Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune/inflammatory disease that is characterized by synovial hyperplasia and vascularization, and progressive destruction of cartilage and bone [1]. Activated T cells, predominantly CD4+ T cells infiltrating the synovium, are responsible for these RA-associated changes, and this is the reason why RA is considered as a Th-1-associated disease. T cells’ activation, their migration as well as production of several inflammatory cytokines such as interleukin 2 (IL-2), IL-6, IL-16, IL-17, interferon (IFN-γ) and tumor necrosis factor (TNF-α) are important for the initiation and perpetuation of RA-related pathology [2, 3].
The proinflammatory cytokine IL-16 is a lymphocyte chemoattractant factor secreted by CD4+, CD8+ T lymphocytes, monocytes, epithelial cells and fibroblasts [3]. After binding to its receptor, IL-16 stimulates CD4+ T cell and monocyte migration to inflamed sites and also promotes proliferation of these cells [4]. IL-16 is generally considered to play a decisive role in both immune and inflammatory responses. This cytokine plays a special role not only in the development of arthritis but also systemic RA manifestations; it also participates in the transformation of acute inflammation into a chronic process [5, 6].
IL-17 inflammatory cytokine plays a key role in the propagation of joint inflammation, cartilage destruction, and bone erosion [7, 8]. It promotes recruitment of both neutrophils and monocytes by means of inducing various chemokines and is associated with severity of synovial inflammation of RA patients. Cho et al. [9] reported that IL-17 can induce the production of IL-16 in RA patients.
MicroRNAs (miRNAs) are an abundant class of evolutionarily conserved, single-stranded RNA molecules that have emerged as important posttranscriptional regulators of gene expression [10]. Gene expression by miRNAs has recently been identified to be important for basic function of the immune system [10, 11]. Moreover, miRNAs contribute to the terminal differentiation of mature lymphocytes [12]. Many miRNAs are expressed in a tissue-specific manner, suggesting a role of the miRNAs in the specification of the tissue during differentiation. miR-206, a vertebrate-specific miRNA, is called a skeletal muscle specific myomiR [12, 13]. It shares the same seed sequence and at least some targets with miR-1, which also belongs to the myomiR family along with miR-133a, miR-133b, miR-208a, miR-208b and miR-499 [14]. miR-206 is unique among the myomiR family in that it is specifically expressed in skeletal muscle, being absent or expressed at relatively low levels in other tissues. It is being intensively studied in several conditions such as skeletal muscle development, heart failure [14], various types of cancers and other diseases.
A positive correlation in expression of miR-133b/miR-206 and IL-17 was found. miR-206 and miR-133b are co-transcribed together with IL-17 when IL-23 is used, which is specific for T-lymphocytes since these miRNAs were clustered nearly 45 kb upstream of the IL-17a/f locus. It appears that miR-206 regulation of IL-17 is context dependent [15]. Proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-17 tend to be osteoclastogenic. Knowing that altered miR-206 expression has been observed in several muscle disorders, we postulated that miRNAs might participate with some inflammatory cytokines in the pathogenesis of RA. To address this hypothesis, we studied the correlation between miR-206 expression and other circulatory levels of IL-16 and IL-17 proinflammatory cytokines in RA patients.
Material and methods
Thirty patients with RA diagnosed according to current American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria were included in the study. Demographic and clinical characteristics were collected through a structured interview and physical examination. Patients with infection, malignancy as well as with other co-existing autoimmune diseases were excluded from this study.
Disease activity was assessed using the Disease Activity Score 28 (DAS28). Laboratory tests such as rheumatoid factor (RF) and the erythrocyte sedimentation rate (ESR) and other routine biochemistry tests were performed. Functional ability was assessed using the modified version of the Health Assessment Questionnaire (mHAQ) [16]. Radiographs of the hands and feet with the evaluation of erosive disease using scoring based on the Sharp score (ShS) was done by standard radiographs for all studied patients. At least one definite erosive change on any of the hands or feet radiographs was sufficient for inclusion of such a patient in the “erosive” group. Twenty healthy subjects with no previous history of autoimmune disease were included in the control group. The present study was performed according to the Ministry of Health, Health and Human Ethical Clearance Committee guidelines for Clinical Research and with approval of Cairo University local Ethics Committee. From all study subjects (patients and healthy controls) written consent was obtained.
Measurement of miR-206 by quantitative real-time RT-PCR (qRT-PCR)
Samples of 5 ml of blood were withdrawn in ethylene diamine tetra acetic acid (EDTA) sterile tubes. Isolation of peripheral blood mononuclear cells (PBMCs) by Ficoll-Hypaque density gradient centrifugation, isolation of total RNA from freshly obtained PBMCs using the TRIzol isolation protocol (Thermo Fisher Scientific, Waltham, MA, USA) and qRT-PCR for the detection of Hs_miR-206 that was carried out in 25 μl PCR reactions using the miScript SYBR Green PCR Kit and miScript Primer Assays (Qiagen) were performed as previously described [17].
