Published online Sep 26, 2014. doi: 10.4330/wjc.v6.i9.929
Revised: March 12, 2014
Accepted: July 17, 2014
Published online: September 26, 2014
Primary percutaneous coronary intervention is the preferred reperfusion strategy for patients presenting with ST-segment elevation myocardial infarction (STEMI). First generation drug-eluting stents (DES), (sirolimus drug-eluting stents and paclitaxel drug-eluting stents), reduce the risk of restenosis and target vessel revascularization compared to bare metal stents. However, stent thrombosis emerged as a major safety concern with first generation DES. In response to these safety issues, second generation DES were developed with different drugs, improved stent platforms and more biocompatible durable or bioabsorbable polymeric coating. This article presents an overview of safety and efficacy of the first and second generation DES in STEMI.
Core tip: Primary percutaneous coronary intervention is the preferred reperfusion strategy for patients presenting with ST-segment elevation myocardial infarction (STEMI). First-generation drug-eluting stents (DES) reduce restenosis and target vessel revascularization compared to bare metal stents at the expense of an increased stent thrombosis rate. Recent improvements in second-generation DES have overcome these safety concerns. This article presents an overview of safety and efficacy of the DES in STEMI.
- Citation: Otsuki S, Sabaté M. Drug-eluting stents and acute myocardial infarction: A lethal combination or friends? World J Cardiol 2014; 6(9): 929-938
- URL: https://www.wjgnet.com/1949-8462/full/v6/i9/929.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i9.929
Primary percutaneous coronary intervention (PCI) has become a well-established reperfusion strategy for patients presenting with acute ST-segment elevation myocardial infarction (STEMI)[1,2]. In this setting, bare-metal stents (BMS) reduced the risk of recurrent ischemia and restenosis compared to balloon angioplasty[3]. First-generation drug-eluting stents (DES)-sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES)-were also able to reduce the risk of restenosis and target-vessel revascularization (TVR) compared to BMS in this context[4,5]. However, stent thrombosis emerged as a major safety concern[6]. In response, second-generation DES were developed with different drugs, more biocompatible durable polymers or bioabsorbable polymeric coatings, and new stent platforms, including fully bioresorbable vascular scaffolds.
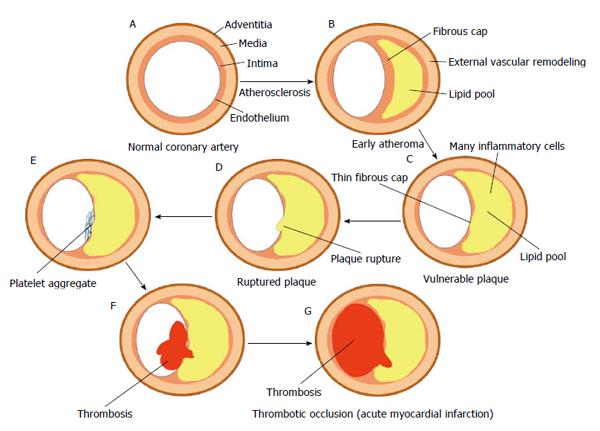
As shown in Figure 1, STEMI is an event related to atherosclerotic plaque rupture, ulceration, fissuring, erosion, or dissection that results in intraluminal thrombus in one or more of the coronary arteries, leading to decreased myocardial blood flow or distal platelet emboli with ensuing myocyte necrosis[7,8]. During the early years after the introduction of coronary stents, it was thought that implanting a metallic device under a thrombotic environment in the acute phase of STEMI could increase the risk of adverse outcome. However, refinement of stent implantation technique and the development of new antithrombotic regimen have overcome those initial concerns.
