INTRODUCTION
Thymic epithelial tumors (TETs) are rare in the general population, with an estimated incidence in Netherlands of 3.2 cases per 1 000 000 inhabitants[1]. In spite of their rarity, TETs are the most frequently diagnosed primary malignant tumor of the anterior mediastinum, accounting for about 40% of mediastinal masses in adult patients[2]. Stage and histology are predictive of survival[3]. At histological examination, TETs present both epithelial and lymphocytic cells. Epithelial cells are responsible for neoplastic growth and can regulate maturation of the accompanying lymphocytic population, which is involved in the typical associated autoimmune syndromes[4]. Surgery, radiotherapy, and chemotherapy offer a possibility of cure for patients with localized or locally advanced TETs. Metastatic disease is virtually incurable but highly responsive to chemotherapy, which can significantly prolong survival and palliate symptoms. TETs are responsive to several anti-neoplastic agents including platinum compounds[5], anthracyclines[5], and pyrimidine analogs[6]. Among targeted drugs, somatostatin analogs, employed in combination with prednisone, provided radiological disease stabilization in TETs patients and improvement of the associated auto-immune syndromes[7].
The biology of TETs is presently unknown. Immunohistochemistry data showing expression of c-KIT and epidermal growth factor receptor (EGFR) in TETs prompted experimentation of imatinib and cetuximab in TETs[8,9]. Multi tyrosine kinases inhibitors(such as sorafenib and sunitinib) and histone deacetylase inhibitors (such as bolinostat) were also tested in TETs[10]. Unfortunately, results obtained with targeted therapy, with the exception of somatostatin analogues, were overall modest and can presently be considered inconclusive and not applicable to clinical practice.
Everolimus (Afinitor®, Novartis Pharma, Basel, Switzerland) is an oral inhibitor of the mTOR pathway with multiple applications in transplantation medicine, cardiology and oncology[11]. In two phase III randomized, placebo-controlled trials, everolimus, administered at 10 mg daily, successfully prolonged progression free survival in patients with advanced kidney cancer and neuroendrocrine pancreatic cancer[11]. Of all retrospectively reviewed patients treated for advanced TETs at our Institution in the last five years, records of two cases who received everolimus were identified. Everolimus was obtained for compassionate use with approval of the local ethics committee on the grounds of its broad range of anti-tumor activity and in view of lack of medical options of established efficacy. Both patients gave their informed consent to treatment. Although they had been heavily pretreated, they obtained a remarkable, long-lasting clinical improvement with everolimus, with improved quality of life, a durable radiographic response and manageable side effects, as detailed below.
CASE REPORT
Case 1
In October 2005, the patient, a 51-year old woman from Genua, with no relevant medical history, was incidentally diagnosed with a mediastinal mass on chest X-ray. A chest computed tomography scan with contrast showed the presence of a 6 cm mass in the upper-anterior mediastinum, with multiple bilateral pleural metastases. An ultrasound-guided trans-thoracic core biopsy was performed at the “Gallino” Hospital of Genua. Histologic analysis was diagnostic of B2 thymic epithelial tumor (World Health Organization 2004). Additional histologic analyses, performed at the Department of Pathology of Regina Elena (Rome), revealed strong EGFR expression on immunohistochemistry. An 111In-DTPA-pentetreotide scintigraphy (Octreoscan) showed intense radioactivity uptake in the mediastinal and pleural lesions.
