Introduction
It is estimated that 1% of the European Union population suffers from rheumatoid arthritis (RA), which gives approximately 5 million patients. Despite increasingly effective therapies, RA not only often leads to disability, but also shortens the life expectancy of patients by about 7 years [1].
An important cause of this unfavorable prognosis is accelerated atherosclerosis and cardiovascular complications [2]. Myocardial infarction or stroke is estimated to occur in patients with RA twice as often as in the population without RA. The incidence of some traditional coronary factors in patients with RA is increased [3].
Physical activity is low due to pain and impaired joint mobility. In some studies, a slightly increased incidence of hypertension was also found. This does not fully explain the increased incidence of cardiovascular disease (CVD). In 15–20% of patients with normal traditional cardiovascular risk factors (also with normal cholesterol levels and in non-smokers) early heart attacks and strokes occur. It is believed that the cause of their occurrence is chronic inflammation, which is characterized by RA [2].
In patients with this disease we observe a high concentration of C-reactive protein (CRP), a number of proinflammatory cytokines such as IL-1, IL-6, IL-18, and tumor necrosis factor (TNF) [4]. An increase in these factors has also been observed in many CVDs (hypertension, coronary heart disease [CHD], heart failure), and it has been shown to accelerate the atherosclerotic process [5].
The long-term use of nonsteroidal anti-inflammatory drugs and steroids increases the incidence of heart disease, while methotrexate and biological therapy significantly reduce the incidence of these diseases [2, 6]. However, in some countries, biological treatment is not available to many patients with RA. There are also publications indicating that such treatment promotes the development of arrhythmia [6, 7].
Methotrexate therapy is often discontinued due to side effects. In the first year of using this drug, 10–30% of patients abandon this therapy, complaining about numerous side effects [8, 9]. These situations cause that chronic inflammation is still conductive to the occurrence of CVD, which are still the main cause of premature death in patients with RA [2].
Recently another factor contributing to accelerated atherosclerosis in patients with RA has been pointed out. It is the increased concentration of homocysteine (HCY) in the blood serum [10, 11].
Metabolism of homocysteine
Homocysteine is formed in the body through the demethylation of methionine, in which the donor of the methyl group is a folic acid (FA) derivative – N5-methyl-tetrahydrofolate. The cofactor in this reaction is vitamin B12. In the liver, the reaction of HCY remethylation takes place with the participation of methyl groups, donated by betaine.
Another route of HCY metabolism is transsulfuration in the presence of a cofactor of vitamin B6 [12]. Many studies have shown that elevated levels of HCY in the serum disturb endothelial function by reducing nitric oxide synthesis [13, 14].
Moreover, HCY is considered to reduce the activity of the enzyme that decomposes the asymmetric dimethylarginine [15]. The increased concentration of the latter compound is already observed in the early stages of atherosclerosis.
According to many authors, HCY may bind with proteins, which in consequence changes their structure and biochemical properties, which increases their susceptibility to oxidation (so-called protein homocysteinylation). Proteins thus modified can become an autoantigen and stimulate a humoral immune response [16].
Homocysteine enhances the synthesis of free radicals, which leads to further endothelial impairment and increases the synthesis of vasoconstrictive thromboxane [17]. Also HCY accelerates atherosclerosis through increased expression of adhesion molecules and through increased monocyte proliferation intensifies inflammation.
It also enhances IL-6 and IL-8 production in synoviocytes in patients with RA, so it may contribute to joint damage [18]. Finally, HCY has a prothrombotic effect [19] increasing the incidence of arterial and venous thrombosis.
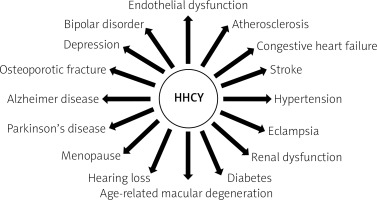
Currently, it is indicated that hyperhomocysteinemia (HHCY) is a risk factor for the development of complications both from the cardiovascular system and from the areas of neurology, psychiatry and bone metabolism (Fig. 1).
A lot of clinical trials have confirmed the influence of HHCY on the development of atherosclerotic cardiovascular disease. It has been shown during seven years of observation that in the general population, an increased HCY level in serum increases the risk of stroke by 24% and myocardial infarction by 15%.
