IPMN display slow growth. A branched pancreatic duct type is commonly observed in IPMN, whereas ITPN derived from the branched pancreatic duct has been reported in a limited number of cases; hence, its pathogenesis remains unclear.
Here, we present the case of a patient with ITPN localized in a branched pancreatic duct, with poorly controlled irritable bowel syndrome. A contrast-enhanced computed tomography scan of the abdomen incidentally revealed a 5-mm oligemic nodule-like change in the body of the pancreas. Endoscopic ultrasound (EUS) indicated a 10-mm hypoechoic mass without any cystic structures that had grown within 2 mo. EUS-guided fine needle aspiration was performed for definitive diagnosis, and the findings suggested ductal papillary carcinoma. Distal pancreatectomy was performed, and the tumor was pathologically diagnosed as ITPN with an invasive cancerous component, pT3N1aM0, pStage IIB (International Cancer Control, 8th edition). The patient underwent treatment with postoperative adjuvant chemotherapy (S-1 monotherapy); however, relapse was observed 1 year and 10 mo after surgical resection, and subsequent treatment involving a combination of chemotherapy and radiotherapy was administered. Maintenance therapy has since facilitated a stable disease state.
Regardless of the microscopic size of the neoplasm, early diagnosis of ITPN with EUS-guided fine needle aspiration and surgical resection are crucial.
Core Tip: Intraductal tubulopapillary neoplasm (ITPN), a relatively rare intraductal pancreatic cancer, is frequently derived from the main pancreatic duct rather than from the branching ducts. Although tumors with larger diameters are more likely to develop into cancer, we reported a case of small ITPN originating from a bifurcated pancreatic duct rapidly developing into invasive cancer. The tumor was diagnosed and managed with endoscopic ultrasound-guided fine needle aspiration and surgery. However, relapse occurred 1 year and 10 mo postoperatively and was managed using chemotherapy and radiotherapy. Our case suggests the importance of early diagnosis and surgical resection even of a small ITPN.
- Citation: Yamamoto K, Takada Y, Kobayashi T, Ito R, Ikeda Y, Ota S, Adachi K, Shimada Y, Hayashi M, Itani T, Asai S, Nakamura K. Rapid transformation of branched pancreatic duct-derived intraductal tubulopapillary neoplasm into an invasive carcinoma: A case report. World J Clin Oncol 2023; 14(12): 620-627
- URL: https://www.wjgnet.com/2218-4333/full/v14/i12/620.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i12.620
Intraductal tubulopapillary neoplasm (ITPN) is a recently reported type of intraductal pancreatic tumor[1,2]. ITPN is extremely rare, accounting for approximately 3% of all intraductal tumors; its pathogenesis remains unclear[1]. Most ITPNs have a primary pancreatic ductal origin, while those growing exclusively in the branching duct are rare[3]. Despite ITPN being considered a precursor to invasive ductal adenocarcinoma, with 70% of cases associated with adenocarcinoma at diagnosis[4,5], ITPN progresses more slowly than conventional pancreatic ductal carcinoma. Further, even with invasive lesions, the prognosis is generally better than that for ductal carcinoma.
Herein, we present the case of a 59-year-old male with ITPN localized in a branched pancreatic duct.
A 59-year-old male was admitted to our hospital with a 3-4-year history of diarrhea and abdominal pain.
He had intermittent abdominal pain in the lower abdomen.
He was taking medication for irritable bowel syndrome, which was poorly controlled.
He had no family history of malignancy.
The abdomen was soft and flat and showed no tenderness.
