the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Altitude and management affect soil fertility, leaf nutrient status and Xanthomonas wilt prevalence in enset gardens
Sabura Shara
Rony Swennen
Jozef Deckers
Fantahun Weldesenbet
Laura Vercammen
Fassil Eshetu
Feleke Woldeyes
Guy Blomme
Roel Merckx
Karen Vancampenhout
Enset (Ensete ventricosum) is a productive, drought-tolerant and multipurpose food security crop grown in the densely populated Ethiopian highlands. It is a so-called orphan crop, and its production suffers from a lack of information on proper soil fertility management and its interaction with bacterial wilt disease caused by the pathogen Xanthomonas campestris pv. musacearum. The aim of this study was therefore to assess soil–plant nutrient variation within enset home gardens at three altitudes (ranging from 2000 to 3000 m above sea level – a.s.l.) in the Gamo highlands and investigate whether this variation affects disease prevalence. Altitude in the rift valley covaries with soil leaching, and plant available P, Ca and Mg in soils significantly raised with decreasing altitude. Soil carbon and most nutrients reached very high levels in the gardens, whereas the more distant outfields were severely nutrient deprived. Differences in management intensity within the garden caused soil pH, conductivity, total organic carbon, total N and available P, K, Ca, Mg, Mn and Fe levels to significantly decline with distance from the house; yet, this decrease in soil nutrients was not mirrored in a response of foliar nutrient content, except for N. Hence, over-fertilization is likely, and establishing evidence-based nutrient recommendations for enset would benefit soil quality and productivity both in the gardens and in the outfields. Disease prevalence was high in the study area, with one-third of the farms affected in the recent past. Although more experimental work is needed to exclude confounding factors, our data indicate that the effects of altitude, P fertilization, micronutrients and K-Ca-Mg balance are promising avenues for further investigation into Xanthomonas wilt disease susceptibility.
- Article
(10252 KB) - Full-text XML
-
Supplement
(194 KB) - BibTeX
- EndNote
The global sustainable development goals (SDGs) aim for zero hunger and stress the urgency of combatting climate change impacts on agriculture (SDGs 2 and 13; Mariño and Banga, 2016; Rosegrant et al., 2003). Indigenous crops with tolerance to marginal conditions and resilience to climatic stress are therefore regarded as an increasingly important avenue for achieving food security and agro-ecosystem resilience in future tropical climate conditions (Allemann et al., 2004; Nayar, 2014; Renard and Tilman, 2019). A large discrepancy exists globally between the potential and the current use of such crops, which is partly attributed to limited international scientific attention and investments (Manners and van Etten, 2018; Naylor et al., 2004). One of these so-called orphan crops is enset or false banana (Ensete ventricosum (Welw.) Cheesman).
Enset is a perennial multipurpose crop grown for food, feed, fibre and medicine (Brandt et al., 1997; Nurfeta et al., 2008; Tesfaye and Lüdders, 2003). It is one of the oldest cultivated plants in Ethiopia (Brandt et al., 1997), feeding about 20 million people (Brandt et al., 1997; Merga et al., 2019; Yemataw et al., 2014). Unlike other plants from the banana family, enset takes 5 to 7 years to mature, performs best from 2000 to 2750 m above sea level (a.s.l.; Brandt et al., 1997) and is not grown for fruit bunches. Instead, the processed pseudostem and corm are consumed, and leaves are fed to the cattle (Andeta et al., 2018; Atlabachew and Chandravanshi, 2008; Tsegaye and Struik, 2002). Nicknamed the “tree against hunger”, enset can withstand prolonged periods of moisture stress (Brandt et al., 1997; Quimio and Tezera, 1996), and the food yield per hectare is higher than any other crop cultivated in Ethiopia, with the fresh weight of the fermented product ranging from 19 to 33 (Tsegaye and Struik, 2001). The dense leaf canopy, moreover, is an asset in reducing soil erosion and in sequestering carbon (Brandt et al., 1997; Lal, 2003; Tamire and Argaw, 2015). Despite their potential for increasing agricultural resilience in future climates, enset farming systems remain under-researched, leaving issues in soil fertility management and disease control largely unresolved (Borrel et al., 2019).
Enset typically grows in gardens on weathered tropical soils, and animal manure and compost from household refuse are used as soil amendments (Elias et al., 1998; Tamire and Argaw, 2015; Tsgaye and Struik, 2002). However, the supply of these organic inputs is limited and mainly acquired from free-range cattle, which puts an additional strain of overgrazing on the already degraded communal lands (Amede and Taboge, 2007; Assefa and Bork, 2017; Elias et al., 1998; Garedew and Ayiza, 2018). Hence, the optimal use of scarce nutrient resources is vital, yet there are no generally accepted recommendations to the enset farmers in the region. Moreover, information is scanty on how current management has affected soil fertility in existing enset farms. We therefore advocate that soil–plant nutrient interactions should be studied on farms first so as to better align agronomical research with farmers' practices. Moreover, given the considerable ecological amplitude of enset, we hypothesize that these interactions might change with altitude.
Enset Xanthomonas wilt (EXW), a bacterial wilting disease caused by Xanthomonas campestris pv. musacearum (Xcm) causes significant damage to enset gardens (Garedew and Ayiza, 2018; Yemataw et al., 2017; Yirgou and Bradbury, 1968). It can cause yield losses up to 100% (Yemataw et al., 2016), and all cultivated varieties are susceptible (Merga et al., 2019), albeit some variation in tolerance occurs (Handoro and Michael, 2007; Welde-Michael et al., 2008a; Wolde et al., 2016). Basic phytosanitary practices involve the removal of diseased plants, disinfection of farm tools and use of clean planting material (Tadesse et al., 2003; Welde-Michael et al., 2008b). Yet, without access to disease-free plantlets, these measures have little effect in curbing the disease, and alternative disease control strategies need to be established (Negash et al., 2000; Welde-Michael et al., 2008a, b; Wolde et al., 2016). Recent studies in banana crops indicate a promising link between soil fertility management, plant nutrition and bacterial wilt incidence (Atim et al., 2013; Mburu et al., 2016), but this avenue remains to be explored for enset (Huber et al., 2012; Huber and Graham, 1999; Huber and Haneklaus, 2007). We therefore hypothesize that an insight into soil–plant and plant–pathogen interactions at different altitudes might yield a complementing path for EXW control.
Using an on-farm observational approach, this study therefore aims to increase the knowledge base required to attain the full potential of this orphan crop and to improve the food security and livelihood of enset-dependent farm households, namely by assessing soil–plant nutrient interactions in typical enset farms and by exploring potential inferences for Xanthomonas wilt prevalence. More specifically, we assessed the impact of altitude and management on soil fertility by comparing soil properties in enset gardens across different altitudes. We also compared soil properties within the garden (inner and outer garden) and between the garden and the surrounding fields (outfield). Furthermore, we related the observed variation in soil nutrients to plant nutrition by comparing soil nutrient levels to leaf nutrient status. Next, we surveyed the prevalence and distribution of EXW symptomatic enset gardens and related the distribution of symptomatic gardens to altitude, soil properties and leaf nutrient contents. The Gamo highlands were chosen as a particularly relevant study area, as they have a long history of enset cultivation (Cartledge, 1999; Olmstead, 1974) and EXW is common. Moreover, local altitude gradients represent much of the agro-ecological diversity found in the Ethiopian rift area.
2.1 Study area
The study was carried out in the Chencha catchment of the Gamo highlands in Southern Nations, Nationalities and Peoples' regional state of Ethiopia (between 6∘05′–6∘35′ N and 37∘30′–37∘45′ E). The area rises up from the base of Lake Abaya at 1100–3250 over a distance of 20 km (Assefa and Bork, 2017; Coltorti et al., 2019). Located in the western part of the southern Ethiopian Rift Valley escarpment, this landscape is characterized by flat plateaus bordered by steep slopes and dissected by concave valleys and gullies due to erosion (Coltorti et al., 2019). The parent material is mainly made up of continental flood basalts buried under thick ignimbrites, rhyolites and trachytic flows comprising of lava flows, pyroclastic and lacustrine deposits (Ayalew et al., 2002; Tefera et al., 1996). Dominant soil types are reddish, deeply weathered Nitisols and Luvisols (Coltorti et al., 2019; IUSS Working Group WRB, 2015).