Patient was completely asymptomatic at the time of the diagnosis, with an Eastern Cooperative Oncology Group performance status of 0. In view of the advanced stage of the disease (stage IVa according to the Masaoka staging system), patient was judged to be inoperable and suitable for medical therapy. Since November 2005 to August 2010, patient underwent several lines of treatment, which included cisplatin-doxorubicin-cyclophosphamide, carboplatin-etoposide, capecitabine-gemcitabine and cisplatin-doxorubicin-cyclophosphamide-prednisone. Patient also received single agent cetuximab, single agent imatinib, high dose prednisone and single agent octreotide. Patient received all of these anti-neoplastic regimens at the medical Oncology Department of the “Gallino” Hospital (Genua), except for imatinib, which was delivered at the Department of Molecular and Clinical Oncology and Endocrinology of University Federico II of Naples. Since January 2005 to the present time, patient has uninterruptedly received long-acting somatostatin analogs (octreotide LAR 30 mg or lanreotide LAR 60 mg delivered intramuscularly every month). No unexpected or life-threatening side effects were reported. Heart, liver, kidney and bone marrow functions were periodically monitored during treatment administration. Due to intolerance to iodine contrast, treatment response was periodically evaluated with PET-TAC with FDG, without contrast, approximately every three-four months while receiving treatment and every 6 mo during follow-up. In August 2010, patient had complained about mild dyspnea at rest for the past month which worsened on exertion. Her Eastern Cooperative Oncology Group performance status was 1. With respect to her last scan performed in April 2010, positron emission tomographic/computed tomographic (PET-CT) scan performed in July 2010 showed increased size and SUV of the mediastinal mass (SUVmax = 6.3 vs 4.5) and of the bilateral pleural lesions (SUVmax = 4.5 vs 3.5). Given the amount of chemotherapy received, patient was no longer considered suitable for cytotoxic therapy. She was started on everolimus on August 4, 2010 at the dose of 5 mg/d, which was increased to 10 mg/d after a week. Follow-up during treatment was performed with a complete blood count every two weeks and a complete blood chemistry every month, history and physical examination every two months, with phone contact at need, PET-CT scan approximately every three months, as well as ecocardiographic assessment of heart function every 6 mo.
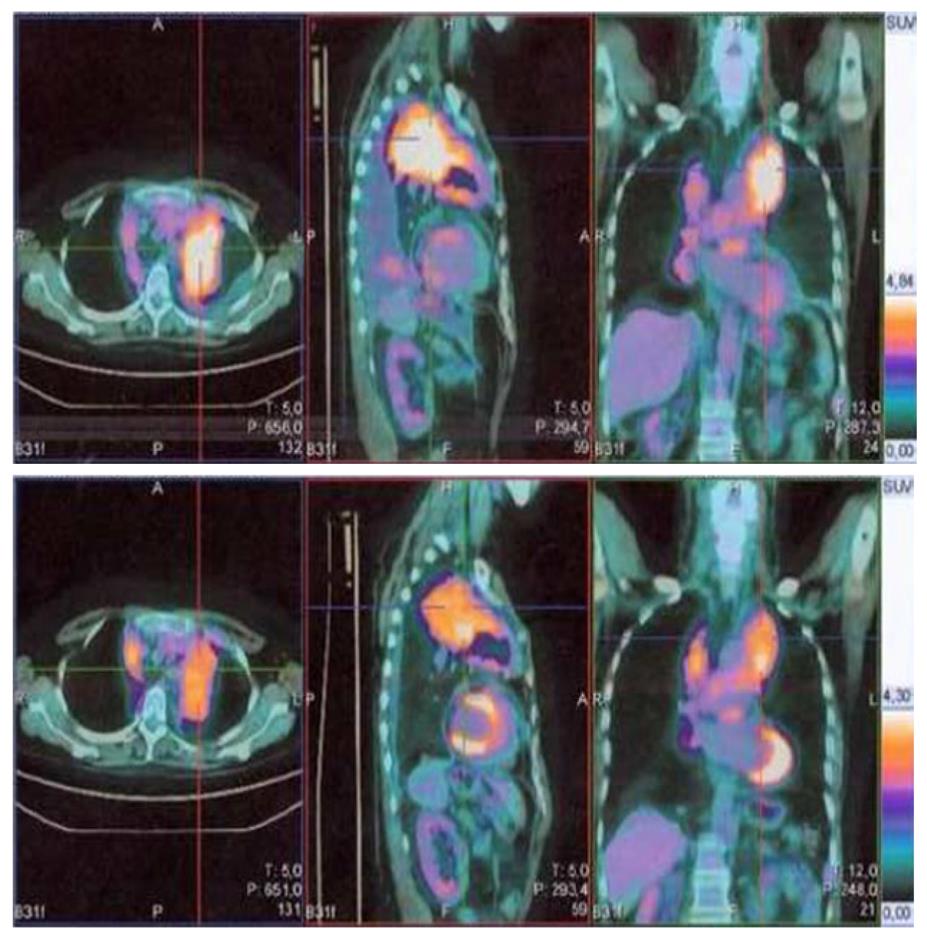
After two weeks’ treatment, patient developed grade 2 oral mucositis with thrush, which resolved with appropriate antifungal and coating agents. Patient also developed grade 1 hyperglycemia, which was easily managed with diet recommendations. After two months’ treatment, patient referred a marked improvement of dyspnea. Her performance status had improved to 0. On October 25th, 2010 patient underwent a PET-CT scan, which showed disappearance of pleural effusion and decreased SUV of all pathologic lesions (Figure 1). Patient underwent subsequent scans in January 2011, May 2011 and August 2011 which indicated lack of progression and progressive decrease in the metabolic activity of the lesions. During treatment with everolimus, patient has reported several episodes grade 1/2 diarrhea, stomatitis, anemia, rash, hypertension. All of these side effects were manageable and were responsible for a total treatment interruption of 7 wk. Dose was never reduced throughout treatment. As of November 30th, 2011 patient has been continuing to take 10 mg everolimus daily, with complete resolution of dyspnea and a performance status of 0.
