Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia that currently affects over 472 million people worldwide [1]. Diabetes is often accompanied by a range of disorders, including obesity [2], atherosclerosis [3], peripheral arterial disease [4], and obstructive sleep apnea [5]. Impaired wound healing is a common complication in patients with diabetes that severely affects life quality [6]. Normal wound healing is a dynamic and complex process involving inflammation, re-epithelization, neovascularization, and tissue remodeling [7]. Persistent inflammation, poor circulation and limited nutrients [8] are associated with impaired diabetic wound healing [9]. Hyperglycemia increased oxidative stress and led to development of chronic low-grade inflammation [10]. A cluster of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), may significantly influence the progression of inflammation in diabetes [11]. Treatments that enhance diabetic wound healing are often associated with reducing inflammatory cytokines in the diabetic wound environment [12]. Although the treatment of diabetic wounds has been greatly improved, the management of severe or chronic wounds is still challenging. Therefore, it is important to develop novel therapies for patients with wound healing difficulties.
Natural products and their phytochemicals have been used for wound healing for a long time. Natural products with wound healing properties have been reported to have anti-inflammatory, antioxidant, antibacterial and procollagen synthesis actions [13]. Musa paradisiaca (Linn). commonly known as banana, belongs to the family Musaceae, which mainly grows in tropical and subtropical regions. Banana fruits and peels are rich in dietary fibers, vitamins, potassium, magnesium, and serotonin [14]. Different parts of this plant have been traditionally used for the treatment of various diseases. The fruits are used for the treatment of diarrhea, diabetes, and intestinal lesions; the leaves are used for the treatment of eczema and burns; the flowers are used for the treatment of dysentery and menorrhagia; the roots are used for anthelmintic treatment; and so on [15]. Recently, pharmacological investigations have shown that some of the bioactive phytochemicals of Musa paradisiaca have antioxidative stress [16], antimicrobial [17], antidiabetic [18], and wound healing properties [19]. It was recently discovered that super green (SG), a water-soluble substance extracted from the leaves, peels and stems of Musa paradisiaca (Linn), seems to have antibacterial effects. The present study investigated the role of SG in the healing of surgical wounds in diabetic rats. The results will be useful for its development as a drug for the enhancement of wound healing.
Material and methods
Experimental animals
Eight-week-old male Wistar rats (250–280 g) were obtained from the National Institute of Legal National Laboratory Experimental Animal Center. The rats were maintained at a controlled temperature of 22 ±2°C on a 12-h light/dark cycle. The experimental protocols were approved by the Institutional Animal Ethics Committee of Tajen University (No. 102-22). All experiments conformed to the Guide for the Care and Use of Laboratory Animals as well as the guidelines of the Animal Welfare Act.
Streptozotocin (STZ)-induced diabetes
Diabetes was induced in the rats by using streptozotocin (Sigma-Aldrich, St. Louis, MO, USA), a compound that has a preferential toxicity toward pancreatic β cells. Diabetic conditions were induced by a single intraperitoneal injection of STZ at a concentration of 60 mg/kg body weight in overnight-fasted rats [20]. After 3 days of induction, blood samples were collected from the tail vein and measured using an automatic analyzer. Animals with fasting blood glucose levels greater than 250 mg/dl were considered diabetic.
Dosing protocol of SG and standard drug
SG was obtained by patented extraction technology and was provided by Natural Well Technical Ltd. Company, Taiwan. The SG stock solution was diluted 1 : 6000 (SG/6000), 1 : 3000 (SG/3000), 1 : 1000 (SG/1000) and 1 : 500 (SG/500) and mixed with ointment base to obtain a 1% SG ointment. An antimicrobial drug, chlorhexidine gluconate solution (Panion & BF Biotech Inc, Taipei, Taiwan), was mixed with the ointment base to obtain a 4% chlorhexidine gluconate (CG) ointment. Additionally, a commercial 1% Sulfasil ointment (silver sulfadiazine, Sinphar, Yilan, Taiwan) was used.
Excision wound model and experimental design
One week after diabetes induction, the diabetic rats were randomly classified into seven groups (n = 6): group 1 (the vehicle-treated diabetic group), group 2 (the CG-treated diabetic group), group 3 (the Sulfasil-treated diabetic group), group 4 (the SG/6000-treated diabetic group), group 5 (the SG/3000-treated diabetic group), group 6 (SG/1000-treated diabetic group), and group 7 (the SG/500-treated diabetic group).