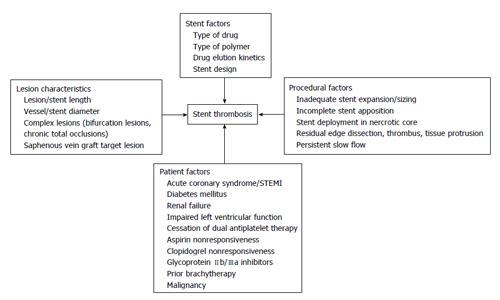
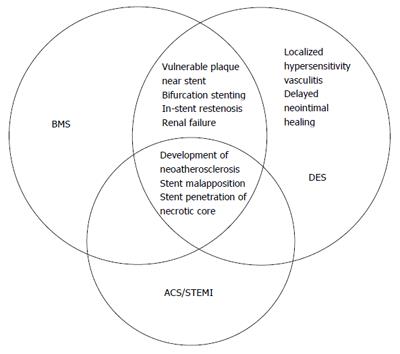
The pathophysiology of stent thrombosis includes procedure-, stent-, and patient-related factors (Figure 2). The PCI procedure for acute coronary syndrome, including STEMI, is one of the most powerful predictors for stent thrombosis in the vast majority of registries[9-14] (Figure 3). Late stent malapposition is common in STEMI patients and may eventually provoke stent thrombosis. Late malapposition may be linked to underdeployment of stents at the time of STEMI treatment, due mainly to dissolution of thrombus behind the struts or undersized vessels due to the spastic condition of the coronary arteries in the acute phase of STEMI[15]. Implanting DES over a necrotic core may also significantly delay healing, due to less neointimal growth and greater inflammation, fibrin deposits, and uncovered struts compared to DES implanted over coronary stable plaques[16,17].
Currently, patients are categorized as having early or late stent thrombosis. Early stent thrombosis is defined as occurring within 30 d of implantation, and is further categorized as acute (events within 24 h) or subacute (events on day 1-30) thrombosis. Events that occur more than 30 d postimplantation are classified as late stent thrombosis, and those occurring beyond 12 mo as very late stent thrombosis[18].
Early and late stent thrombosis differ in their pathophysiology and mechanism. Early stent thrombosis is mainly related to one or more procedural characteristics, such as stent underexpansion, incomplete stent apposition, dissection, thrombus, tissue protrusion, and persistent slow flow. It may occur after either BMS or DES implantation.
Late stent thrombosis may result when neointimal healing is delayed, as this can lead to inadequate neointimal coverage and/or to incomplete stent apposition. Evaluation of angioscopy, optical coherence tomography, and autopsy revealed that first-generation DES are associated with delayed arterial healing due to hypersensitive reactions to polymers that cause chronic inflammation[9,16]. These phenomena are typically observed more than 1 year after implantation.
Twelve randomized controlled trials (RCTs) of first-generation DES outcomes in STEMI have been published[4,5,19-33]. Comparisons were made as follows: BMS vs SES, 7 reports; BMS vs PES, 5 reports; PES vs SES, 2 reports; BMS vs SES vs PES, 1 report (Table 1).
| Study, author (Ref.) | Year | Primary endpoint | Design | Randomized ratio | Maximal length of follow-up | Stent comparators (n) | Results of the primary endpoint |
| Pasceri et al[19] | 2003 | Death, MI, recurrent ischemia at 1 yr | Single center | 1:1 | 1 yr | BMS/SES 65 (33/32) | No significant differences between stents |
| TYPHOON[4] | 2006 | TVF at 1 yr | Multicenter, superiority | 1:1 | 4 yr | BMS/SES 712 (355/357) | SES superior to BMS |
| STRATEGY[20] | 2007 | Death, MI, stroke, binary restenosis at 8 mo | 2-center, superiority | 1:1 | 2 yr | BMS/SES 175 (87/88) | SES superior to BMS |
| SESAMI[21,22] | 2007 | Binary restenosis at 1 yr | Single-center, superiority | 1:1 | 5 yr | BMS/SES 320 (160/160) | SES superior to BMS |
| Díaz de la Llera et al[23] | 2007 | Death, MI, TLR at 1 yr | Single center, superiority | 1:1 | 1 yr | BMS/SES 114 (54/60) | SES superior to BMS |
| MISSION[24] | 2008 | In-segment late luminal loss at 9 mo | Single center, noninferiority | 1:1 | 5 yr | BMS/SES 310 (152/158) | SES superior to BMS |
