Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CARDIOLOGY / EXPERIMENTAL RESEARCH
miR-29a activates expression of α-cardiac myosin heavy chain via β1 thyroid hormone receptor
in neonatal rats
1
Departments of Pediatrics, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, China
Submission date: 2019-09-30
Final revision date: 2019-11-09
Acceptance date: 2019-11-24
Online publication date: 2020-01-08
Publication date: 2024-04-23
Arch Med Sci 2024;20(2):641-654
KEYWORDS
TOPICS
ABSTRACT
Introduction:
MicroRNAs (miRs) are small noncoding RNAs which are regulators of gene expression and also regulate the genes in heart tissues. The aim of the study was to evaluate the effect of miRs on the expression level of myosin heavy chain (MHC), which is responsible for regulation of cardiac functions in neonatal rat ventricular myocytes and mice.
Material and methods:
The miRs were suppressed in neonatal rat ventricular myocytes using small interfering RNAs (siRNAs) against Dicer followed by evaluation of MHC levels. For in vivo study the C57 black/6 Jacksonian mice were subjected to the transverse aortic constriction (TAC) procedure.
Results:
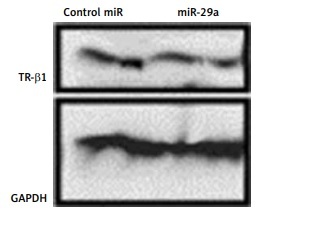
The Dicer siRNA suppressed the endogenous miRs and the α-MHC gene but failed to down-regulate the β-MHC. Among the 17 selected miRs, miR-29a was found to up-regulate the α-MHC gene significantly but not β-MHC. The expression of α-MHC was suppressed by silencing the expression of miR-29a. Bioinformatics study done by TargetScan suggested thyroid hormone receptor-β1 (TR-β1) as a potential target of miR-29a. Additionally, miR-29a was found to regulate the expression of α-MHC via TR-β1 signaling.
Conclusions:
The findings of the present study indicated that miR-29a modulates expression of α-the MHC gene by targeting TR-β1 in cardiac cells. The study may provide a new direction for treating cardiac failure and cardiac hypertrophy.
MicroRNAs (miRs) are small noncoding RNAs which are regulators of gene expression and also regulate the genes in heart tissues. The aim of the study was to evaluate the effect of miRs on the expression level of myosin heavy chain (MHC), which is responsible for regulation of cardiac functions in neonatal rat ventricular myocytes and mice.
Material and methods:
The miRs were suppressed in neonatal rat ventricular myocytes using small interfering RNAs (siRNAs) against Dicer followed by evaluation of MHC levels. For in vivo study the C57 black/6 Jacksonian mice were subjected to the transverse aortic constriction (TAC) procedure.
Results:
The Dicer siRNA suppressed the endogenous miRs and the α-MHC gene but failed to down-regulate the β-MHC. Among the 17 selected miRs, miR-29a was found to up-regulate the α-MHC gene significantly but not β-MHC. The expression of α-MHC was suppressed by silencing the expression of miR-29a. Bioinformatics study done by TargetScan suggested thyroid hormone receptor-β1 (TR-β1) as a potential target of miR-29a. Additionally, miR-29a was found to regulate the expression of α-MHC via TR-β1 signaling.
Conclusions:
The findings of the present study indicated that miR-29a modulates expression of α-the MHC gene by targeting TR-β1 in cardiac cells. The study may provide a new direction for treating cardiac failure and cardiac hypertrophy.
REFERENCES (34)
1.
Szemraj-Rogucka Z, Szemraj J, Masiarek K, Majos A. Circulating microRNAs as biomarkers for myocardial fibrosis in patients with left ventricular non-compaction cardiomyopathy. Arch Med Sci 2019; 15: 376-84.
2.
Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 2008; 27: 5959-74.
3.
van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007; 316: 575-9.
4.
Chen JF, Murchison EP, Tang R, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA 2008; 105: 2111-6.
5.
da Costa Martins PA, Bourajjaj M, Gladka M, et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 2008; 118: 1567-76.
6.
Cheng Y, Ji R, Yue J, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol 2007; 170: 1831-40.
7.
Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. Micro-RNAs play an essential role in the development of cardiac hypertrophy. Circ Res 2007; 100: 416-24.
8.
Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardiol 2008; 45: 185-92.
9.
Tatsuguchi M, Seok HY, Callis TE, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol 2007; 42: 1137-41.
10.
Thum T, Galuppo P, Wolf C, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 2007; 116: 258-67.
11.
Zhao Y, Ransom JF, Li A, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 2007; 129: 303-17.
12.
Liu N, Bezprozvannaya S, Williams AH, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 2008; 22: 3242-54.
13.
Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol 1996; 12: 417-39.
14.
Callis TE, Pandya K, Seok HY, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 2009; 119: 2772-86.
15.
Ojamaa K, Klemperer JD, MacGilvray SS, Klein I, Samarel A. Thyroid hormone and hemodynamic regulation of beta-myosin heavy chain promoter in the heart. Endocrinology 1996; 137: 802-8.
16.
Galli E, Pingitore A, Iervasi G. The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev 2010; 15: 155-69.
17.
Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev 2010; 15: 125-32.
18.
Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res 2008; 103: 1072-83.
19.
Hasegawa K, Meyers MB, Kitsis RN. Transcriptional coactivator p300 stimulates cell type-specific gene expression in cardiac myocytes. J Biol Chem 1997; 272: 20049-54.
20.
Rockman HA, Ross RS, Harris AN, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA 1991; 88: 8277-81.
21.
Pall GS, Codony-Servat C, Byrne J, Ritchie L, Hamilton A. Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by Northern blot. Nucleic Acids Res 2007; 35: e60.
22.
Takaya T, Ono K, Kawamura T, et al. MicroRNA-1 and microRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circ J 2009; 73: 1492-7.
23.
Carè A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med 2007; 13: 613-8.
24.
Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 2007; 4: 721-6.
25.
Ono K, Iwanaga Y, Horie T, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem 2010; 285: 4920-30.
26.
Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol 2007; 43: 388-403.
27.
Kinugawa K, Yonekura K, Ribeiro RC, et al. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res2001; 89: 591-8.
28.
Danzi S, Ojamaa K, Klein I. Triiodothyronine-mediated myosin heavy chain gene transcription in the heart. Am J Physiol Heart Circ Physiol 2003; 284: H2255-62.
29.
Chen J, Murchison EP, Tang R, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA 2008; 105: 2111-6.
30.
Qi M, Ojamaa K, Eleftheriades EG, Klein I, Samarel AM. Regulation of rat ventricular myosin heavy chain expression by serum and contractile activity. Am J Physiol 1994; 267: C520-8.
31.
Kinugawa K, Minobe WA, Wood WM, et al. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation 2001; 103: 1089-94.
32.
Sassi Y, Avramopoulos P, Ramanujam D, et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun 2017; 8: 1614.
33.
Gloss B, Trost S, Bluhm W, et al. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor alpha or beta. Endocrinology 2001; 142: 544-50.
34.
Swanson EA, Gloss B, Belke DD, Kaneshige M, Cheng SY, Dillmann WH. Cardiac expression and function of thyroid hormone receptor beta and its PV mutant. Endocrinology 2003; 144: 4820-5.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.