The local climate is strongly influenced by the complex terrain and is mainly associated with altitudinal gradients (Assefa and Bork, 2017; Berhanu et al., 2013; Jury, 2014; Minda et al., 2018). Mean annual temperature ranges between 23 and 14 ∘C, and the mean annual rainfall is between 750 and 1700 mm in the lowlands and highlands, respectively. On the basis of local agro-ecological zonation, four climate zones were defined. These were kolla (semi-arid; below 1500 ), woyna dega (sub-humid; between 1500 and 2300 ), dega (humid; between 2300 and 3000 ) and wurch (frost; a cold alpine zone above 3000 ; Cartledge, 1999).
In the study area, enset is grown in traditional home gardens surrounding the house (Fig. 1). Each garden typically comprises a multitude of enset varieties or landraces that are unevenly aged and commonly intercropped with coffee, vegetables, pulses, maize, trees, bamboo or sugar cane in complex patterns and associations (Cartledge, 1999; Tesfaye and Lüdders, 2003; Yemataw et al., 2014). Plantlets are multiplied locally, and young plants are densely planted, predominantly at the outer rim of the garden. Plants are transplanted several times and moved wider apart and closer to the house as they mature, yet practices vary considerably for different varieties and between farms. Enset gardens are fertilized with animal manure and composted plant and household waste. The amount of amendments that is applied varies considerably between gardens, depending on financial resources and the amount of land and cattle owned by the farmer. Within the garden, plants near the house (inner garden) receive inputs almost constantly, while plants farther away from the house (outer garden) receive fewer inputs. A typical amount for the outer garden would be a bamboo basket of ca. 10 kg of composted plant waste and cattle manure per one to three enset plants per year (own interviews, performed at 276 visited farms in 2016–2017). Fertilizer is rarely used in gardens, yet it is common in the outfields that surround the gardens and are used for arable cropping. Urea and diammonium phosphate (DAP) are most common.
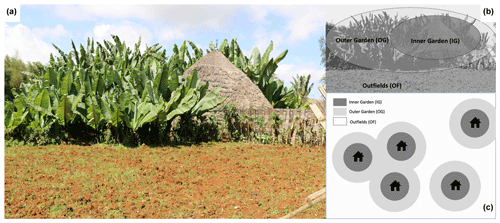
Figure 1(a) Typical enset-based farm in the Gamo highlands surrounding a traditional hut. (b) Illustration of the different farm zones. The area closest to the house is called the inner garden (IG) and receives more organic inputs, while the remaining part of enset garden (outer garden – OG) receives fewer organic inputs. The garden is often surrounded by a plot devoted to the cultivation of annuals that are fertilized mainly with chemical fertilizer (outfield – OF). (c) Schematic illustration of the spatial arrangement of enset gardens in a landscape. In more densely populated areas, gardens are closer together and may not have outfields.
2.2 Sampling and data collection
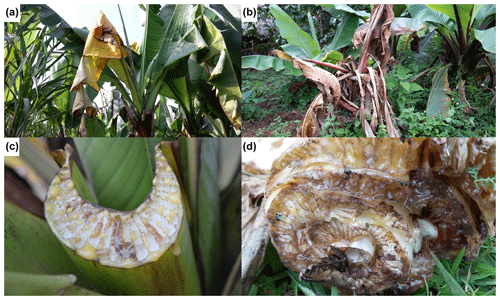
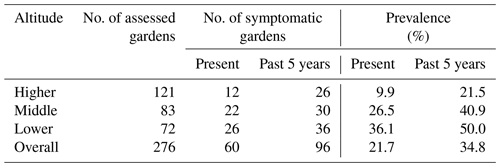
Based on reconnaissance field visits and discussions with farmers, the larger catchment was divided into three altitude zones, namely higher (2600–3000 ), middle (2300–2600 ) and lower (2000–2300 ). In these higher, middle and lower altitude zones, enset gardens were randomly chosen (121, 83 and 72, respectively). Each garden was recorded as symptomatic (EXW symptoms present) or non-symptomatic (no EXW symptoms observed), and the altitude was noted. Symptoms attributed to EXW were leaf wilting, leaf yellowing and slimy yellow bacterial ooze inside the petiole and leaf sheath tissues (Fig. 2). The observations were made between June 2016 and March 2017. Based on interviews with the farmer, the presence or absence of symptomatic plants in the previous 5 years was also registered. It was not possible to accurately assess the number or location of affected plants in the garden, as farmers commonly uproot and remove symptomatic plants and do not keep records.
Soil samples were acquired for a subset of 40 farms. The subset was selected to obtain similar amounts of farms per agro-ecological zone (i.e. 14 in the higher, 15 in the middle and 11 in the lower zone) and per occurrence of EXW symptoms (i.e. 13 farms with symptomatic enset at the time of sampling, 13 farms with no symptomatic enset at the time of sampling, but where symptomatic plants were present in the last 5 years, and 14 farms with no symptomatic enset in the last 5 years; Table S3 in the Supplement). To address intra- and inter-garden variability, each garden was divided into an inner garden (IG) and an outer garden (OG; Fig. 1). Further division of these zones was not opportune as the average size of an enset garden is about 0.13 ha. If present, the outfield (OF) zone of the farm surrounding the enset garden was also sampled (this was the case for 28 farms; see Table S1 for a summary). Four bulk soil samples were taken and combined into one composite bulk sample per farm zone. Sampling depth comprised the upper 25 cm of soil, where most of the enset cord roots are typically distributed (Blomme et al., 2008; Zewdie et al., 2008).
Finally, three sets of leaf samples were taken in order to (i) compare soil nutrient status to leaf nutrient concentrations, (ii) compare nutrient concentrations in the leaves of symptomatic and non-symptomatic plants and (iii) document typical nutrient concentrations in enset leaves as this information is currently not available in the literature. Since no standard leaf sampling method is available for enset, the common method for banana crops was adapted (Martin-Prével, 1977) in which the central 10 cm of the whole lamina was collected on both sides of the midrib in the second fully open leaf. The first set was collected in the same garden as the soil samples if mature (5–7 year old) and non-symptomatic plants from the most common enset variety (locally named maze or mazia; enset varieties do not have standardized names) were present in the inner and the outer garden. This was the case for 19 of the 40 gardens in the subset (two samples per garden, i.e. 38 samples in total). For the second set, leaf samples from 20 pairs of symptomatic and non-symptomatic plants, each pair belonging to the same garden and variety (total of 40 samples), were sampled in 12 gardens. Finally, additional leaf samples of non-symptomatic plants were collected from a range of local varieties (locally named maze or mazia, chamo, checho, falake, geena, katame, katise, kunka, phello and sorghe) to expand the data set on non-symptomatic plants to 218 samples from 58 gardens (Table S1 provides a summary).
2.3 Laboratory analysis
Soil texture was determined by laser diffraction particle size analysis after pre-treatment with HCl and H2O2 (LS 13 320; Beckman Coulter; ISRIC, 2002). Soil pH and electrical conductivity (EC) were measured by using a 1:5 soil to water ratio. Plant-available Kav, Mgav, Caav, Feav, Alav, Mnav and Pav were extracted from soil samples by an ammonium lactate solution (Egner et al., 1960) and analysed by inductively coupled plasma–optical emission spectroscopy (Winge et al., 1979). Soil and leaf total organic carbon and total nitrogen were measured by total combustion (EA1110 elemental analyser; Carlo Erba; Kirsten and Hesselius, 1983). Leaf samples were oven-dried at 60–70 ∘C and finely ground. Approximately 50 mg of each ground leaf sample was extracted by 1 mL HNO3 in an acid-washed glass tube. Quantification of P, K, Ca, Mg, Zn, Cu, Fe, Mn, Al, Mo, Ni and Co was made by inductively coupled plasma–mass spectroscopy (Date and Gray, 1983).