According to Alomari et al. [20], an increase of HCY concentration in serum by 5 µmol/l doubles the risk of CVD. This is confirmed by two meta-analyses published in 2002 [21, 22]. The relationship between HHCY and cardiovascular complications is independent of other interfering factors.
According to Waśkiewicz et al. [23], HCY concentration in blood already above 10.5 µmol/l is associated in Poland with a significant increase in mortality. In most studies, it is believed that HHCY can be diagnosed when the serum HCY concentration exceeds 15 µmol/l.
Increased serum HCY levels are found in individuals with decreased serum folate levels, in persons carrying the TT genotype in the MTHFR (methylenetetrahydrofolate reductase) gene, in patients with diabetes, renal failure, RA, as well as the elderly, or after a stroke [24].
Folic acid in treatment of stroke
Since the beginning of this century, attempts have been made to reduce the adverse effect of HHCY on CVD by administering group B vitamins (FA, betaine, vitamin B6, vitamin B12), which significantly reduce the concentration of this compound [25].
However, the interest in this treatment quickly ended, as the first randomized studies did not show any reduction in cardiovascular complications despite a 25% decrease in serum HCY levels. Thus, the NORVIT (The Norwegian Vitamin Trial) study of 3000 patients was discontinued in 2006, because administration of FA at a dose of 0.8 mg/day and vitamin B12 at 0.4 mg/day did not reduce the incidence of cardiovascular complications [26].
In a further VITATOPS (the VITAmins TO Prevent Stroke) study involving 8,000 patients, the complex endpoint of the study was defined as stroke, heart attack, or death from vascular causes. After three years of vitamin B12 therapy at a dose of 0.5 mg/day and 2 mg of FA/day, no significant reduction in cardiovascular complications was observed (15% in the group treated with group B vitamins and 17% in the group treated with placebo) [27]. In later studies, however, it was noted that the administration of FA (alone or with other group B vitamins) significantly reduces the incidence of stroke, although it does not reduce the incidence of heart attacks.
A breakthrough was the Chinese work of Huo et al. [28], who showed in a very large group of participants that 0.8 mg of FA administered in primary prevention reduces the incidence of stroke. This study, with the acronym CSPPT (China Stroke Primary Prevention Trial) involving 20,000 patients with hypertension, but without stroke and CVD in their medical history, showed that the administration of enalapril with FA to patients with hypertension significantly reduced the risk of ischemic stroke in comparison to patients treated with enalapril only.
However, the administration of FA did not affect the risk of myocardial infarction. The greatest benefit was obtained by patients who initially showed a high level of HCY in the serum. In the subanalysis of this study, it was noted that the addition of FA in subjects with high serum cholesterol levels reduced the risk of stroke by up to 31%, while the addition of FA in people with low cholesterol levels had only a slight impact on reducing the risk of stroke [29].
In 2016, a meta-analysis by Li et al. [30] was published, which included 30 randomized studies and showed that FA administration in fact substantially reduces the incidence of stroke by 10%, CVD by 4%, and does not significantly affect the incidence of myocardial infarction.
In the following years, several large meta-analyses were published, including many randomized studies, in which the statistically significant 10–15% reduction of ischemic stroke following FA treatment was confirmed again, while the risk of myocardial infarction did not change.
Also a meta-analysis by Martí-Carvajal et al. [31] from 2017 including 15 randomized analyses published in the Cochrane Library and covering more than 4,400 patients with hypertension showed that brain stroke prevention with group B vitamin is effective and the risk of this disease is significantly reduced by 10%.
In the same year, a meta-analysis by Wang et al. [32] was published, which compared stroke risk reduction in patients with hypertension treated not only with enalapril but also with other blood pressure-lowering medicines (calcium antagonists, benazepril) or treated with FA and the above mentioned hypotensive drugs. The results of this meta-analysis showed that FA addition to drugs lowering blood pressure significantly reduces the risk of cardiovascular complications by 12.9%. This positive effect was observed in those patients who received FA for more than 12 weeks and the blood HCY concentration decreased by at least 25%.
The meta-analysis of Tian et al. [33] is noteworthy as it is focused on the effect of FA on stroke reduction in patients already burdened with CVD. The administration of FA significantly reduced the number of strokes by 10%. Patients with HCY concentration reduced by more than 25% (15% stroke reduction), patients with low (less than 2 milligrams) intake of folates in food (22% stroke reduction) and those in countries with no additional folates in food (13% stroke reduction) benefited more.