The rats were anesthetized with 2% isoflurane to minimize suffering. After the hair on the back of each rat had been removed, the skin was sterilized with 70% alcohol, and a full-thickness excisional wound (1.5 cm × 1.5 cm) was created on the dorsal skin. The drugs were applied topically once a day for 15 consecutive days of treatment. Each rat was individually caged. All rats had free access to food and water.
Wound healing analysis
Rat wounds were photographed under isoflurane anesthesia on day 0, day 3, day 7, day 10 and day 15 after induction. A standard ruler was placed on the side of the wounds. The wound areas were measured using ImageJ Software, and the area observed on day 0 was considered 100%.
Histopathological examination
At the end of the experiment, rats were anesthetized with 2% isoflurane, and the wound area was sampled for histological examination. Skin tissue samples were formalin-fixed and embedded in paraffin. The section thickness of skin tissue was 5 µm. After hematoxylin and eosin (HE) staining, wound healing was evaluated in terms of the extent of dermal inflammation, angiogenesis, presence of granulation tissue, and re-epithelialization [21]. The parameters used to assess healing were as follows: for dermal inflammation, “–” – no dermal inflammation, “+” – mild dermal inflammation, “++” – moderate dermal inflammation; and “+++” – marked dermal inflammation; for angiogenesis, “–” – no angiogenesis, “+” – mild angiogenesis (< 5 vessels per high-power field), “++” – moderate angiogenesis (6–10 vessels per high-power field), and “+++” – marked angiogenesis (> 10 vessels per high-power field); for granulation tissue thickness, “–” – none, “+” – scant, “++” – moderate, and “+++” – marked; for re-epithelialization, “–” – no re-epithelialization, “+” – < 50% of the wound, “++” – > 50% of the wound, and “+++” – complete re-epithelialization [22, 23].
Collagen deposition in diabetic wounds was assessed by Masson’s trichrome staining [24]. Collagen fibers were stained blue, and muscle and connective tissues were stained red. HE staining or Masson’s staining was performed according to the manufacturer’s protocol. Samples were observed using optical microscopy. The collagen deposition volume and other parameters were quantified using ImageJ. Five different fields of visions were randomly selected.
Real-time quantitative PCR detection
The expression of collagen I, collagen III, IL-1β, IL-6 and TNF-α in wound tissues in STZ-treated rats was measured using RT-PCR. Total RNA was extracted from tissue samples using the TRIzol method (Thermo Fisher Scientific, Waltham, MA, USA) and processed according to the manufacturer’s instructions. Five micrograms of RNA were reverse transcribed to cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Amplification and detection were performed with a LightCycler 480 system (Roche). Gene expression was quantified using the relative standard curve method. β-Actin was used to normalize the RT-PCR results. The primer sequences used were as follows:
collagen I(α1) F: 5′-CTGGCAAGAACGGAGATGAT-3′;
collagen I(α1) R: 5′-AATCCACGAGCACCCTGA-3′;
collagen III(α1) F: 5′-CAGGAAACCCAGGAGAAAGG-3′;
collagen III(α1) R: 5′-CCAGCTACTCCTATAGGTCCTG-3′;
TNF-α F: 5′-CAGCCTCTTCTCCTTCCTGAT-3′;
TNF-α R: 5′-GCCAGAGGGCTGATTAGAGA-3′;
IL-6 F: 5′-GATGAGTACAAAAGTCCTGATCCA-3′;
IL-6 R: 5′-CTGCAGCCACTGGTTCTGT-3′;
IL-1β F: 5′-CAGCCAATCTTCATTGCTCA-3′;
IL-1β R: 5′-AGTCATCCTCATTGCCACTGT-3′;
β-actin F: 5′-CTAAGGCCAACCGTGAAAAG-3′;
β-actin R: 5′-GCCTGGATGGCTACGTACA-3′.