Detection of circulating interleukin 16 and 17
Total concentrations of IL-16 and IL-17A were detected using commercial enzyme linked immunosorbent assay (ELISA) kits (R&D Systems Inc., Minneapolis, MN) according to the manufacturer’s recommendations. Absorbencies were measured at 450 nm using an ELISA plate reader (Sunrise, Tecan Group Ltd.). The ELISA reader-controlling software (Magellan 71, Tecan Group Ltd.) processes the digital data of raw absorbance values into a standard curve from which cytokine concentrations of unknown samples can be derived directly. Results – cytokine concentration were expressed in picograms per milliliter (pg/ml).
Statistical analysis
All statistical analyses were performed using SPSS version 19 (SPSS, Inc., Chicago, IL). Data were statistically described in terms of mean ±standard deviation (±SD), and frequencies when appropriate. For the non-parametric tests the Spearman rank correlation was used, Student’s t-test was used for comparison of numerical variables between the study groups for quantitative variables and also the one-way ANOVA test was used; p-value < 0.05 was considered as significant.
Results
Demographic and laboratory characteristics of RA patients and control group
The 30 RA patients included 27 female and 3 males, F/M ratio 9 : 1 with age from 22 to 62 years (mean age 39.10 ±10.43 years). The patients’ age at the time of disease onset ranged from 18 to 60 years and disease duration ranged from 1 to 9 years. Healthy individuals in the control group were optimally matched in terms of gender and age (mean age of 28.28 ±8.77 years). The descriptive data of the patients and healthy control are shown in Table I. Patients were classified as having active (low, moderate or high activity) or inactive disease on the basis of the DAS28.
Table I
Demographic and laboratory characteristics of patients with rheumatoid arthritis (RA)
miR-206 expression levels in RA patients and control group
The data presented in Figure 1 demonstrate a significant increase (p < 0.001) in the fold change expression levels of miR-206 in PBMCs of RA patients compared with the control group. A receiver operating characteristic (ROC) curve (Fig. 2) was constructed for the studied markers to estimate the best cut-off which could improve the specificity and sensitivity of miR-206 to differentiate between the studied groups. The ROC curve for the RA group vs. the control group showed a cutoff value of 2.75, miR-206 with 70% sensitivity and 85% specificity, the area under the curve 0.802 (p < 0.001) with 95% confidence interval (CI): 0.676–0.927.
Fig. 1
Relative fold change expression levels of miR-206 in rheumatoid arthritis (RA) and normal controls.
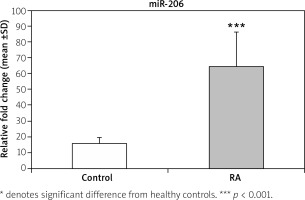
Fig. 2
Area under curve of receiver operating characteristic (ROC) of the miR-206 to discriminate RA patients from normal controls.
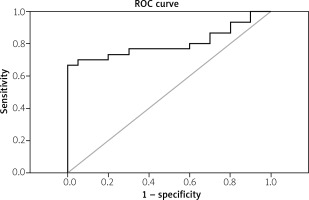
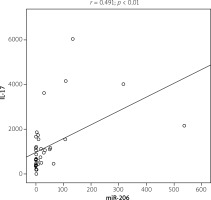
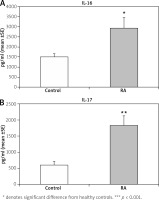
Figure 3 shows a significant increase of IL-16 (p < 0.05) and IL-17 (p < 0.01) serum level in RA patients compared with the control group. A positive correlation was found between miR-206 and IL-17 (r = 0.491; p < 0.01) (Fig. 4). No correlation was found between miR-206 and IL-16 concentration.
Fig. 3
Interleukin 16 and interleukin 17 concentration in rheumatoid arthritis (RA) and normal controls.
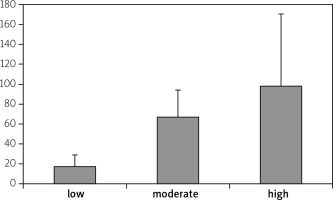
Disease activity of RA patients was classified into low, moderate and high depending on DAS28 values. There were no statistically significant correlation between high expression levels and low expression levels of miR-206 and disease activity, which is presented in Figure 5. There were no significant correlations between miR-206, IL-16 and IL-17 and clinical manifestation of RA.