This meta-analysis indicates that FA supplementation is also effective for stroke prevention in patients with CVD. In the last meta-analysis of Hsu et al. [34] the administration of FA with a low dose of vitamin B12 (< 0.05 mg/day) reduced the number of strokes by 25%, while increasing the dose of vitamin B12 to > 0.4 mg/day canceled the beneficial effect of FA.
In the biggest meta-analysis of Wang et al. [35] from 2019, taking into account 12 randomized controlled trials, which involved 47,523 participants, it was found that FA supplementation reduced the risk of stroke by 15% in patients with CVD, but it did not have an impact on either all-cause mortality or cardiovascular mortality and coronary heart disease.
It should be emphasized that all the meta-analyses and studies presented above concerned patients in countries where FA is not added to food. For 100 years, FA has been added in the amount of 140 µg/100 γ of cereal grains in the United States, Canada, and Australia. Already in 2006, Yang et al. [36] demonstrated that in countries with mandatory FA supplementation, stroke-related mortality has significantly decreased.
It should also be noted that most of the studies presented above were concerned about the primary prevention of stroke. The authors of most of the cited studies emphasize that FA reduces the risk of stroke, whereas in the case of CHD the beneficial effect is insignificant or nonexistent. The most frequent interpretation in the literature assumes that FA mainly reduces atherosclerotic changes in small blood vessels (brain, kidneys, retina), while it has a minimal impact on the development of atherosclerosis in large vessels, e.g. in coronary vessels.
Stroke in rheumatoid arthritis
In Tanaka’s et al. [37] epidemiological study, stroke is 68% more common in patients with RA than in the general population. Similar conclusions can be found in the study by Wiseman et al. [38]. According to these authors, in patients with RA stroke is 64% more frequent than in the control group (without RA). Hemorrhagic stroke is 68% more frequent and in total strokes in patients with RA under 50 years of age are 79% more frequent [38].
It has also been demonstrated that the use of non-selective nonsteroidal anti-inflammatory drugs also increases the incidence of stroke by 39% [39]. In patients with RA recurrent strokes are also more frequent.
In a large Chen et al. [40] study involving 3,190 patients after a stroke or transient ischemic attack, 638 patients were also diagnosed with RA and 2,552 strokes were reported in patients without RA. In patients with no RA, the quoted authors showed significantly less frequent recurrence of strokes or transient ischemic attacks.
The influence of age on the occurrence of strokes in patients with RA was studied by Tiosano et al. [41]. Their study included more than 11,000 patients with RA and more than 57,000 age- and gender-matched control subjects. Patients were divided according to their age above and below 65 years of age. In patients with RA below 65 years of age, strokes were more frequent (3.74% vs. 2.20%; p < 0.001) than in the control group [41].
Homocysteine in rheumatoid arthritis
A number of factors can lead to an increased incidence of stroke in patients with RA. These include hypertension, chronic inflammation, high cholesterol level, and decreased physical activity caused by changes in joints. These factors also include an increased concentration of homocysteine in the blood serum, which accelerates the atherosclerotic and thrombotic process.
Increased serum HCY levels in patients with RA, in comparison to the general population, have been reported by many authors [42–44].
According to Obradović-Tomasević et al. [45], excessively high levels of HCY occur in 39%, and according to Bouchti et al. [46] in 20–40% of patients with RA. One of the reasons for this elevated HCY concentration is that the MTHFR TT gene polymorphism is more prevalent in these patients than in the general population [44]. Homocysteine levels are also higher in patients treated with methotrexate. It has also been shown that HHCY in patients with RA significantly increases the risk of CVD [46]. High levels of HCY in serum are also important prognostic factors.
After 6.5 years of observation Berglund et al. [47] already noticed significantly more frequent CVD in patients with elevated serum HCY levels than in patients with a normal concentration of this compound. The obtained correlations were independent of the age and gender of the subjects.
The analysis by Cisternas et al. [48] from 2002 showed that there were higher HCY levels in RA patients with a history of CVD than in those without. In another study published by Chung et al. [49], it was found that in unadjusted comparisons, HHCY was more common in RA patients with coronary artery calcification than in those without.
Considering the more frequent occurrence of HHCY and the higher incidence of stroke in patients with RA, the use of FA should be considered in the primary prevention of this disease. This is even more justified, as strokes are more common in young patients with RA, and FA most effectively reduces the risk of strokes as the primary prevention in patients with elevated HCY levels.