Figure 1 The marked decrease in fluoro-2-deoxy-D-glucose uptake in positron emission tomography/computed tomography performed in October, 2010 (below) in comparison to positron emission tomography/computed tomography performed in August, 2010 (above) in patient 1.
Color scales are approximately the same.
Case 2
In January 2007, the patient, a 42-year old woman from Naples, complaining of severe tiredness and showing palpebral ptosis, was referred a neurologist for suspected myasthenia gravis. Diagnosis was confirmed by electromiography and serum anti-acetilcholinesterase antibodies levels and patient was started on pyridostigmine. A whole body CT scan with and without contrast showed a mediastinal mass (4.2 cm × 6.5 cm), which extended from the right paratracheal space to the aorto-pulmonary window and was attached to the esophagus and the left atrium. A pericardial effusion was detected along with several pleural lesions in the right costovertebral space and on diaphragmatic pleura. Multiple biopsies of the mediastinal lesion were obtained via thoracotomy, performed at the “Antonio Cardarelli” Hospital in February 2007. Histology analysis performed at the same Institution was diagnostic of B3 thymoma. Immunohistochemistry was positive for p53, ki67, CD1a and CD5 and negative for TTF1 and CK7. Additional immunohistochemistry analysis performed at the Department of Pathology of Regina Elena (Rome) was negative for c-kit and positive for EGFR. Given the presence of multiple pleural metastases, patient was not considered suitable for surgery or radiotherapy. Since February 2007 to July 2007, patient underwent eight cycles of cisplatin-doxorubicin-cyclophosphamide, achieving disappearance of all pleural lesions and shrinkage of the mediastinal mass on CT scan. Given the partial response obtained, surgery was deemed to be feasible, but it was refused by the patient, so she received single agent octreotide LAR as maintenance treatment (Octreoscan was positive). In September 2008, a CT scan with contrast showed progressive disease, with enlargement of the mediastinal mass (55 mm × 12 mm), and recurrence of several pleural lesions. Since October 2008, she underwent 17 cycles of capecitabine-gemcitabine, which was interrupted in November 2009 for unacceptable toxicity. Since January 2010 to July 2010, patient was enrolled in the TETIMAX study and was treated with imatinib. Imatinib was interrupted for progressive disease on CT scan, which showed a mediastinal mass measuring 18 mm × 59 mm, with multiple pleural metastases (largest diameter, 27 mm × 38 mm). Patient subsequently received everolimus, according to the same dosing and schedule adopted for case 1. After one month’s treatment pyridostigmine dose was slowly tapered and was suspended after three months. A CT scan was scheduled for October 2011, but a subsequent CT scan was performed in February 2011, due to patient’s poor compliance. Stable disease was achieved, with the mediastinal mass measuring 18 mm × 51 mm, shrinkage of the largest pleural metastasis, which measured 16 mm × 9 mm, and shrinkage or stability of the others. Subsequent CT scans performed in June and November 2011 confirmed stable disease. As of November 30th, 2011 patient is free of progression and continuing treatment with everolimus.