Blood tests on admission showed an increased alkaline phosphatase level (363 IU/L, normal range: 38–113 IU/L); however, no other hepatobiliary enzyme levels were elevated. The tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9 were within normal limits (Table 1).
| Test | Result |
| Hematology | |
| WBC | 6300/μL |
| RBC | 562 × 104/μL |
| Hb | 16.7 g/dL |
| Ht | 49.5% |
| Plt | 23.4 × 104/μL |
| PT-INR | 0.9 |
| APTT | 29.0 S |
| Biochemistry | |
| Blood sugar | 92 mg/dL |
| CRP | 0.0 mg/dL |
| TP | 6.8 g/dL |
| Alb | 4.4 g/dL |
| T-Bil | 0.1 mg/dL |
| AST | 19 IU/L |
| ALT | 29 IU/L |
| γ-GTP | 35 IU/L |
| ALP | 363 IU/L |
| LDH | 172 IU/L |
| AMY | 60 IU/L |
| BUN | 23 mg/dL |
| Cre | 0.84 mg/dL |
| Na | 139 mEq/L |
| K | 4.5 mEq/L |
| Cl | 105 mEq/L |
| Tumor maker | |
| CEA | 3.4 ng/mL |
| CA19-9 | < 2.0 ng/dL |
| DUPAN-2 | 36 U/mL |
| Span-1 antigen | < 3 U/mL |
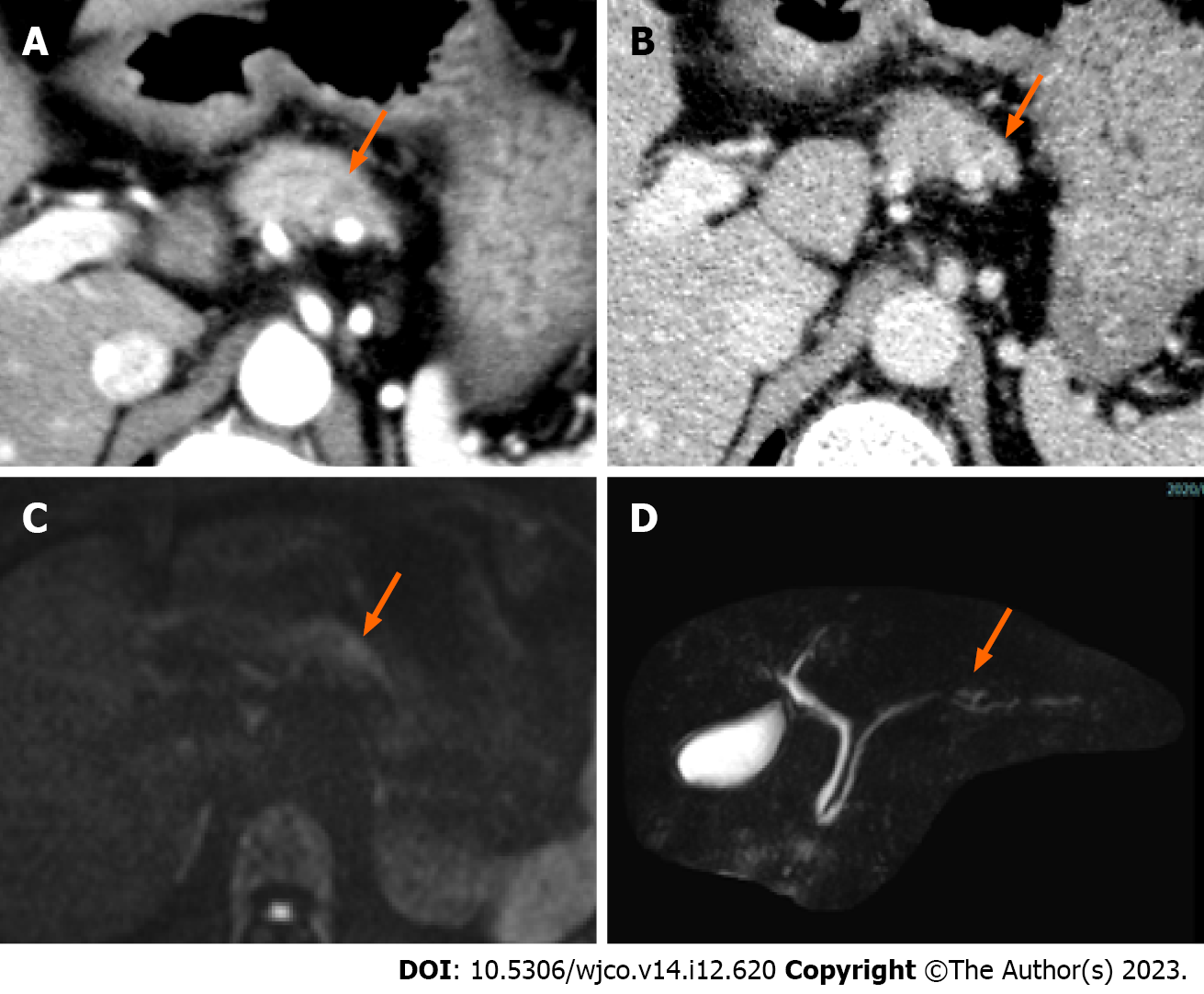
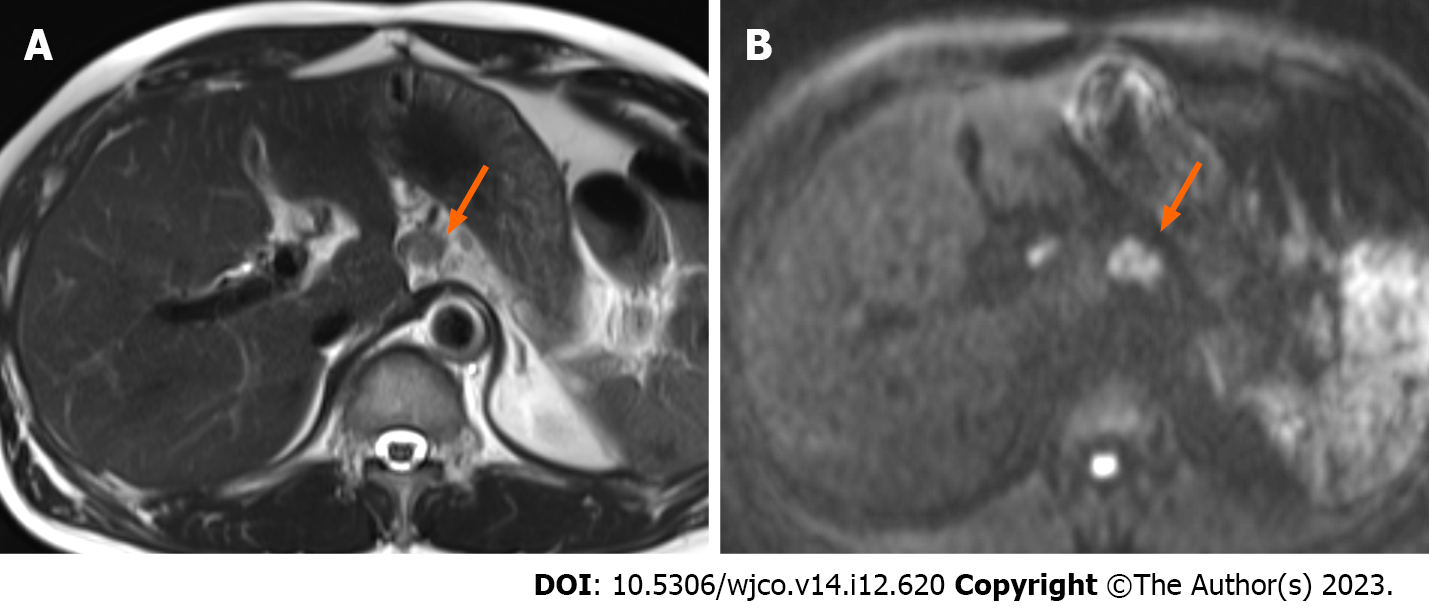
Contrast-enhanced computed tomography of the abdomen incidentally revealed a 5-mm nodule-like mass in the pancreatic body. The nodule was not contrast-enhancing in the arterial phase and was faintly contrast-enhancing in the late phase (Figure 1A and B). Magnetic resonance imaging findings indicated a slightly high-intensity signal on diffusion-weighted images in the same area as that identified on computed tomography. However, identification of obvious neoplastic lesions or abnormal signal areas using T1- weighted and T2-weighted images and dynamic studies was difficult, and there was no change in the caliber of the main pancreatic duct (Figure 1C and D). Endoscopic ultrasound (EUS) performed 2 mo after the initial consultation revealed a 10-mm hypoechoic mass without a cystic structure in the body of the pancreas (Figure 2A). Using Sonazoid® (GE Healthcare, Chicago, IL, United States), a contrast agent, the hypoechoic mass was identified as a hypovascular mass without contrast effect (Figure 2B).