| MULTISTRATEGY[25] | 2008 | Death, MI, clinically driven TVR at 8 mo | Multicenter, superiority | 1:1 | 3 yr | BMS/SES 744 (372/372) | SES superior to BMS |
| HAAMU-STENT[26] | 2006 | Death, MI, late lumen loss, TVR at 1 yr | Single center, superiority | 1:1 | 1 yr | BMS/PES 164 (82/82) | PES superior to BMS |
| SELECTION[27] | 2007 | Neointimal proliferation by IVUS at 7 mo | Single-center, superiority | 1:1 | 7 mo | BMS/PES 76 (39/37) | PES superior to BMS |
| PASSION[28] | 2008 | Cardiac death, MI, TLR at 2 yr | 2-center, superiority | 1:1 | 5 yr | BMS/PES 619 (310/309) | Superiority not demonstrated |
| HORIZONS-AMI[5,29] | 2009 | TLR Death, MI, stroke, or ST at 1 yr | Multicenter, superiority (TLR) Noninferiority (Death, MI, stroke, ST) | 3:1 | 3 yr | BMS/PES 3006 (2257/749) | PES superior for TLR and noninferior for clinical endpoints |
| GRACIA-3[30] | 2010 | In-segment binary restenosis, myocardial flow at 1 yr | Multicenter, noninferiority | 1:1 | 1 yr | BMS/PES 419 (210/209) | BMS noninferior to PES |
| PROSIT[31] | 2008 | Death, MI, TVR, ST at 1 yr | Multicenter, superiority | 1:1 | 3 yr | PES/SES 308 (154/154) | Superiority not demonstrated |
| Juwana et al[32] | 2009 | Late lumen loss at 9 mo | Single center, superiority | 1:1 | 1 yr | PES/SES 397 (196/201) | SES superior to PES |
| PASEO[33] | 2009 | TLR at 12 mo | Single-center, superiority | 1:1:1 | 4 yr | BMS/PES/SES 270 (90/90/90) | PES and SES superior to BMS |
The TYPHOON study[4] was the largest RCT to consider SES, enrolling 712 patients to assess the effectiveness and safety of SES vs BMS at 1 year. Target-vessel failure was significantly lower in the SES (7.3%) than in the BMS (14.3%) group (P = 0.004), driven by a decrease in the rate of TVR (5.6% vs 13.4%, respectively; P < 0.001). There was no significant difference between the two groups in the rates of mortality (2.3% vs 2.2%; P = 1.00), repeat myocardial infarction (MI) (1.1% vs 1.4%; P = 1.00), or stent thrombosis (3.4% vs 3.6%; P = 1.00). At 4-year follow-up[4], freedom from target lesion revascularization was significantly better in the SES group, compared to BMS (92.4% vs 85.1%; P = 0.002). However, no differences were observed, respectively, in freedom from cardiac death (97.6% vs 95.9%; P = 0.37), freedom from repeat MI (94.8% vs 95.6%; P = 0.85), or definite/probable stent thrombosis (4.4% vs 4.8%, P = 0.83). Other studies have also reported that SES was superior or noninferior to BMS in mortality, repeat MI, TVR, and stent thrombosis rates[20-25,33] (Table 1).
With regard to PES, the HORIZONS-AMI study was the largest RCT[5]. A total of 3006 patients were enrolled in this 12-mo trial to assess the effectiveness and safety of PES vs BMS. The PES group had significantly lower 12-mo rates of ischemia-driven target lesion revascularization (4.5% vs 7.5%; P = 0.002) and TVR (5.8% vs 8.7%; P = 0.006). There were no significant differences between the PES and BMS groups in 12-mo rates of mortality (3.5% vs 3.5%; P = 0.98) and stent thrombosis (3.2% vs 3.4%; P = 0.77). At the 3-year follow-up[29], the PES group had lower rates of ischemia-driven target lesion revascularization (9.4% vs 15.1%; P < 0.0001), but did not differ from the BMS group in mortality, repeat MI, stroke, or stent thrombosis rates. Stent thrombosis was high (≥ 4.5%) in both groups. Other studies have also shown that PES was superior or noninferior to BMS in mortality, repeat MI, TVR, and stent thrombosis rates[26,27,30,33] (Table 1).