Discussion
miRNAs are epigenetic regulatory mechanisms that impart broad and long-term changes in gene expression. They are able to modulate target gene translation via binding to the 3′-untranslated region (UTR) of miRNAs, thus affecting multiple protein-encoding genes at the posttranscriptional level, and they are implicated in the control of a wide range of biological functions [18, 19].
Documented data have shown that some miRNAs regulate bone homeostasis, including bone formation, resorption, remodeling, repair and bone-related diseases, by regulating the expression of certain cytokines and transcription factors [20]. miR-206 was originally found to be a specific miRNA for muscle differentiation under physiological conditions [21]. In muscular dystrophies, miR-206 is up regulated, as a form of “self-treating” significance. It has been recently discovered that miR-206 is also expressed in osteoblasts and its expression decreased during osteoblast differentiation. Overexpression of miR-206 inhibits osteoblast differentiation while the knockout of miR-206 promotes osteoblast differentiation [22]. miR-206 is referred to as a negative regulator of osteoblast differentiation [23]. Rheumatoid arthritis is a systemic, chronic inflammatory disease characterized by increase in bone resorption and impaired bone formation leading to joint destruction, deformity, and disability. Osteoblasts probably play a role in the pathogenesis of bone erosions in RA [24].
Patients with RA have alterations in their cellular miRNAs [25]. Dysregulation of miRNAs in effector cells in joint destruction such as PBMCs, T lymphocytes, synovial fibroblasts and osteoclasts was shown to contribute to inflammation, extracellular matrix degradation, and invasiveness of resident cells [26]. Concerning this idea, the role of miR-206 in regulation of osteoblast/osteoclast in RA as a bone-related disease needs to be fully elucidated; moreover there have been no documented articles that identify the role of miR-206 in RA patients till now.
In this study we used qRT-PCR to demonstrate the expression of miR-206 levels in PBMCs of RA patients compared with healthy controls. Our findings showed a highly significant increase in the expression levels of miR-206 in RA patients compared with the normal control group. Despite the absence of RA studies, there are some studies about miR-206 in other autoimmune diseases. In systemic lupus erythematosus (SLE) and dermatomyositis (DM) [27, 28], the expression of miR-206 in the PBMCs of patients was significantly decreased compared with that of healthy controls.
The osteoclast is a target of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-17 and is an effector in initiating bone destruction and joint erosion in RA. This suggests that osteoclastogenesis should be carefully controlled to maintain normal bone remodeling or to reduce the progression of ectopic bone resorption and erosion in RA. Since the first demonstration that IL-17 is crucially involved in RA, scores of papers during the last decade have confirmed its role, and implication in RA. Our results showed that IL-17 was present at high levels in sera of RA patients; the majority of studies of IL-17 in RA have confirmed our data [29]. In accordance with Haas et al. [15] a positive correlation in expression of miR-206 and IL-17 production was found to confirm their contribution and interplaying role in RA pathology.
IL-17 stimulates the production of several inflammatory mediators, including IL-6, IL-8, granulocyte-macrophage colony stimulating factor (GM-CSF), and prostaglandin E2 (PGE2) in synoviocytes. IL-17 accelerates bone metabolism by stimulating osteogenic differentiation of mesenchymal stem cells and osteoblasts and promoting pro-osteoclastogenic molecules on these cells. It supports osteoclastogenesis dependent on the receptor activator of nuclear factor κ and its ligand, RANK/RANKL, signaling pathway. It has been reported that IL-17 activity is synergistically increased with other pro-inflammatory cytokines and ultimately resulted in joint inflammation and damage [30].
One of these proinflammatory cytokines is IL-16, which has been induced by IL-17 in RA patients. IL-17 and the specific ligand of TLR2 were shown to be the major stimuli that induced IL-16 expression and production in fibroblast-like synoviocytes (FLS) [9]. This might explain the elevation of both IL-16 and Il-17 in our study. Blaschke et al. [31] demonstrated that IL-16 was detected at significantly higher levels in sera and synovial fluid (SF) of patients with RA in comparison to osteoarthritis (OA). Interesting contrary results were presented by Klimiuk et al. and in their study IL-16 acted as an immunomodulatory mediator and IL-16 administration in a murine model of RA decreased the production of IFN-γ, IL-1β and TNF-α [32]. What is worth emphasizing, in the literature there is no described research on the correlation between IL-16 and the expression of miR-206, which makes the present study promising in this topic.
Conclusions
Taking together, our results suggest that the elevated expression of miR-206, and proinflammatory cytokines (such as IL-16 and IL-17) might be involved in RA pathogenesis. A better understanding of the pathologic role of myomiRs such as miR-206 and their interplay with proinflammatory cytokines which influence bone metabolism will provide greater insight into the osteolytic process and ensure future development of therapies to ameliorate bone loss in RA. Thus, further investigations are warranted to explain this important aspect of RA pathophysiology.