2.4 Statistical analysis
Data analysis was executed using the JMP Pro 14 statistical software package (SAS Institute Inc., 2018). First, an explorative principal component analysis was computed on soil properties to obtain a first appreciation of what explains most of the variation in the data set and to identify interrelationships among the variables. Then, a one-way analysis of variance was used to determine variation in soil properties among altitudes and between symptomatic and non-symptomatic gardens. On-farm variability in soil properties among the garden zones was determined by a linear mixed model. A paired sample t test was employed to determine the variation in plant nutrient levels within enset gardens and symptomatic and non-symptomatic plants. Levene's test was used to test for heteroscedasticity. A Shapiro–Wilk test and the normal quantile plot were used to check for normality assumptions. Data were log transformed, except for pH and EC. Multiple comparisons of significant means were determined using the Tukey–Kramer honestly significant difference (HSD) post hoc test. When unequal variance was observed, a Wilcoxon test was used with a Steel–Dwass post hoc test. All means were separated at 5 % probability level. Disease prevalence (in percent) was computed as follows:
3.1 Variability in soil properties between farms
Factor loadings of the first two principal components (PCs) explained 57 % of the variation in the data set (Fig. 3). PC1 (39 % of the variation) showed positive loadings for most soil nutrients, soil carbon and pH, whereas Al has negative loadings on this component (Fig. 3a). PC2 (18 % of the variation) has positive loadings for sand and silt and negative loadings for clay content. Hence, a higher score on PC1 reflects lower exchangeable soil acidity and higher soil nutrient status, while the score of a plot on PC2 reflects its soil texture. Garden scores (Fig. 3b) on PC1 are negatively correlated with farm altitude, yet this correlation is only marginally significant (Spearman rank correlation; p<0.1). Symptomatic gardens have significantly higher scores on PC1 compared to non-symptomatic gardens (Kruskal–Wallis; p<0.05).
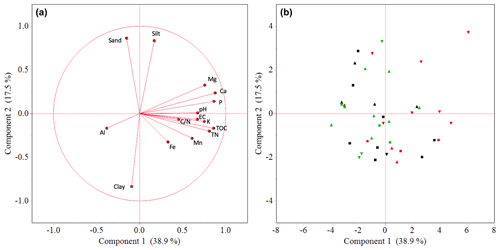
Figure 3The distribution patterns of 40 enset gardens, showing the loading plot (a) in relation to the score plot (b) of soil properties (inner and outer garden data pooled) over two principal components. Shapes in (b) denote enset gardens at higher (△; n=14), middle (□; n=15) and lower (▿; n=11) altitudes, while colours represent gardens that were symptomatic at the time of sampling (red; n=13), were not symptomatic at the time of sampling but had been symptomatic in the past 5 years (black; n=13) and were not symptomatic in the past 5 years (green; n=14).
3.2 Effect of altitude on soil properties of enset gardens
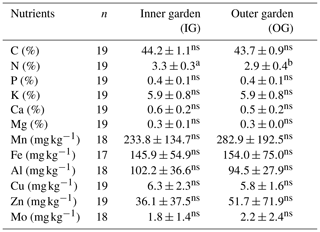
Soil texture in enset gardens did not differ with altitude, and the dominant class of the soil texture was clay (Table 1). In line with the principal component analysis (PCA) results, most soil chemical properties showed an increasing trend with decreasing altitude, yet this trend was significant only for Pav (p<0.05), Caav (p<0.001) and Mgav (p<0.01). Pav was 65 % higher at the lower than at the higher altitude. Levels of Caav and Mgav were 25 % and 16 % larger at the lower than at the middle altitude. In contrast, significantly (p<0.001) higher levels of Alav were observed at the higher altitude compared to the middle and lower altitudes. Levels of Alav at the higher altitude were 14 % and 17 % larger than that at the middle and at the lower altitudes, respectively.
Table 1Variation in soil properties between enset gardens (IG and OG zones pooled) with respect to altitude (higher – 2600–3000 , n=14; middle – 2300–2600 , n=15; lower – 2000–2300 , n=11). Soil nutrients refer to available fractions.
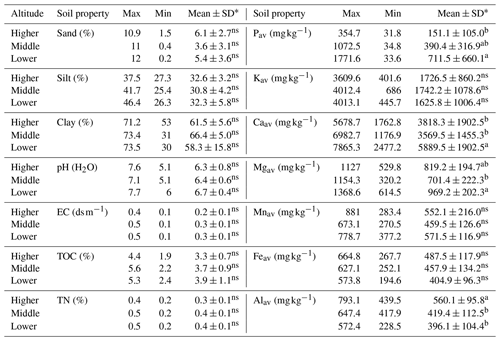
* Different letters within a column and for the same soil property indicate significant differences in mean soil property values between altitudinal zones (p<0.05). Soil properties that were not significantly different are marked with ns.
3.3 Variation in soil properties between inner gardens, outer gardens and outfields
Apart from soil texture, all measured soil properties change significantly (p<0.01) from the garden to the outfields (Table 2), and pH, electrical conductivity (EC), total organic carbon (TOC), total N (TN), Pav, Kav, Caav, Kav and Mgav decrease significantly from the inner garden to the outer garden and from the outer garden to the outfields. The ratio C ∕ N decreased significantly from the garden to the outfields, and Mnav and Feav were significantly higher in the inner garden compared to the rest of the farm. Alav was significantly higher in the outfields as compared to the inner garden.
Table 2Variation in soil properties (mean ± SD) within a farm, showing average levels and standard deviations for the gardens (inner and outer garden) and the outfield (annually cropped plot surrounding the enset garden).
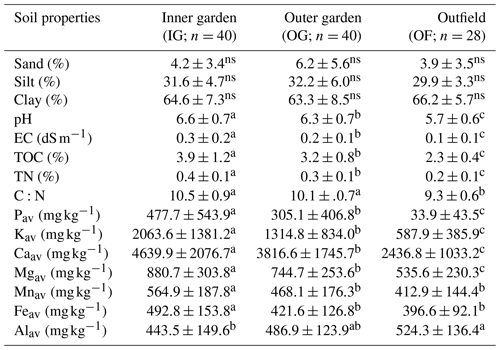
Different letters denote significant differences within a row. Non-significant differences are denoted with ns (p<0.05).
3.4 Leaf nutrient status: variation within the garden
Despite the observed significant differences in soil nutrient levels between inner and outer garden, there was very little difference in leaf nutrient levels (Table 3). Only leaf total N was significantly (p<0.01) higher (6 %) in leaves from plants from the inner compared to the outer garden. Ranges for foliar macronutrient and Mo levels were relatively narrow, whereas larger ranges were observed for micronutrients (Mn, Fe, Zn and Cu; Table St2 in the Supplement). When levels of N, Mn and Fe in both garden zones were compared to optimal and deficiency ranges based on banana crops as a reference (Fig. Sf1 in the Supplement), they generally fall within the optimum range, whereas levels of Ca, Mg and Cu in both zones fall within the deficiency range. However, P, K and Zn levels in both zones were above the optimum.
3.5 Prevalence and distribution of symptomatic enset gardens
Of the 276 enset gardens (including the 40 gardens in which soil properties were studied), 60 gardens (22 %) were currently symptomatic, whereas 96 gardens (35 %) had disease symptoms in the recent past (Table 4). Disease prevalence increased with decreasing altitude irrespective of time periods. At present, the number of symptomatic gardens in the middle and lower altitudes was 2.6- to 3.6-fold higher than in the higher altitude. Moreover, in the 40 enset gardens where soils were sampled, a similar trend of disease prevalence with altitude was observed (Table S3).
3.6 Association between soil and leaf nutrients vs. disease prevalence
Currently symptomatic gardens had significantly (p<0.05) higher levels of Pav and Caav compared to non-symptomatic gardens (Table 5). The other nutrients and texture could not be linked to the presence or absence of EXW symptoms when all altitudes were pooled. When the gardens of the lower altitude zone (where the incidence is highest) are analysed separately, currently symptomatic gardens have significantly (p<0.05) higher pH, Pav, Kav and Caav levels compared to non-symptomatic gardens (Tables S4–6 in the Supplement). When the last 5 years were considered, TOC and TN were also significantly (p<0.05) higher for symptomatic than for non-symptomatic gardens (data not shown).
Table 5Comparison of soil properties between symptomatic and non-symptomatic enset gardens (n=40) from the Chencha catchment, Gamo highlands, Ethiopia.