Moreover, it has been shown that FA reduces insulin resistance, reduces oxidative stress, and improves endothelial function [50–52]. In a meta-analysis from 2019, Fatahi et al. [50] demonstrated that FA slightly, but statistically highly significantly reduces CRP levels. This reduction of CRP in serum is slightly larger in women and diabetic patients. So far, the concentration of CRP after FA administration in patients with RA has not been studied. The use of FA is safe, but it is important to remember that administering this drug to patients with vitamin B12 deficiency may alleviate megaloblastic anemia, but at the same time, it may intensify brain changes caused by a lack of vitamin B12.
There are some studies suggesting that FA supplementation increases the risk of developing cancer because of folate’s role in de novo biosynthesis of purine and thymine nucleotides. In Baggott’s [53] meta-analysis, it was found that cancer incidence rates were higher in the FA-fortification groups than the non-FA-supplemented groups.
According to a meta-analysis conducted by Vollset et al. [54] the doses of FA used in this trial were higher than those from fortification. This study showed that FA supplementation does not substantially increase or decrease the incidence of cancer. In another Chinese study, the results were similar. A trial covering more than 20,000 hypertensive adults without stroke or myocardial infarction (MI) in their medical history showed that there is no evidence that 0.8 mg daily FA supplementation can increase the risk of cancer [55]. Additionally, this study suggested a protective effect FA in subjects with MTHFR TT genotype and low folate levels.
Methotrexate and folic acid
The use of FA in patients treated with methotrexate also needs to be discussed. In 2004 Hornung et al. [56] already recommended the use of small doses of FA to reduce the adverse effects associated with methotrexate therapy. It is known that the use of this medicine increases the concentration of HCY by disturbing the synthesis of FA.
In 2013 a large meta-analysis was published, assessing the effect of FA on the reduction of methotrexate side effects [57]. According to this meta-analysis, FA administration substantially reduces nausea, vomiting, and abdominal pain in patients treated with methotrexate, prevents the increase of transaminases and significantly reduces the need to discontinue further treatment with this drug [57].
According to Koh et al. [58], a dose of both 5 and 30 mg of FA, within a week has the same protective effect against the occurrence of toxic symptoms caused by methotrexate therapy. A high level of HCY in the blood may, according to the opinion of Chaabane et al. [59], be a useful marker of the toxic effects of this drug.
The use of FA in patients treated with methotrexate may additionally protect them from a stroke. It was observed that the combination of methotrexate and FA resulted in reduced mortality hazard ratio of 0.2, compared with 0.5 for methotrexate alone in patients with rheumatoid arthritis.
Stroke in rheumatoid arthritis and folic acid
Given the more frequent occurrence of serious CVD and cerebral strokes at an already younger age in patients with RA, it is necessary to implement early and effective prevention in the form of smoking cessation, lowering blood cholesterol levels and maintaining normal blood pressure values. Frequent occurrence of elevated HCY concentration, which is conducive to, among other things, increased risk of stroke, indicates the usefulness of determining the serum level of HCY. This study is not only prognostic but also by administering low doses of FA (0.8 mg/day) it can protect 10–15% of patients from stroke.
It is confirmed by the results of a study among residents of the United States conducted by Sonawane et al. [60], published in 2019. These researchers compared the incidence of CVD in patients with RA above 20 years of age, depending on the use of FA. One group of patients received FA for more than 6 months, because of side effects caused by drugs modifying the course of RA (DMARD). The second group of patients, despite using the same DMARD drugs, was not additionally supplemented with FA.
In patients from the first group, a statistically significant 15% decrease in the frequency of CVD was observed, in comparison with patients treated only with DMARD. The authors of this study did not analyze to what extent the reduction of CVD concerned only stroke. In clinical practice, such treatment should be considered in patients with RA for primary stroke prevention and secondary prevention in patients who do not tolerate, or for other reasons do not use, aspirin.
However, large-scale and long-term homocysteine-lowering clinical trials with foliate are warranted to definitely confirm the effectiveness of this treatment.
Conclusions
Therapy with FA (lowering the concentration of HCY in the blood serum) reduces the incidence of stroke. The concentration of HCY in the blood serum of patients with RA is higher than in the general population.
Strokes in patients with RA are more common than in the general population. More frequent prophylactic use of folic acid in patients with RA may significantly reduce the incidence of stroke.