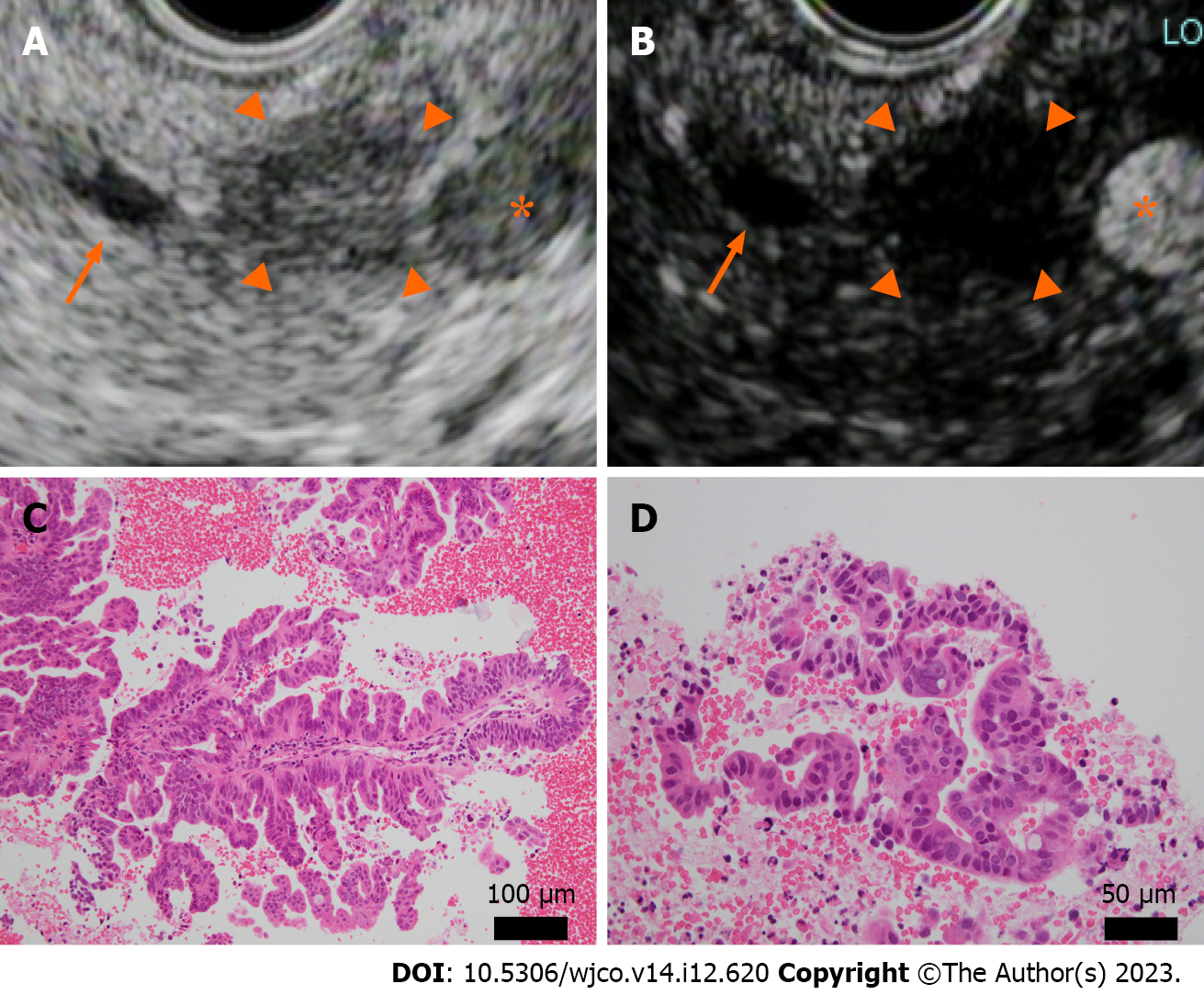
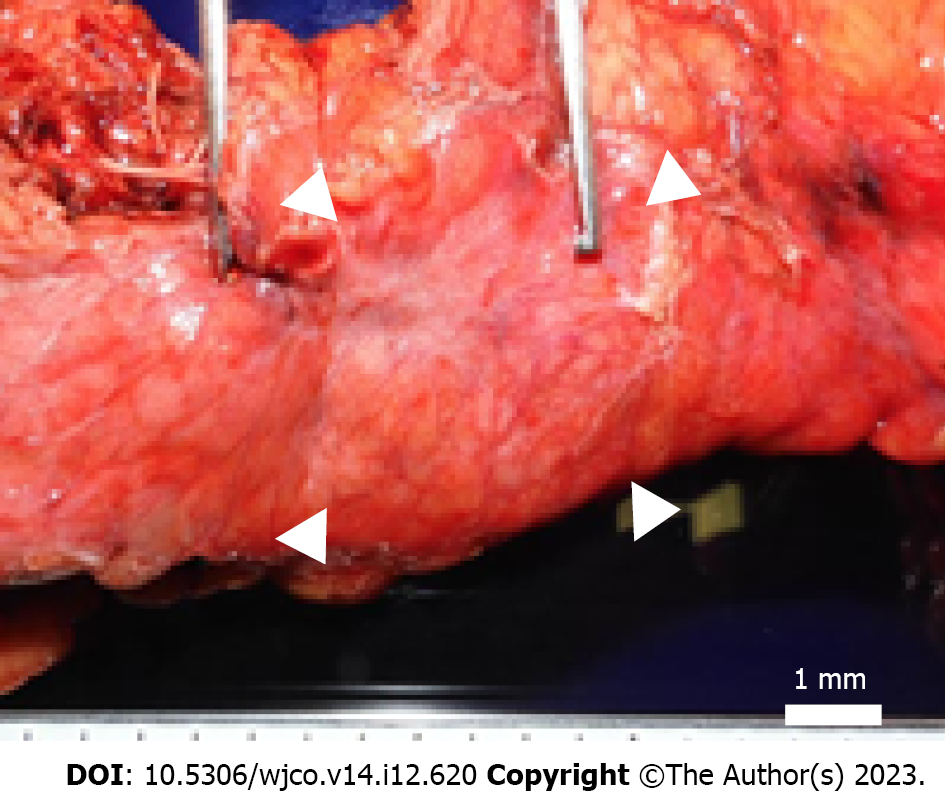
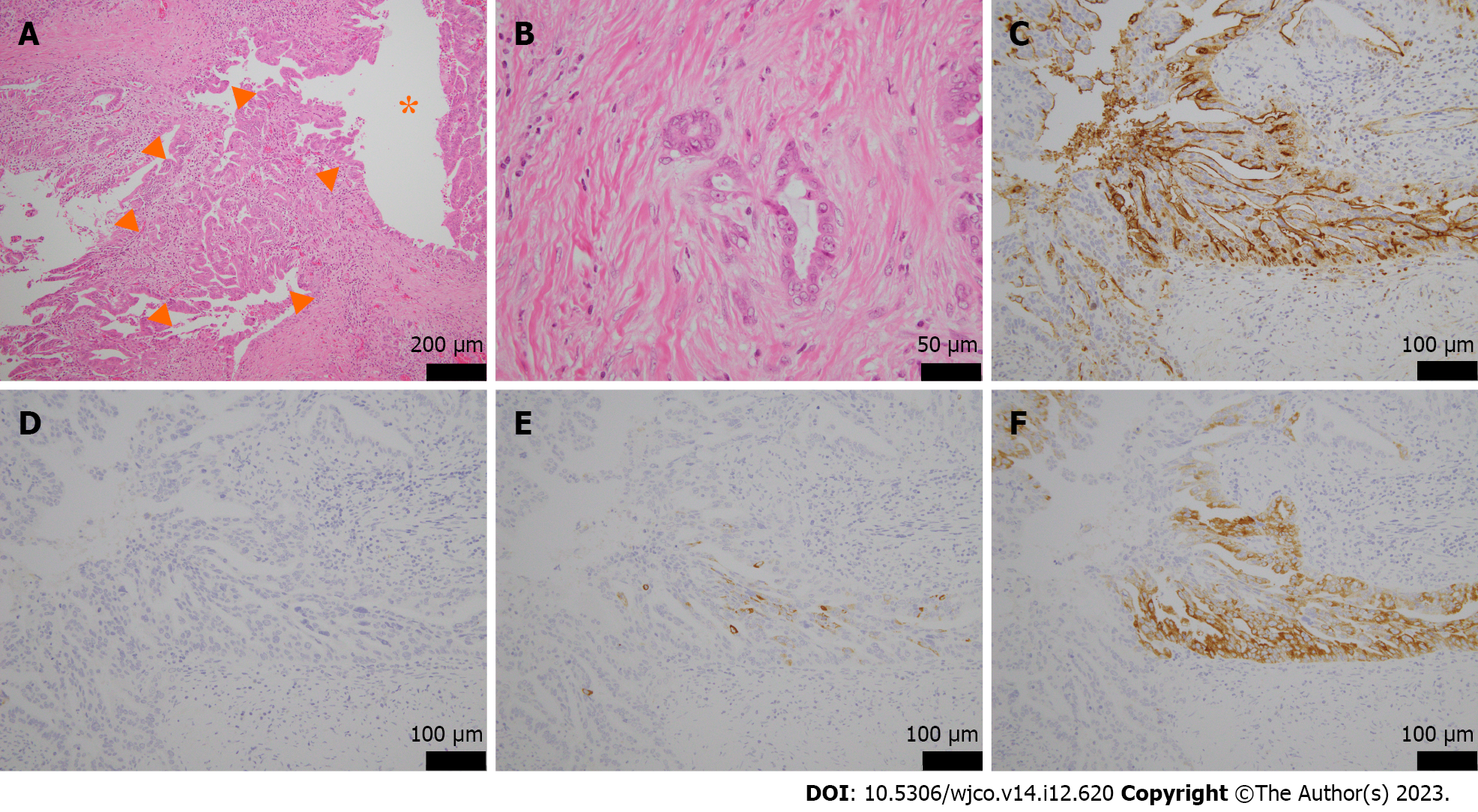
Because of the rapid increase in size within a short period and the possibility of malignancy, we performed EUS-guided fine needle aspiration (EUS-FNA) to obtain a definitive diagnosis. Histopathological examination revealed an atypical epithelium with papillary growth without a mucinous component. Some of the epithelium showed strong nuclear atypia, raising suspicion of an intraductal papillary carcinoma (Figure 2C and D). This was considered an indication for surgery, and a combined distal pancreatectomy and splenic resection were performed 5 mo after the initial detection of the tumor. Macroscopically, a 4 mm × 4 mm borderline brownish nodule and surrounding fibrosis measuring 12 mm × 8 mm × 12 mm were observed at a distance of 3.5 cm from the proximal lateral section of the pancreatic tail. No continuity with the main pancreatic duct was observed, and there was no dilation of the main pancreatic duct or notable changes in the surrounding pancreas (Figure 3). Microscopically, the tumor was located within the branched pancreatic duct and exhibited intraductal tubulopapillary growth with stromal invasion around the branched pancreatic duct (Figure 4A and B). There was no mucus production or cyst formation, and immunohistochemistry was positive for Mucin 1 (MUC1), negative for MUC2, partially positive for MUC5AC, and positive for MUC6 (Figure 4C-F). Mild perineural invasion but no lymphatic or venous invasion with two direct infiltrations in the peripancreatic lymph nodes were observed. Both dissection and pancreatic margins were negative.
The pathological diagnosis was ITPN (pT3N1aM0, pStageIIB, according to the eighth edition of the Union for International Cancer Control) with an invasive cancer component. The patient had no postoperative complications and was discharged from the hospital on postoperative day 13.