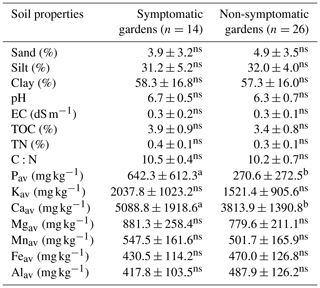
Different letters denote significant differences within a row. Non-significant differences are denoted with ns (p<0.05).
Comparison of foliar nutrient levels between symptomatic and non-symptomatic plants was significant (p<0.05) only for K and Cu (Table 6). K and Cu were higher in symptomatic and non-symptomatic plants, respectively.
Table 6Pairwise comparison of leaf nutrient status (mean ± SD) between symptomatic and non-symptomatic plants with each of the same pairs of 10 local varieties (chamo, checho, falake, geena, katame, katise, kunka, maze, phello and sorghe).
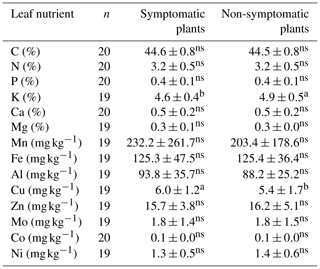
Different letters denote significant differences within a row. Non-significant differences are denoted with ns (p<0.05).
4.1 Soil fertility in relation to agro-ecological zones and management practices
Agro-ecological zones in the study area were mainly determined by altitude, which also affects temperature and rainfall, as precipitation decreases with decreasing altitude in tropical highlands (Berhanu et al., 2013; Cartledge, 1999; Minda et al., 2018). In rift situations, the most weathered soils typically occur at the highest level in the landscape, which is evidenced in the elevated Alav content and decreased soil bases (Caav and Mgav; Fig. 3, Table 1). Acid- and Al-rich Nitisols tend to strongly fix P, which is in line with lower Pav levels at higher altitudes. Slower decomposition of soil organic matter, erosion and land degradation can also contribute to this effect (Elias, 2017; Shigaki et al., 2007; Vancampenhout et al., 2006). Soil texture typically varies with localized differences in Si content of the volcanic parent material, which explains the lack of correlation with altitude or distance from the garden. For most of the measured soil properties, however, intra-farm variability was more prominent than inter-farm variability, and nutrient levels covaried with TOC levels (Table 2), reflecting the paramount influence of management on soil properties in the study area. Continuous application of manure and organic waste was common within the gardens, but not in the outfields, and decreased with distance from the house. This explains the clear intra-garden soil fertility gradient (Amede and Taboge, 2007; Elias et al., 1998; Haileslassie et al., 2006; Tensaye et al., 1998) and the sharp contrast between gardens and outfields. Similar observations have been made for banana crops in Kenyan smallholder farms (Okumu et al., 2011). The pH measured in the enset gardens is comparable to the optimum range suggested for enset, i.e. 5.6–7.3 (Brandt et al., 1997), and is significantly higher than in the outfields, most likely due to the liming effect of organic resources such as manure, compost and ashes applied in the gardens (Abdala et al., 2015; Agbede and Adekiya, 2012; Mokolobate and Haynes, 2002; Whalen et al., 2000). On the other hand, the outfields only receive urea and DAP, which can lower soil pH (Elias et al., 1998; Zelleke et al., 2010). Levels of TOC, TN, Pav, Kav, Caav, Mgav, Mnav and Feav in the enset gardens were much higher than typical values reported in the literature (Ayenew et al., 2018; Elias; 2017; Hengl et al., 2017; Mamo et al., 2002, 2014; Moges and Holden, 2008; Nabhan et al., 1999; Roy et al., 2006) and for banana farms (Ndabamenye et al., 2013; Nyombi et al., 2010). For soil nutrients, recommended levels are not available for enset gardens, although farmers typically expect an increase in growth with higher organic inputs (Amede and Taboge, 2007). A shift from free-range to stabled cattle due to increasing population densities is a trend observed in our study area and may amplify the flux of nutrients to the inner gardens (own interviews).
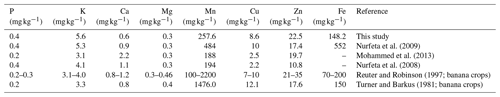
Table 7Comparison of mean foliar nutrient levels in our study as against reported values for enset (Mohammed et al., 2013; Nurfeta et al., 2008, 2009) and banana crops (Reuter and Robinson, 1997; Turner and Barkus, 1981).
Applying more organic inputs closer to the house obviously is more practical but also serves a purpose. Enset varieties grown for the fermented product of the pseudostem are transplanted to the fertile inner zone. As a result, they grow vigorously and produce a higher pseudostem and corm biomass. On the other hand, varieties meant for eating the cooked corm remain in the outer, less fertile zone and receive manure only during their earlier growth stages, as slower growth is said to improve the texture and taste of the cooked product (own interviews). Nevertheless, in our study area, the high nutrient levels suggest that more inputs than required are applied in the gardens, while the outfields suffer from a lack of soil carbon and nutrients (Table 2). This hypothesis is supported by the lack of variation observed in foliar nutrient levels between the inner and outer garden zone (Table 3), despite significant differences in soil nutrient status. If an increase in soil nutrients is not mirrored in an increase in foliar nutrients, it can be considered a sign of inefficient plant nutrient uptake and, therefore, non-optimal soil nutrient management. Hence, agronomical research to determine optimal enset nutrient requirements is needed to optimize input use in the infields and curb soil degradation and low arable yields in the outfields.
Foliar N, P and K in our study were comparable to earlier reports by Uloro and Mengel (1994) for enset grown with inorganic nitrogen, phosphorus and potassium (NPK). We further compared our leaf nutrient contents (Fig. Sf1) to the available literature for enset and standards in banana crops (Table 7). Our results were largely comparable to Nurfeta et al. (2008), but P and K levels were higher for enset than banana crops (Lahav and Turner, 1989; Reuter and Robinson, 1997; Turner and Barkus, 1981). Considering standards in banana crops, our results were comparable for N, Mn and Fe, above optimum for P and K, and deficient for Ca, Mg and micronutrients such as Cu and Zn. Our results suggest a potential additional drawback of over-fertilization, as low Ca and Mg could be linked to a reduced absorption caused by high K levels (Baker and Pilbeam, 2007; Hiltunen and White, 2002; Lahav and Turner, 1989), and deficiency in micronutrients may be induced by excessive rates of P (Huang et al., 2000; Singh et al., 1988; Soltangheisi et al., 2013). A comparison to reported enset leaf nutrients in the literature confirms the high K and low Ca levels in our study area (Table 7). Nevertheless, these results need to be interpreted with caution, as optimal leaf sampling methods for enset leaves are not known and optimal enset nutrient levels may differ substantially from those reported for banana crops. Hence, dedicated research to infer optimal foliar nutrient status in enset would be an important scope for future agronomical research. Considering the complexity of establishing yield or crop performance for enset (Negash et al., 2013; Tsegaye and Struik, 2000, 2001), research based on foliar analysis will be especially important to complement the scanty long-term agronomical trials for this crop.
4.2 Effects of altitude and nutrients on Xanthomonas wilt disease incidence
In our study area, Xanthomonas wilt incidence was high; over one-third of the visited farms had lost plants to the disease in the last 5 years (Table 4). As enset takes between 5–7 years to mature, these losses are an important threat to food security in the area (own interviews). Lower-lying areas had a significantly higher prevalence of affected gardens, which is in line with results reported by Wolde et al. (2016) and Zerfu et al. (2018) and can be typically attributed to faster disease progression in the warmer climate at lower altitudes (Berhanu et al., 2013; Cartledge, 1999). In contrast, Ocimati et al. (2019) did not find a significant effect of altitude and temperature on Xanthomonas wilt spread in banana farms. However, as enset is cultivated at higher altitudes than banana crops, our results indicate that altitude may be more of a determining factor for enset.