DISCUSSION
Platinum-based chemotherapy is the standard approach for TETs not amenable to loco-regional treatments. Cisplatin-doxorubicin-cyclophosphamide[12], cisplatin-etoposide[13] and carboplatin-paclitaxel[14] are among the combination regimens tested as first-line treatment for thymomas, with a response rate of 42.9%-56% and a PFS of 16.7-26.4 mo. Poor evidence about second-line treatment is available in literature. We showed that combination of capecitabine and gemcitabine was able to provide a PFS of 11 mo (95% confidence interval 4-17), with an overall response rate of 40% and excellent tolerance in cisplatin-pretreated patients[6]. Single-agent pemetrexed also provided a promising PFS of 45.4 wk in patients with previously treated thymoma[15].
Somatostatin analogues, employed either alone or in combination with prednisone, were the first targeted agents to prove to be active in TETs[16], with a PFS of 9.2 mo (95% CI, 8.1 mo to 13.9 mo), obtained with the combination regimen in a phase II trial[7]. Although several other targeted agents, including cetuximab, cixutumumab, sorafenib and sunitinib, yielded promising results in TETs in either single cases or small series, no conclusive evidence is presently available for any of these drugs[10]. Of note, one of the largest phase II trial on targeted agents was conducted on hystone deacetilase inhibitor belinostat in 25 thymoma patients, with a response rate of 8% only, but a promising TTP of 11.4 mo[10].
The mTOR pathway plays a critical role for neoplastic growth via phosphorylation of the p70 S6 kinase, a key enzyme in protein synthesis, and it is also implicated in regulation of actin cytoskeleton, angiogenesis and cellular response to hypoxia and energy depletion[11]. The mTOR complex mediates downstream signaling of many soluble factors, including cytokines and growth factors, such as the epidermal growth factor and the insulin-like growth factor-1 (IGF-1)[11]. Both of these soluble factors may have a role in TETs biology. In fact, a retrospective analysis of 111 histology samples showed that 22 cases (20%) were positive for IGF-1R on immunohistochemistry[17]. Similarly, EGFR was detected in 23 of 31 of TETs on immunohistochemistry[18]. Conversely, anti-IGF-1R antibody cixutumumab and anti-EGFR antibody cetuximab demonstrated activity in TETs[9,19]. Although unsupported by in vitro experiences, these findings suggest that stimuli transduced by TK receptors, and consequently by mTOR, are important for thymoma growth, thus providing the biological rationale to support the use of everolimus in TETs. Of note, investigational mTOR inhibitor ridaforolimus recently provided a prolonged disease stabilization (> 16 wk) in a patient with thymic carcinoma in a phase I trial[20].
The two cases reported here present several interesting points worthy of discussion. First, the PFS longer than one year and the incontrovertible and marked improvement of clinical conditions and quality of life obtained in both of these two patients strongly suggest that everolimus merits additional clinical investigations in TETs patients. Furthermore, as cixutumumab may increase signaling via the mTOR pathway, experimentation of the combination of everolimus plus cixutumumab appears to be of interest[19]. Second, patient 2 also showed remission of myasthenia gravis, and pyridostigmine could be suspended. Such effect may be due to the immunosuppressant properties of everolimus coupled with its anti-tumor efficacy. Third, patient 1 was evaluated with PET/CT without iodine contrast during treatment with everolimus and decrease in FDG uptake as shown in Figure 1 was concordant with clinical benefit. We previously reported that PET results were concordant results of CT scans with iodine contrast in 6 of 9 patients for whom both examinations were performed in the TETIMAX trial[8]. PET/CT could be useful for evaluation of response in TETs, but additional data are required.
In conclusion, these two are the first reported cases of everolimus in patients with TETs. We believe that the prolonged clinical benefit shown in these patients should encourage experimentation of everolimus in this disease, either alone or in combination with other promising agents, such as cixutumumab.