Results
Effect of SG on wound healing in diabetic rats
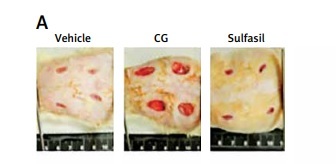
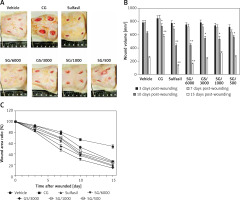
To evaluate the effect of SG ointment on lesion area, we compared wound healing in diabetic rats treated with different drugs. In morphological microanalysis, the wounds appeared clean without signs of irritation or bleeding throughout the experiment (Figure 1 A). On day 10, the topical application of SG at various dilutions and Sulfasil significantly decreased wound size compared to that in the vehicle-treated group. On day 15, the wound closure effect of SG at a dilution of 1/6000 was more effective than that of other dilutions of SG and was comparable with the effect of Sulfasil (Figure 1 B). Figure 1 C shows the wound area ratio of each group during the wound healing process. Healing was promoted after SG and Sulfasil treatment, and the wound area ratio of the SG/6000-treated group was more decreased between day 7 and day 10 than that of the other groups. However, CG failed to affect wound contraction compared with that in other groups.
Figure 1
Effect of SG on skin wound healing in diabetic rats. A – Representative images of the lesion area after treatment with different drugs on postwounding day 15. B – Wound volumes on various days during the healing process. Lesion area measurement of wounds from each group. C – Wound closure rate (%). The measurements obtained on day 0 were considered 100% of the lesion
The values are expressed as the means ± SEM s (n = 6). *P < 0.05, **p < 0.01 compared with the vehicle-treated group.
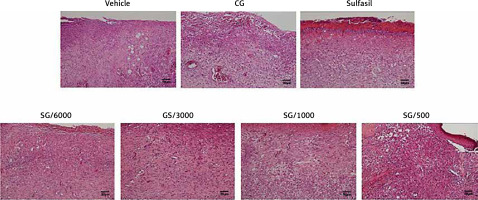
Histopathological examination
Images of HE-stained wound sections of skin on day 15 are shown in Figure 2. Inflammatory cell infiltration (inflammation), epidermal cell regeneration (re-epithelization), granulomas (granulation), and the number of blood vessels (angiogenesis) are presented in Table I. Inflammatory cell infiltration was observed in all diabetic groups; however, SG/6000, SG/3000 and Sulfasil treatment decreased the inflammatory effects. Among these treatments, SG/6000 was the most effective. The number of new blood vessels in the lesions was increased in the SG/6000–, SG/3000– and Sulfasil-treated groups. To evaluate the kinetics of wound closure, the number of granulomas was measured. The SG/6000- and Sulfasil-treated groups exhibited more granulomas than the other groups. Moreover, the SG/6000-treated group showed higher re-epithelialization levels than those of the other groups. After treatment with CG, the wound size of the STZ-treated diabetic rats was not obviously improved.
Figure 2
HE-stained skin tissue sections 15 days after induction in diabetic rats treated with different drugs (scale bar: 50 μm). The parameters used to assess the healing of excision wounds in the diabetic rats are shown in Table I
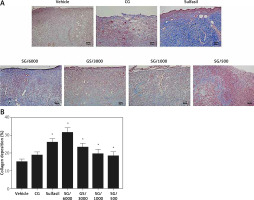
Analysis of collagen deposition
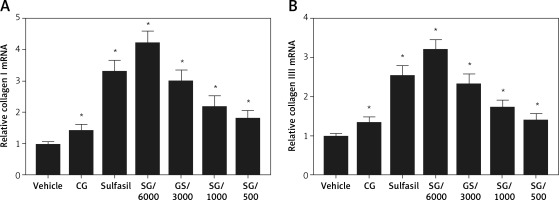
Total collagen content was analyzed using Masson’s trichrome staining. Our results indicated that vehicle- and CG-treated diabetic wounds had poor collagen deposition (Figure 3 A). The SG- and Sulfasil-treated type 1 diabetic groups exhibited significantly increased collagen deposition on day 15. Compared with the groups treated with other dilutions of SG, the SG/6000-treated group showed the highest level of collagen deposition (Figure 3 B). Moreover, the gene expression levels of collagen I and collagen III were markedly increased in the SG/6000-treated group compared with the vehicle-treated diabetic group (Figure 4).
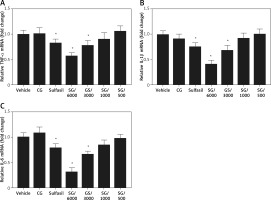
Effect of SG on mRNA expression levels of inflammatory cytokines
To investigate the molecular mechanism of SG-induced wound healing activity, the expression levels of related inflammatory cytokines were examined. TNF-α, IL-1β, and IL-6 are the major genes that are generally involved in wound healing. On day 3, a significant decrease in the expression of TNF-α, IL-1β, and IL-6 in the wound tissue of SG/6000-treated rats compared with vehicle-treated rats was noted (Figure 5). Meanwhile, SG/3000 and Sulfasil treatment showed modest effects.