Although RCTs did not identify any safety issues with first-generation DES, this topic became a firestorm during the 2006 European Society of Cardiology Annual Meeting, held in Barcelona. Meta-analysis of pooled data showed that first-generation DES increased mortality and repeat MI compared to BMS[34]. High rates of early and late stent thrombosis after discontinuation of dual antiplatelet agents in patients treated with first-generation DES also raised safety concerns[35,36]. Pathology studies demonstrated that the durable polymers used in first-generation DES could cause a delay in arterial healing, characterized by persistent fibrin deposits, delayed hypersensitivity reactions, and poor endothelialization of the vessel wall, all of which increased the thrombotic risk[37].
Second-generation DES were developed to resolve these issues. Stent design and polymeric coating were improved by the use of biocompatible or bioabsorbable polymers. Two RCTs have been published about zotarolimus-eluting stents (ZES) implantation in STEMI patients[38,39] (Table 2).
| Study | Year | Primary endpoint | Design | Randomized ratio | Maximal length of follow-up | Stent comparisons (n) | Results of the primary endpoint |
| ZEST-AMI[38] | 2009 | Death, MI, and ischemia-driven TVR at 1 yr | Multicenter, safety study | 1:1:1 | 1 yr | PES/SES/PC-ZES 328 (110/110/108) | No significant differences between stents |
| KOMER[39] | 2011 | Cardiac death, MI, ischemia-driven TLR at 1 yr | Multicenter, safety study | 1:1:1 | 18 mo | PES/SES/PC-ZES 611 (202/204/205) | PC-ZES as safe as SES and PES |
| EXAMINATION[40,41] | 2011 | Death, MI, any revascularization at 1 yr | Multicenter, superiority | 1:1 | 2 yr | CoCr-EES/BMS 1504 (751/747) | CoCr-EES superior to BMS |
| XAMI[42] | 2012 | Cardiac death, MI, TVR at 1 yr | Multicenter, noninferiority | 2:1 | 1 yr | EES/SES 625 (404/221) | EES noninferior to SES |
| COMFORTABLE AMI[43] | 2012 | cardiac death, reinfarction, and TLR at 1 yr | Multicenter, superiority | 1:1 | 1 yr | EES/BMS 1161 (575/582) | BES superior to BMS |
The multicenter, prospectively randomized, ZEST-AMI trial included 328 patients who were randomly assigned to ZES (n =108), SES (n =110), or PES (n =110) groups[38]. Mortality, MI, and ischemia-driven TVR rates at 12 mo were 11.3%, 8.2%, and 8.2%, respectively (P = 0.834); there were no differences in mortality, recurrent MI, and ischemia-driven TVR rates. The SES group had 2 acute and 2 subacute cases of stent thrombosis. In the PES group, 3 patients had subacute thrombosis.
The KOMER study was also a multicenter, prospective, single-blind RCT[39]. The 611 participants were STEMI patients undergoing primary PCI. They were randomized to treatment with ZES (n =205), SES (n =204), or PES (n =202). At 12-mo follow-up, the incidence of cardiac death, MI, or ischemia-driven target lesion revascularization was 5.9% in the ZES group, 3.4% in the SES group, and 5.7% in the PES group, respectively (P = 0.457). The rate of stent thrombosis was similar in all 3 groups (approximately 2%).
Two RCTs have studied the use of everolimus-eluting stents (EES) implantation in STEMI patients[40-42]. The EXAMINATION study was a multicenter, prospective, randomized, all-comer, controlled trial. In this trial, 1498 patients were randomly assigned to receive EES (n =751) or BMS (n =747)[40]. At 1-year follow-up, target lesion and vessel revascularization were significantly lower in the EES group (2.1% vs 5.0%; P = 0.003, and 3.7% vs 6.8%; P = 0.0077). There were no differences between the EES and BMS groups in all-cause (3.5% vs 3.5%, P = 1.00) or cardiac death (3.2% vs 2.8%, P = 0.76) or repeat-MI (1.3% vs 2.0%, P = 0.32). Stent thrombosis rates differed significantly between EES and BMS groups for both “definite” and “definite or probable” diagnoses (0.5% vs 1.9% and 0.9% vs 2.5%, respectively; both P = 0.019). At the 2-year follow-up, there were significantly fewer target lesion revascularizations in the EES group (2.9% vs 5.6% for BMS; P = 0.009)[41]. Composite of all-cause death, any MI, or revascularization did not differ between groups (14.4% vs 17.3%, respectively; P = 0.11). Definite and probable stent thrombosis rates were significantly lower in the EES group (1.3% vs 2.8%; P = 0.03).