The relation between plant nutrition and Xanthomonas wilt is typically more difficult to infer when plants are nutrient deficient; their susceptibility to diseases may increase (Graham, 1983; Thongbai et al., 1993), yet an excessive amount of some nutrients has also been reported to have negative effects (Dordas, 2008; Huber and Graham, 1999). In our study area, a first complication is that both disease incidence and soil nutrient levels were highest at the lowest altitudes, so these factors seem confounded. Nevertheless, when assessing disease prevalence at the lower, most affected altitude only, soils of symptomatic farms still have significantly higher levels of certain nutrients. From our observations, two potential mechanisms on how nutrient levels may influence Xanthomonas susceptibility are in line with the data. First, excessive rates of P may interfere with micronutrient uptake (Huang et al., 2000; Singh et al., 1988; Soltangheisi et al., 2013), and micronutrient deficiencies have been shown to increase susceptibility to Fusarium wilt in banana crops (Hecht-Buchholz et al., 1998). In our study area, a significantly higher Pav was observed in the soils of symptomatic farms, while an imbalance in micronutrients is likely based on the leaf analysis reported (Tables 3 and 7; Fig. Sf1). Nevertheless, as the effect of nutrients on a plant's response to disease is often species specific (Ghorbani et al., 2009; Spann et al., 2009), the role of micronutrients in enset is an important avenue for future research. Second, interferences in the uptake between K, Mg and Ca have been evidenced to influence plant health in banana crops (e.g. Atim et al., 2013; Freitas et al., 2015, 2016). In our study, symptomatic gardens could be linked to increased levels of Caav in the soil of all 40 farms and also to both Caav and Kav in the subset of the lower altitudes (Tables 5 and S4–6). Leaf analysis indicates that plants in our study area had the highest K values reported and K content was significantly different in symptomatic plants, while Ca levels in the leaves were the lowest reported so far (Tables 6 and 7). The dynamics of those cations in the soil and plant should therefore be further researched in enset, especially in view of the observed over-fertilization with compost and manure.
An alternative explanation is that the organic composts used to fertilize the enset garden may be a source of inoculum for EXW and, hence, explain the correlation between certain soil nutrients and EXW incidence. Although no specific information is available for EXW, other Xanthomonas species have been reported to be heat-sensitive and have been easily eliminated during composting (Elorrieta et al., 2003; Mwebaze et al., 2006; Silva et al., 2012; Wichuk et al., 2011).
In this study, we conducted a reconnaissance observational study into soil fertility and EXW prevalence in enset gardens in the Gamo highlands of Ethiopia. Our results indicate that soil fertility was strongly influenced by altitude and management, with sharp contrasts within enset gardens and between enset gardens and outfields. Gardens in the study area show very high levels for most nutrients, yet an increase in soil nutrients is not mirrored in a response of foliar nutrient content, except for N. Hence, over-fertilization is likely, and establishing evidence-based nutrient recommendations for enset would benefit soil quality and productivity both in the gardens and in the outfields. Disease prevalence was high in the study area, with one-third of the farms affected in the recent past. Although more experimental work is needed to exclude confounding factors, our data indicate that the effects of altitude, P-fertilization, micronutrients and K-Ca-Mg balance are promising avenues for further investigation into EXW disease susceptibility.
Data are publicly accessible via https://doi.org/10.25502/apce-ng55/d (Sabura et al., 2019).
The supplement related to this article is available online at: https://doi.org/10.5194/soil-7-1-2021-supplement.
KV, RS, FWo, JD, GB and FWe designed the observational setup, KV and RM designed the soil and nutrient components of the research, and RS designed the plant and disease-related components. SS and LV collected and analysed the data, and SS compiled the paper, supervised by KV, FE and RS. All authors contributed to the interpretation and discussion of the results.
The authors declare that they have no conflict of interest.
The authors greatly acknowledge the Flemish Interuniversity Council for University Development Cooperation (VLIR-UOS) for funding this research (Enset Team Project; grant no. ZEIN 2015PR407). We thank the farmers of Chencha, who shared their experience. We want to extend our gratitude to Mathilde Vantyghem and Ieben Broeckhoven, for their help with the leaf sampling and nutrient analysis, Alene Abeje and Azmera Walche, for their help during field data gathering, and to Luc Vancampenhout, for language advice. The authors would like to thank laboratory technicians Lore Fondu and Kim Vekemans for their help in laboratory measurements. The authors thank all donors who supported this work through their contributions to the CGIAR Fund (https://www.cgiar.org/funders/, last access: 16 December 2020) and, in particular, to the CGIAR Research Program on Roots, Tubers and Bananas (RTB-CRP).
This research has been supported by the VLIR-UOS (grant no. ZEIN 2015PR407).
This paper was edited by Jose Alfonso Gomez and reviewed by three anonymous referees.
Abdala, D. B., da Silva, I. R., Vergütz, L., and Sparks, D. L.: Long-term manure application effects on phosphorus speciation, kinetics and distribution in highly weathered agricultural soils, Chemosphere, 119, 504–514, https://doi.org/10.1016/j.chemosphere.2014.07.029, 2015.
Agbede, T. M. and Adekiya, A. O.: Effect of wood ash, poultry manure and NPK fertilizer on soil and leaf nutrient composition, growth and yield of okra (Abelmoschus esculentus), Emirates J. Food Agric., 24, 314–321, 2012.
Allemann, J., Laurie, S. M., Thiart, S., Vorster, H. J., and Bornman, C. H.: Sustainable production of root and tuber crops (potato, sweet potato, indigenous potato, cassava) in southern Africa, S. Afr. J. Bot., 70, 60–66, https://doi.org/10.1016/S0254-6299(15)30307-0, 2004.
Amede, T. and Taboge, E.: Optimizing soil fertility gradients in the Enset (Ensete ventricosum) systems of the Ethiopian Highlands: Trade-offs and local innovations, in: Advances in Integrated Soil Fertility management in Sub-Saharan Africa: Challenges and Opportunities, Springer, Dordrecht, 289–297, 2007.
Andeta, A. F., Vandeweyer, D., Woldesenbet, F., Eshetu, F., Hailemicael, A., Woldeyes, S., Crauwels., B, Lievens., J, Ceusters., K, Vancampenhout and Van Campenhout, L.: Fermentation of enset (Ensete ventricosum) in the Gamo highlands of Ethiopia: physicochemical and microbial community dynamics, Food Microbiol., 73, 342–350, https://doi.org/10.1016/j.fm.2018.02.011, 2018.
Assefa, E. and Bork, H.-R.: Indigenous resource management practices in the Gamo Highlands of Ethiopia: challenges and prospects for sustainable resource management, Sustain. Sci., 12, 695–709, https://doi.org/10.1007/s11625-017-0468-7, 2017.
Atim, M., Beed, F., Tusiime, G., Tripathi, L., and van Asten, P.: High potassium, calcium, and nitrogen application reduce susceptibility to banana Xanthomonas wilt caused by Xanthomonas campestris pv. musacearum, Plant Disease, 97, 123–130, https://doi.org/10.1094/PDIS-07-12-0646-RE, 2013.
Atlabachew, M. and Chandravanshi, B. S.: Levels of major, minor and trace elements in commercially available enset (Ensete ventricosum (Welw.), Cheesman) food products (kocho and bulla) in Ethiopia, J. Food Compos. Anal., 21, 545–552, https://doi.org/10.1016/j.jfca.2008.05.001, 2008.
Ayalew, D., Barbey, P., Marty, B., Reisberg, L., Yirgu, G., and Pik, R.: Source, genesis, and timing of giant ignimbrite deposits associated with Ethiopian continental flood basalts, Geochim. Cosmochim. Acta, 66, 1429–1448, https://doi.org/10.1016/S0016-7037(01)00834-1, 2002.
Ayenew, B., Tadesse, A. M., Kibret, K., and Melese, A.: Phosphorus status and adsorption characteristics of acid soils from Cheha and Dinsho districts, southern highlands of Ethiopia, Environ. Syst. Res., 7, 1–14, https://doi.org/10.1186/s40068-018-0121-1, 2018.
Barker, A. V. and Pilbeam, D. J.: Handbook of plant nutrition, CRC Press, Taylor and Francis Group, Boca Raton, Florida, 613, 2007.
Berhanu, B., Melesse, A. M., and Seleshi, Y.: GIS-based hydrological zones and soil geodatabase of Ethiopia, Catena, 104, 21–31, https://doi.org/10.1016/j.catena.2012.12.007, 2013.
Blomme, G., Sebuwufu, G., Addis, T., and Turyagyenda, L. F.: Relative performance of root and shoot development in enset and east African highland bananas, Afr. Crop Sci. J., 16, 51–57, https://doi.org/10.4314/acsj.v16i1.54339, 2008.