The patient underwent treatment with postoperative adjuvant chemotherapy [S-1 monotherapy (80 mg/day)] for 6 mo following the treatment protocol for pancreatic cancer in Japan, and recurrence-free survival was maintained for 10 mo.
Magnetic resonance imaging performed at 1 year and 10 mo postoperatively revealed recurrence in the retroperitoneal lymph nodes at the surgical site (Figure 5). This postoperative recurrence was treated with oral S-1 in combination with radiation therapy (S-1: 120 mg for 4 wk, 100 mg for 2 wk, and 80 mg for 2 wk), and a reduction in the tumor size at the site of recurrence was observed. Following completion of radiotherapy (total 50 Gy), gemcitabine plus nab-paclitaxel (1000 mg/m2, 125 mg/m2) therapy was continued. To date, 2 years and 6 mo postoperatively, the patient’s condition has remained in a stable state of disease[6].
ITPN was described for the first time in 2009 by Yamaguchi et al[1] and is characterized by papillary growth in the pancreatic duct without mucous production, unlike intraductal papillary mucinous neoplasm (IPMN), which typically involves excessive mucin production. ITPN is a highly rare disease, with a frequency of 0.9% among pancreatic exocrine tumors and 3% among intraductal tumors[1]. The average patient age is 58 (25–82) years, and there are no sex differences[1,7]. The 5-year survival rate of ITPN is 81.5%, which is higher than that of pancreatic ductal carcinoma[3]. This type of tumor tends to grow slowly, and the average tumor diameter at the time of detection is 4.5 (0.5–15.0) cm[4].
ITPN is frequently compared to IPMN. Both are intraductal pancreatic tumors with a common intraductal growth pattern and the potential for malignant transformation; however, ITPN and IPMN differ in their immunohistological features. In the gross view, ITPN is a tumor that fills, expands, and proliferates in the pancreatic duct[1,2]. Although the caudal pancreatic duct may dilate due to its obstruction, the tumor does not have a cystic appearance or mucus deposits as in IPMN[1,2]. The Vaters papilla is not enlarged, and mucus outflow is not as observed in IPMN[1,2].
In IPMN, branched pancreatic duct types are common (88%), whereas in ITPN, this is a relatively rare feature (14%)[8]. Additionally, IPMN is positive for MUC5AC in all subtypes, including the oncocytic subtype. Conversely, ITPN is positive for MUC1 and MUC6 and negative for MUC2 and MUC5AC[9], which is consistent with our findings.
Surgical resection is the recommended treatment for ITPN[1,4,7,10]. Date et al[10] reported that among 37 patients with ITPN, the 1-year, 3-year, and 5-year overall survival (OS) rates after surgery were 97.3%, 80.7%, and 80.7%, respectively. Patients with ITPN-associated carcinoma had a favorable 5-year OS of 81.5% compared with the OS of those with ductal adenocarcinoma of the pancreas or IPMN-associated pancreatic cancer. Regarding ITPN prognosis, the recurrence-free survival rate tends to differ according to tumor size (< 4.0 cm vs > 4.0 cm) and Ki-67 labeling index (< 20% vs > 20%); however, these differences are not statistically significant[11]. Currently, there are no comprehensive reports on the efficacy of chemotherapy for ITPN. In the present case, when ITPN with invasive cancer was finally diagnosed, S-1 was administered as postoperative adjuvant chemotherapy following the standard treatment for conventional pancreatic ductal carcinoma in Japan[12]. It should be noted that the efficacy of S-1 for ITPN treatment is unknown.
Our report described a rare case of ITPN originating from a branched pancreatic duct that was incidentally observed in imaging studies and diagnosed using EUS-FNA. In this case, the tumor was small (3 cm in diameter), and the Ki-67 labeling index was approximately 50%, although it varied depending on the site. Despite being microscopic, the tumor rapidly increased in size, and the resected specimen demonstrated an invasive cancerous component. Thus, despite the general tendency of ITPN to grow slowly, in this case, the tumor rapidly progressed to invasive cancer and recurred 1 year and 10 mo postoperatively. As the occurrence of ITPN in the branched pancreatic duct is extremely rare, the nature of the tumor remains unclear.