The XAMI trial randomized 625 patients with acute myocardial infarction (2:1) to receive EES or SES[42]. Death, nonfatal MI, or any TVR at 1 year was lower at 4.0% for EES vs 7.7% for SES (P = 0.048) and 1-year incidence of definite and/or probable stent thrombosis was 1.2% for EES vs 2.7% for SES (P = 0.21).
The COMFORTABLE AMI is the only RCT by the use of biolimus-eluting stents (BES) in STEMI patients[43]. A total of 1161 patients were randomized 1:1 to receive BES (n =575) or BMS (n =582). Major adverse cardiac events at 1 year occurred in 24 patients (4.3%) receiving BES and in 49 patients (8.7%) receiving BMS (P = 0.004). The difference was driven by a lower risk of target vessel-related repeat MI [3 (0.5%) vs 15 (2.7%); P = 0.01] and ischemia-driven target-lesion revascularization [9 (1.6%) vs 32 (5.7%); P = 0.001] in patients receiving BES compared with those receiving BMS. Rates of cardiac death were not significantly different [16 (2.9%) vs 20 (3.5%), P = 0.53]. Definite stent thrombosis occurred in 5 patients (0.9%) treated with BES and 12 patients (2.1%, P = 0.10) treated with BMS.
Recent meta-analyses also showed that EES were associated with significantly lower rates of stent thrombosis than both BMS and PES at 1-year follow-up. In addition, EES were associated with significantly lower rates of cardiac death or MI compared with PES[44,45].
Pathological analysis also showed that late and very late stent thrombosis occurred less often in the EES (4%) than in the SES (21%; P = 0.029) and PES groups (26%; P = 0.008). The percentage of uncovered struts was lower in the EES (media n = 2.6%) than in SES (18.0%; P < 0.0005) or PES groups (18.7%; P < 0.0005). Furthermore, EES was associated with less inflammation, no hypersensitivity, and less fibrin deposit than both SES and PES[46].
A new, self-apposing stent has been developed to reduce malapposition, which may eventually provoke stent thrombosis. In the APPOSITION II study, optical coherence tomography at 3 d after implantation showed a lower rate of malapposed stent struts in the self-apposing BMS group than in the balloon-expandable group (0.58% vs 5.46%, P = 0.001)[47]. In the APPOSITION IV study, patients treated with a self-apposing SES had better apposition (P = 0.001) and better coverage at 4-mo follow-up than the balloon-expandable ZES (31.6% vs 3.8%; P = 0.03)[48].
The micronet-mesh-covered stent has been developed to prevent distal embolization. In the MASTER study, complete ST-segment resolution was significantly improved in patients treated with micronet-mesh-covered stent, compared with commercially available BMS or SES (57.8% vs 44.7%; P = 0.008)[49].
Nonpolymeric stents have been developed to avoid polymer-related delayed neointimal healing and late stent thrombosis, and several have undergone clinical investigation (Table 3). However, most of the clinical data have been gathered in low-risk patients without STEMI[50-56]. A small study showed that polymer-free PES (PF-PES) were noninferior to polymer-based PES (PB-PES) in patients with STEMI, both in terms of target lesion failure (10.9% PB-SES vs 12.0% PF-PES; P = 0.861) and definite or probable stent thrombosis (1.8% PB-SES vs 2.0% PF-PES; P = 1.000) at one year[57].