Borrell, J. S., Biswas, M. K., Goodwin, M., Blomme, G., Schwarzacher, T., Heslop-Harrison, Wendawek, A. M., Berhanu, A., Kallow, S., Janssens, S., Molla, E. L., Davis, A. P., Woldeyes, F., Willis, K., Demissew, S., and Wilkin, P.: Enset in Ethiopia: a poorly characterized but resilient starch staple, Ann. Bot., XX, 1–20, https://doi.org/10.1093/aob/mcy214, 2019.
Brandt, S. A., Spring, A., Hiebsch, C., McCabe, J. T., Tabogie, E., Diro, M., and Tesfaye, S.: The tree against hunger. Enset-based agricultural systems in Ethiopia, American Association for the Advancement of Science, Washington DC, 56, 1997.
Cartledge, D. M.: The management of Ensete ventricosum in the Gamo Highlands of southwest Ethiopia, Cult. Agric., 21, 35–38, https://doi.org/10.1525/cag.1999.21.1.35, 1999.
Coltorti, M., Pieruccini, P., Arthur, K. J., Arthur, J., and Curtis, M.: Geomorphology, soils and palaeosols of the Chencha area (Gamo Gofa, southwestern Ethiopian highlands), J. Afr. Earth Sci., 151, 225–240, https://doi.org/10.1016/j.jafrearsci.2018.12.018, 2019.
Date, A. R. and Gray, A. L.: Development progress in plasma source mass spectrometry, Analyst, 108, 159–165, https://doi.org/10.1039/AN9830800159, 1983.
Dordas, C.: Role of nutrients in controlling plant diseases in sustainable agriculture. A review, Agron. Sustain. Dev., 28, 33–46, https://doi.org/10.1051/agro:2007051, 2008.
Egner, H., Riehm, H., and Domingo, W. R.: Investigations of the chemical soil analysis as a basis for the evaluation of nutrient status in soil. II. Chemical extraction methods for phosphorus and potassium determination, Kungliga Lantbrukshügskolans Annaler, 26, 199–215, 1960.
Elias, E., Morse, S., and Belshaw, D. G. R.: Nitrogen and phosphorus balances of Kindo Koisha farms in southern Ethiopia, Agric. Ecosyst. Environ., 71, 93–113, https://doi.org/10.1016/S0167-8809(98)00134-0, 1998.
Elias, E.: Characteristics of Nitisol profiles as affected by land use type and slope class in some Ethiopian highlands, Environ. Syst. Res., 6, 1–20, https://doi.org/10.1186/s40068-017-0097-2, 2017.
Elorrieta, M. A., Suarez-Estrella, F., Lopez, M. J., Vargas-Garcııa, M. C., and Moreno, J.: Survival of phytopathogenic bacteria during waste composting, Agric. Ecosyst. Environ., 96, 141–146, https://doi.org/10.1016/S0167-8809(02)00170-6, 2003.
Freitas, A. S., Pozza, E. A., Pozza, A. A. A., Oliveira, M. G. F., Silva, H. R., Rocha, H. S., and Galv ao, L. R,: Impact of nutritional deficiency on Yellow Sigatoka of banana, Australasian Plant Pathol., 44, 583–590, 2015.
Freitas, A. S., Pozza, E. A., Alves, M. C., Coelho, G., Rocha, H. S., and Pozza, A. A. A.: Spatial distribution of Yellow Sigatoka Leaf Spot correlated with soil fertility and plant nutrition, Precis. Agric., 17, 93–107, 2016.
Garedew, B. and Ayiza, A.: Major Constraints of Enset (Ensete ventricosum) production and management in Masha district, southwest Ethiopia, Int. J. Agric. Res., 13, 87–94, https://doi.org/10.3923/ijar.2018.87.94, 2018.
Ghorbani, R., Wilcockson, S., Koocheki, A., and Leifert, C.: Soil management for sustainable crop disease control: a review. In Organic farming, pest control and remediation of soil pollutants, Springer, Dordrecht, 177–201, 2009.
Graham, R. D.: Effects of nutrient stress on the susceptibility of plants to disease with particular reference to the trace elements, in: Advances in botanical research, vol. 10, Academic Press, Cambridge, Massachusetts, 221–276, https://doi.org/10.1016/S0065-2296(08)60261-X, 1983.
Haileslassie, A., Priess, J. A., Veldkamp, E., and Lesschen, J. P.: Smallholders' soil fertility management in the central highlands of Ethiopia: implications for nutrient stocks, balances and sustainability of agroecosystems, Nutr. Cycl. Agroecosyst., 75, 135–146, https://doi.org/10.1007/s10705-006-9017-y, 2006.
Handoro, F. and Michael, G. W.: Evaluation of enset clone meziya against enset bacterial wilt, in: 8th African Crop Science Society Conference, El-Minia, Egypt, 27–31 October 2007, 887–890, 2007.
Hecht-Buchholz, C., Borges-Pérez, A., Fernandez Falcon, M., and Borges, A. A.: Influence of zinc nutrition on Fusarium wilt of banana-an electron microscopic investigation, Acta Horticult., 490, 277–284, https://doi.org/10.17660/ActaHortic.1998.490.27, 1998.
Hengl, T., Leenaars, J. G. B, Shepherd, K. D., Walsh, M. G., Heuvelink, G. B. M, Mamo, T., Tilahun, H., Berkhout, E., Cooper, M., Fegraus, E., Wheeler, I., and Kwabena, N. A.: Soil nutrient maps of Sub-Saharan Africa: assessment of soil nutrient content at 250 m spatial resolution using machine learning, Nutr. Cycl. Agroecosyst., 109, 77–102, https://doi.org/10.1007/s10705-017-9870-x, 2017.
Hiltunen, L. H. and White, J. G.: Cavity spot of carrot (Daucus carota), Ann. Appl. Biol., 141, 201–223, https://doi.org/10.1111/j.1744-7348.2002.tb00213.x, 2002.
Huang, C., Barker, S. J., Langridge, P., Smith, F. W., and Graham, R. D.: Zinc deficiency up-regulates the expression of high-affinity phosphate transporter genes in both phosphate-sufficient and deficient barley roots, Plant Physiol., 124, 415–422, https://doi.org/10.1104/pp.124.1.415, 2000.
Huber, D. M. and Graham, R. D.: The role of nutrition in crop resistance and tolerance to diseases, in: Mineral nutrition of crops: fundamental mechanisms and implications, Food Product Press, New York, 169–204, 1999.
Huber, D. M. and Haneklaus, S.: Managing nutrition to control plant disease, Landbauforschung Volkenröde, 57, 313–322, 2007.
Huber, D., Römheld, V., and Weinmann, M.: Relationship between nutrition, plant diseases and pests, in: Marschner's mineral nutrition of higher plants, Academic Press, Cambridge, Massachusetts, 283–298, https://doi.org/10.1016/B978-0-12-384905-2.00010-8, 2012.
ISRIC: Procedures for soil analysis, 6th ed., Wageningen, the Netherlands, 2002.
IUSS Working Group WRB. 2015. World Reference Base for Soil Resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. 192. FAO, Rome.
Jury, M. R.: Southern Ethiopia rift valley lake fluctuations and climate, Sci. Res. Essays, 9, 794–805, https://doi.org/10.5897/SRE2014.6062, 2014.
Kirsten, W. J. and Hesselius, G. U.: Rapid, automatic, high capacity Dumas determination of nitrogen, Microchem. J., 28, 529–547, https://doi.org/10.1016/0026-265X(83)90011-5, 1983.
Lahav, E. and Turner, D. W.: Fertilizing for high yield banana, International Potash Institute, Berne/Switzerland, IPI Bulletin, 62, 1989.
Lal, R.: Soil erosion and the global carbon budget, Environ. Int., 29, 437–450, https://doi.org/10.1016/S01604120(02)00192-7, 2003.
Mamo, T., Karltun, E., and Bekele, T.: Soil fertility status and fertilizer recommendation atlas for Tigray Regional State, Ethiopia, Ministry of Agriculture and Ethiopian Agricultural Transformation Agency, Addis Abeba, 91, 2014.
Mamo, T., Richter, C., and Heiligtag, B.: Phosphorus availability studies on ten Ethiopian Vertisols, J. Agric. Rural Dev. Trop. Subtrop., 103, 177–183, 2002.