Discussion
SG is a water-soluble natural product obtained from Musa paradisiaca plants. It has been documented that extracts of Musa species have antibacterial and antioxidant activities [25], and promote the healing of burn wounds [19]. Impaired wound healing is a common and potentially serious complication in diabetes [26]. In the present study, we used excisional wound models to investigate the role of SG in diabetic rats [27]. Furthermore, the wound healing effects of SG at a 1 : 6000 dilution were superior to those of SG at other concentrations in the initial stage of wound healing in STZ-induced diabetic rats. In contrast, the higher dilution of SG had better effectiveness in promoting wound healing in the late stage. However, CG did not promote wound-healing activity in STZ-induced diabetic rats. The findings of the present study indicated that SG/6000 was beneficial to skin wound healing in diabetic rats.
The wounds of diabetic rats in the present investigation showed prolonged inflammation, microvasculature aberrations that result in irregular circulation and diminished angiogenesis and decreased collagen production, which was similar to the previous study [28]. Many factors, including a hyperglycemic environment, chronic inflammation, wound infection, vascular insufficiency, and hypoxia, have been shown to impair wound healing in diabetes [29]. In the wound tissue of diabetic mice, the absence of the expression of various growth factors delays re-epithelization and granulation tissue formation [30]. In our histopathological studies, SG/6000 significantly decreased inflammation and increased granulation tissue formation and neovascularization of excision wounds in diabetic rats. Moreover, SG/6000 improved collagen deposition compared with that in other groups. Collagen is an essential and main component of granulation tissue that contributes to wound strength and the healing process [31]. It has been reported that collagen deposition in acute wounds is impaired in type 1 diabetes, which might be due to the inhibition of fibroblast proliferation [32]. During the early stages of healing, the levels of type III collagen are increased [33]. In the remodeling phase, collagen III that is produced rapidly in the extracellular matrix is replaced by collagen I, which has a higher tensile strength but takes more time to be deposited [34]. In the current study, SG/6000 significantly increased collagen deposition and elevated the gene expression of collagen type I and collagen type III. Therefore, SG/6000 has been suggested to improve diabetic wound healing in terms of increasing collagen formation.
In the present study, SG/6000 treatment significantly decreased the number of inflammatory cytokines compared with that in other groups. Persistent inflammation in diabetes is associated with delayed wound healing. In diabetes, high glucose promotes inflammation through endothelial cell damage, the release of proinflammatory cytokines, and capillary permeability [35]. In the inflammatory phase, activated macrophages produce growth factors and inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [34]. In the wound healing process, proinflammatory cytokines are involved in cell proliferation and differentiation as well [36]. IL-6 plays a crucial role in many wound healing phases, promoting the migration and proliferation of keratinocytes [37]. In the early stages of wound healing, TNF-α and IL-1β stimulate granulation tissue formation and neovascularization. TNF-α has been reported to regulate vascular endothelial growth factor levels and collagen synthesis in wound healing [38, 39]. IL-1β inhibits type I collagen synthesis during the repair phase of the wound healing response [40]. However, the persistence of inflammatory cytokines impairs the healing of diabetic wounds. SG treatment regulated these mediators by reducing proinflammatory cytokines at the early stage of wound healing and promoting wound repair at the late postinjury stages.
Our results indicated that SG/6000 has more potent anti-inflammatory wound healing stimulating properties than silver sulfadiazine. Silver sulfadiazine has been utilized in advanced wound care to improve the microenvironment of wounds [41]. The application of heparan sulfate can accelerate tissue regeneration in the early phase of tissue repair [42]. Nonetheless, a recent study indicated that silver dressings do not decrease the amount of bacteria in diabetic wounds [43]. A previous study documented that extracts of Musa paradisiaca have antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus [44] and can increase the viability of collagen fibrils by increasing the strength of collagen fibers [45]. In addition, Musa species show antidiabetic and better ulcer healing effects in diabetic rats with cooccurring gastric ulcers [46]. Therefore, SG might be a target for the treatment of diabetes.
In conclusion, the present study showed that SG improves the healing process in diabetic rats through its anti-inflammatory effect and may help us cure wound injury in the clinic in the future. SG ointment has the potential to be a new alternative therapeutic strategy for wound healing.