Based on this case, we suggest that ITPN of the branched pancreatic duct type may have a worse prognosis than that of the main pancreatic duct. Further investigation of such cases is warranted. Moreover, early diagnosis of ITPN through EUS-FNA and subsequent surgical resection of the tumor are crucial. Further studies, especially multicenter studies, are required to validate our findings.
We would like to express our appreciation to Dr. Yutaka Takada for his thoughtful guidance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen Z, China; Gupta MK, Germany; Sun GH, China S-Editor: Lin C L-Editor: Filipodia P-Editor: Lin C
| 1. | Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K, Morikawa T, Motoi F, Unno M, Kanno A, Satoh K, Shimosegawa T, Orikasa H, Watanabe T, Nishimura K, Ebihara Y, Koike N, Furukawa T. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Adsay NVKG, Kloppel G, Fukushima N. WHO Classification for Tumors of the Digestive Systems. In: Intraductal Neoplasms of the Pancreas. Bosman FT, Carneiro F, Hruban RH, Theise ND, editor. Lyon: IARC Publication, 2010: 304-313. [Cited in This Article: ] |
| 3. | Fritz S, Küper-Steffen R, Feilhauer K, Sommer CM, Richter GM, Bosse A, Hennig R, Köninger J. Intraductal tubular papillary neoplasm (ITPN), a novel entity of pancreatic epithelial neoplasms and precursor of cancer: A case report and review of the literature. Int J Surg Case Rep. 2019;55:187-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Basturk O, Adsay V, Askan G, Dhall D, Zamboni G, Shimizu M, Cymes K, Carneiro F, Balci S, Sigel C, Reid MD, Esposito I, Baldaia H, Allen P, Klöppel G, Klimstra DS. Intraductal Tubulopapillary Neoplasm of the Pancreas: A Clinicopathologic and Immunohistochemical Analysis of 33 Cases. Am J Surg Pathol. 2017;41:313-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Nabrinsky E, Baste CL, Gonzalez M, James E. Intraductal Tubulopapillary Neoplasm of the Pancreas Presenting as a Localized Pancreatic Tumor in a 52-Year-Old Woman: Focusing on a Rare Pancreatic Malignancy and Contrasting to Intrapapillary Mucinous Neoplasm. Cureus. 2020;12:e8548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15860] [Cited by in F6Publishing: 19179] [Article Influence: 1278.6] [Reference Citation Analysis (1)] |
| 7. | Kim H, Ro JY. Intraductal Tubulopapillary Neoplasm of the Pancreas: An Overview. Arch Pathol Lab Med. 2018;142:420-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Fritz S, Klauss M, Bergmann F, Strobel O, Schneider L, Werner J, Hackert T, Büchler MW. Pancreatic main-duct involvement in branch-duct IPMNs: an underestimated risk. Ann Surg. 2014;260:848-55; discussion 855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Maghrebi H, Makni A, Rhaeim R, Zehani A, Bensafta Z. Intraductal Tubulopapillary Neoplasm: A New Entity in the Spectrum of Pancreatic Intraductal Neoplasms. J Clin Diagn Res. 2017;11:PD14-PD16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Date K, Okabayashi T, Shima Y, Iwata J, Sumiyoshi T, Kozuki A, Morita S, Hata Y, Noda Y, Nishioka A, Matsumoto M. Clinicopathological features and surgical outcomes of intraductal tubulopapillary neoplasm of the pancreas: a systematic review. Langenbecks Arch Surg. 2016;401:439-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Kölby D, Thilén J, Andersson R, Sasor A, Ansari D. Multifocal intraductal tubulopapillary neoplasm of the pancreas with total pancreatectomy: report of a case and review of literature. Int J Clin Exp Pathol. 2015;8:9672-9680. [PubMed] [Cited in This Article: ] |
| 12. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 645] [Article Influence: 80.6] [Reference Citation Analysis (0)] |