Manners R. and van Etten, J.: Are agricultural researchers working on the right crops to enable food and nutrition security under future climates?, Global Environ. Change, 53, 182–194, https://doi.org/10.1016/j.gloenvcha.2018.09.010, 2018.
Mariño, R. and Banga, R. S.: UN sustainable development goals (SDGs): A time to act, J. Oral Res., 5, 5–6, https://doi.org/10.17126/joralres.2016.002, 2016.
Martin-Prével, P.: Sampling of banana for foliar analysis: Consequences of differences in techniques, Fruits, 32, 151–166, 1977.
Mburu, K., Oduor, R., Mgutu, A., and Tripathi, L.: Silicon application enhances resistance to Xanthomonas wilt disease in banana, Plant Pathol., 65, 807–818, https://doi.org/10.1111/ppa.12468, 2016.
Merga, I. F., Tripathi, L., Hvoslef-Eide, A. K., and Gebre, E.: Application of genetic engineering for control of bacterial wilt disease of enset, Ethiopia's sustainability crop, Front. Plant Sci., 10, 1–8, https://doi.org/10.3389/fpls.2019.00133, 2019.
Minda, T. T., Van Der Molen, M. K., Struik, P. C., Combe, M., Jiménez, P. A., Khan, M. S., and De Arellano, J. V. G.: The combined effect of altitude and meteorology on potato crop dynamics: A 10-year study in the Gamo Highlands, Ethiopia, Agric. For. Meteorol., 262, 166–177, https://doi.org/10.1016/j.agrformet.2018.07.009, 2018.
Moges, A. and Holden, N. M.: Soil fertility in relation to slope position and agricultural land use: A case study of Umbulo catchment in southern Ethiopia, Environ. Manage., 42, 753–763, https://doi.org/10.1007/s00267-008-9157-8, 2008.
Mohammed, B., Gabel, M., and Karlsson, L. M.: Nutritive values of the drought-tolerant food and fodder crop enset, Afr. J. Agric. Res., 8, 2326–2333, https://doi.org/10.5897/AJAR12.1296, 2013.
Mokolobate, M. and R. Haynes.: Comparative liming effect of four organic residues applied to an acid soil, Biol. Fert. Soils, 35, 79–85, https://doi.org/10.1007/s00374-001-0439-z, 2002.
Mwebaze, J. M., Tusiime, G., Teshemereirwe, W. K., and Kubiriba, J.: The survival of Xanthomonas campestris pv. musacearum in soil and plant debris, Afr. Crop Sci. J., 14, 121–127, 2006.
Nabhan, H., Mashali, A. M., and Mermut, A. R.: Integrated soil management for sustainable agriculture and food security in Southern and East Africa, Proceedings of the expert consultation, Harare, Zimbabwe, Food and Agriculture Organization of the United Nations, Rome, Italy, 415, 1999.
Nayar, N. M.: The contribution of tropical tuber crops towards food security, J. Root Crops, 40, 3–14, 2014.
Naylor, R. L., Falcon W. P., Goodman, R. M., Jahn, M. M., Sengooba, T., Tefera, H., Nelson R. J.: Biotechnology in the developing world: a case for increased investments in orphan crops, Food Policy, 29, 15–44, https://doi.org/10.1016/j.foodpol.2004.01.002, 2004.
Ndabamenye, T., Van Asten, P. J., Blomme, G., Vanlauwe, B., Uzayisenga, B., Annandale, J. G., and Barnard, R. O.: Nutrient imbalance and yield limiting factors of low input East African highland banana (Musa spp. AAA-EA) cropping systems, Field Crops Res., 147, 68–78, https://doi.org/10.1016/j.fcr.2013.04.001, 2013.
Negash, A., Puite, K., Schaart, J., Visser, B., and Krens, F.: In vitro regeneration and micro-propagation of enset from southwestern Ethiopia, Plant Cell, Tissue and Organ Culture, 62, 153–158, https://doi.org/10.1023/A:1026701419739, 2000.
Negash, M., Starr, M., and Kanninen, M.: Allometric equations for biomass estimation of Enset (Ensete ventricosum) grown in indigenous agroforestry systems in the Rift Valley escarpment of southern-eastern Ethiopia, Agroforest. Syst., 87, 571–581, https://doi.org/10.1007/s10457-012-9577-6, 2013.
Nurfeta, A., Tolera, A., Eik, L. O., and Sundstøl, F.: Yield and mineral content of ten enset (Ensete ventricosum) varieties, Trop. Anim. Health Prod., 40, 299–309, https://doi.org/10.1007/s11250-007-9095-0, 2008.
Nurfeta, A., Tolera, A., Eik, L. O., and Sundstøl, F.: Feeding value of enset (Ensete ventricosum), Desmodium intortum hay and untreated or urea and calcium oxide treated wheat straw for sheep, J. Anim. Physiol. Anim. Nutr., 93, 94–104, https://doi.org/10.1111/j.1439-0396.2007.00784.x, 2009.
Nyombi, K., Van Asten, P. J., Corbeels, M., Taulya, G., Leffelaar, P. A., and Giller, K. E.: Mineral fertilizer response and nutrient use efficiencies of East African highland banana (Musa spp., AAA-EAHB, cv. Kisansa), Field Crops Res., 117, 38–50, https://doi.org/10.1016/j.fcr.2010.01.011, 2010.
Ocimati, W., Bouwmeester, H., Groot, J. C., Tittonell, P., Brown, D., and Blomme, G.: The risk posed by Xanthomonas wilt disease of banana: Mapping of disease hotspots, fronts and vulnerable landscapes, PLOS One, 14, 1–19, https://doi.org/10.1371/journal.pone.0213691, 2019.
Okumu, M. O., van Asten, P. J., Kahangi, E., Okech, S. H., Jefwa, J., and Vanlauwe, B.: Production gradients in smallholder banana (cv. Giant Cavendish) farms in Central Kenya, Sci. Horticult., 127, 475–481, https://doi.org/10.1016/j.scienta.2010.11.005, 2011.
Olmstead, J.: The versatile ensete plant: Its use in the Gamo highlands, J. Ethiop. Stud., 12, 147–158, 1974.
Quimio, A. J. and Tessera, M.: Diseases of enset, in: Enset-based sustainable agriculture in Ethiopia, edited by: Tsedeke, A., Clifton, H., Steven, B. A., Gebre-Mariam, S., in: Proceedings of the International Workshop on enset. Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia, 188–203, 1996.
Renard, D. and Tilman, D.: National food production stabilisation by crop diversity, Nature, 571, 257–260, https://doi.org/10.1038/s41586-019-1316-y, 2019.
Reuter, D. J. and Robinson, J. B.: Fruits, vines and nuts, in: Plant analysis: an interpretation manual, CSIRO Publishing, Collingwood, 354–355, 1997.
Roy, R. N., Finck, A., Blair, G. J., and Tandon H. L. S.: Plant nutrition for food security. A guide for integrated nutrient management, Exp. Agric., 43, 132–132, https://doi.org/10.1017/S0014479706394537, 2006.
Rosegrant, M. W. and Cline, S. A.: Global food security: challenges and policies, Science, 302, 1917–1919, https://doi.org/10.1126/science.1092958, 2003.
Sabura, S., Rony, S., Jozef, D., Fantahun, W., Laura, V., Fassil, E., Feleke, W., Guy, B., Roeland, M., and Karen, V.: Reconnaissance study on ecological niche of Ensete ventricosum, Data set, International Institute of Tropical Agriculture (IITA), https://doi.org/10.25502/APCE-NG55/D, 2019.
SAS Institute Inc.: JMP Pro 14 software, SAS Institute Inc., Cary, North Carolina, USA, 2018.
Shigaki, F., Sharpley, A., and Prochnow, L. I.: Rainfall intensity and phosphorus source effects on phosphorus transport in surface runoff from soil trays, Sci. Total Environ., 373, 334–343, https://doi.org/10.1016/j.scitotenv.2006.10.048, 2007.
Silva, A. M. F., de Menezes, E. F., de Souza, E. B., de Melo, N. F., and Mariano, R.: Survival of Xanthomonas campestris pv. viticola in infected tissues of grapevine, Rev. Brasil. Fruticult., 34, 757–765, https://doi.org/10.1590/S0100-29452012000300015, 2012.
Singh, J. P., Karamanos, R. E., and Stewart, J. W. B.: The mechanism of phosphorus-induced zinc deficiency in bean (Phaseolus vulgaris L.), Can. J. Soil Sci., 68, 345–358, https://doi.org/10.4141/cjss88-032, 1988.
Soltangheisi, A., Ishak, C. F., Musa, H. M., Zakikhani, H., and Rahman, Z. A.: Phosphorus and zinc uptake and their interaction effect on dry matter and chlorophyll content of sweet corn (Zea mays var. saccharata), J. Agron., 12, 187–192, https://doi.org/10.3923/ja.2013.187.192, 2013.
Spann, T. M. and Schumann, A. W.: The role of plant nutrients in disease development with emphasis on citrus and huanglongbing, in: Proceedings of the Florida State Horticultural Society, 122, 169–171, 2009.
Tadesse, M., Bobosha, K., Diro, M., and Gizachew, W. M.: Enset bacterial wilt sanitary control in Gurage zone. Research Report No. 53, Ethiopian Agricultural Research Organization, Ethiopia, 23, 2003.
Tamire, C. and Argaw, M.: Role of Enset (Ensete ventricosum (Welw.) Cheesman) in soil rehabilitation in different agro-ecological zones of Hadiya, Southern Ethiopia, Am. J. Environ. Protect., 4, 285–291, https://doi.org/10.11648/j.ajep.20150406.14, 2015.
Tefera, M., Chernet, T., and Haro, W.: Explanation of the geological map of Ethiopia, Geological Survey Ethiopia, Addis Ababa, Ethiopia, 79, 1996.
Tensaye, A. W., Lindén, B., and Ohlander, L.: Enset farming in Ethiopia: Soil nutrient status in Shoa and Sidamo regions, Commun. Soil Sci. Plant Anal., 29, 193–210, https://doi.org/10.1080/00103629809369938, 1998.
Tesfaye, B. and Lüdders, P.: Diversity and distribution patterns of enset landraces in Sidama, Southern Ethiopia, Genetic Resour. Crop Evol., 50, 359–371, https://doi.org/10.1023/A:1023918919227, 2003.
Thongbai, P., Hannam, R. J., Graham, R. D., and Webb, M. J.: Interaction between zinc nutritional status of cereals and Rhizoctonia root rot severity, Plant Soil, 153, 207–214, https://doi.org/10.1007/BF00012994, 1993.
Tsegaye, A. and Struik, P. C.: Influence of repetitive transplanting and leaf pruning on dry matter and food production of enset (Ensete ventricosum Welw.(Cheesman)), Field Crops Res., 68, 61–74, https://doi.org/10.1016/S0378-4290(00)00111-8, 2000.
Tsegaye, A. and Struik, P. C.: Enset (Ensete ventricosum (Welw.) Cheesman) kocho yield under different crop establishment methods as compared to yields of other carbohydrate-rich food crops, Netherlands J. Agric. Sci., 49, 81–94, https://doi.org/10.1016/S1573-5214(01)80017-8, 2001.
Tsegaye, A. and Struik, P. C.: Analysis of enset (Ensete ventricosum) indigenous production methods and farm-based biodiversity in major enset-growing regions of southern Ethiopia, Exp. Agric., 38, 291–315, https://doi.org/10.1017/S0014479702003046, 2002.
Turner, D. W. and Barkus, B.: Nutrient concentrations in a range of banana varieties grown in the subtropics, Fruits, 36, 217–222, 1981.
Uloro, Y. and Mengel, K.: Response of ensete (Ensete ventricosum W.) to mineral fertilizers in southwest Ethiopia, Fertil. Res., 37, 107–113, https://doi.org/10.1007/BF00748551, 1994.
Vancampenhout, K., Nyssen, J., Gebremichael, D., Deckers, J., Poesen, J., Haile, M., and Moeyersons, J.: Stone bunds for soil conservation in the northern Ethiopian highlands: Impacts on soil fertility and crop yield, Soil Tillage Res., 90, 1–15, https://doi.org/10.1016/j.still.2005.08.004, 2006.
Whalen, J. K., Chang, C., Clayton, G. W., and Carefoot, J. P.: Cattle manure amendments can increase the pH of acid soils, Soil Sci. Soc. Am. J., 64, 962–966, https://doi.org/10.2136/sssaj2000.643962x, 2000.
Winge, R. K., Peterson, V. J., and Fassel, V. A.: Inductively coupled plasma-atomic emission spectroscopy: prominent lines, Appl. Spectrosc., 33, 206–219, https://doi.org/10.1366/0003702794925895, 1979.
Welde-Michael, G., Bobosha, K., Blomme, G., Addis, T., Mengesha, T., and Mekonnen, S.: Evaluation of enset clones against enset bacterial wilt, Afr. Crop Sci. J., 16, 89–95, https://doi.org/10.4314/acsj.v16i1.54348, 2008a.
Welde-Michael, G., Bobosha, K., Addis, T., Blomme, G., Mekonnen, S., and Mengesha, T.: Mechanical transmission and survival of bacterial wilt on enset, Afr. Crop Sci. J., 16, 97–102, https://doi.org/10.4314/acsj.v16i1.54349, 2008b.
Wolde, M., Ayalew, A., and Chala, A.: Assessment of bacterial wilt (Xanthomonas campestris pv. musacearum) of enset in southern Ethiopia, Afr. J. Agric. Res., 11, 1724–1733, https://doi.org/10.5897/AJAR2015.9959, 2016.
Wichuk, K. M., Tewari, J. P., and McCartney, D.: Plant pathogen eradication during composting: a literature review, Compost Sci. Util., 19, 244–266, https://doi.org/10.1080/1065657X.2011.10737008, 2011.
Yemataw, Z., Mohamed, H., Diro, M., Addis, T., and Blomme, G.: Enset (Ensete ventricosum) clone selection by farmers and their cultural practices in southern Ethiopia, Genet. Resour. Crop Evol., 61, 1091–1104, https://doi.org/10.1007/s10722-014-0093-6, 2014.
Yemataw, Z., Tesfaye, K., Zeberga, A., and Blomme, G.: Exploiting indigenous knowledge of subsistence farmers for the management and conservation of Enset (Ensete ventricosum (Welw.) cheesman) (Musaceae family) diversity on-farm, J. Ethnobiol. Ethnomed., 12, 1–25, https://doi.org/10.1186/s13002-016-0109-8, 2016.
Yemataw, Z., Mekonen, A., Chala, A., Tesfaye, K., Mekonen, K., Studholme, D. J., and Sharma, K.: Farmers' knowledge and perception of enset Xanthomonas wilt in southern Ethiopia, Agric. Food Secur., 6, 1–12, https://doi.org/10.1186/s40066-017-0146-0, 2017.
Yirgou, D. and Bradbury, J. F.: Bacterial wilt of Enset (Ensete ventricosum) incited by Xanthomonas musacearum sp., Phytopathology, 58, 111–112, 1968.
Zelleke, G., Getachew, A., Abera, D., and Rashid, S.: Fertilizer and soil fertility potential in Ethiopia: Constraints and opportunities for enhancing the system, IFPRI, Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia, 63, 2010.
Zerfu, A., Gebre, S. L., Berecha, G., and Getahun, K.: Assessment of spatial distribution of enset plant diversity and enset bacteria wilt using geostatistical techniques in Yem special district, Southern Ethiopia, Environ. Syst. Res., 7, 1–13, https://doi.org/10.1186/s40068-018-0126-9, 2018.
Zewdie, S., Fetene, M., and Olsson, M.: Fine root vertical distribution and temporal dynamics in mature stands of two enset (Ensete ventricosum Welw Cheesman) clones, Plant Soil, 305, 227–236, https://doi.org/10.1007/s11104-008-9554-z, 2008.
tree against hunger, enset (Ensete ventricosum) is an important multipurpose crop for the farming systems of the densely populated Gamo highlands in Ethiopia. Its high productivity and tolerance to droughts are major assets. Nevertheless, enset production is severely threatened by a wilting disease. This observational study aims to assess soil and leaf nutrients in enset gardens at different altitudes to see if fertility management can be linked to disease prevalence.
tree against hunger, enset (Ensete ventricosum) is an important multipurpose crop...