| Stent | Study | Platform | Drug | Primary endpoint | Design | Randomized ratio | Stent comparisons (n) | Result |
| Yukon (Translumina) | ISAR TEST[50] | 316 L microporous surface | Sirolimus + Probucol | MACE/ST at 1yr | RCT | 2:1 | Yukon/R-ZES 3002 (2002/1000) | Noninferior |
| Cre 8 (CID) | NEXT[51] | CoCr abluminal reservoirs | Amphilimus | LL at 6 mo | RCT | 1:1 | Cre 8/PES 323 (162/161) | Superior |
| BioFreedom DCS (Biosensors) | BioFreedom FIM[52] | 316 L microstructured surface | Biolimus A9 | LL at 12 mo | RCT | 1:1:1 | Standard dose/low dose Biofreedom/PES 182 (60/62/60) | Noninferior |
| Vestasync (MIV therapeutics) | VESTASYNC Il[53] | 316 L microporous nanofilm Hap | Sirolimus | LL at 4 and 9 mo | RCT | 2:1 | VESTAsync/BMS 75 (50/25) | Superior |
| Amazonia Pax (Minvasys) | Pax A and Pax B | CoCr nontextured | Paclitaxel | LL at 6 mo | RCT | 1:1 | PAXA/PES 30 (15/15), PAXB = 100 | Noninferior |
| Yinyi (Liaoning Biomed.Mat) | FREEDOM[54] | 316 L micropores | Paclitaxel | MACE/ST/TVR | RCT | 2:1 | Yinyi/SES 1626 (931/449) | Noninferior |
| Bicare+ (Lepu Medical) | BICARE[56] | 316 L | Sirolimus + Probucol | TVF at 30 d | FIM | - | n = 32 | TVF = 9.4%, LL 0.14, ISR = 3.2% |
| Pronova XR (Vascular Concepts) | EURONOVA XR l[55] | Co-Cr | Sirolimus | LL at 6 mo | FIM | - | n = 50 | In-stent LL 0.45 |
| Focus NP (Envision Scientific) | Nano active FIM | 316 L nontextured | Sirolimus nanoparticles | LL at 6 mo | FIM | - | n = 100 | Ongoing |
| Mitsu (Meril Medical) | - | CoCr ultrathin struts | Merilimus | - | - | - | Planned | - |
| Hollow-core DFS (Medtronic) | - | CoCr holes and hollow tube | Sirolimus | - | - | - | Planned | - |
| Nano+ (Lepu medical) | Nano+ | Microporous | Sirolimus | OCT evaluation | FIM | - | n = 45 | Ongoing |
Fully bioresorbable vascular scaffold (BVS) was developed to overcome problems associated with a durable polymer and metallic scaffold. Disappearance of the stent from the treated site might decrease the risk of stent thrombosis. So far, a few studies with short-term follow-up have been published about bioresorbable vascular scaffold in STEMI or acute coronary syndrome[58-61]. Further studies in a larger number of patients and long-term follow-up are planned.
The ongoing ISAR-absorb MI trial (A Prospective, Randomized Trial of BVS vs EES in Patients Undergoing Coronary Stenting for Myocardial Infarction, http://www.clinicaltrial.gov, NCT01942070) tests the clinical performance of the everolimus-eluting BVS vs durable polymer EES in patients undergoing PCI in the setting of acute MI. The primary endpoint is percent diameter stenosis in angiographic follow-up at 6 to 8 mo. Subsequent clinical follow-up will be undertaken up to 5 years.
Another ongoing study is ABSORB STEMI: the TROFI II trial (http://www.clinicaltrial.gov, NCT01986803), a prospective, single-blind, noninferiority, European multicenter RCT. The primary endpoint is to assess the neointimal healing score as evaluated by intracoronary optical frequency domain imaging in patients with STEMI and treated with everolimus-eluting BVS at 6 mo follow-up, compared to that of EES. Furthermore, the safety and feasibility of implanting everolimus-eluting BVS in patients with STEMI will be assessed.
The second-generation DES significantly reduced TVR compared with BMS, without an increase in mortality, MI, or stent thrombosis rates. In patients with STEMI, the use of second-generation DES appears safer and more efficacious than either BMS or first-generation DES. Results of the ongoing ISAR-absorb trial and ABSORB STEMI: the TROFI II trial will shed light on the potential benefits of the new BVS in the context of STEMI.
P- Reviewer: Berenguer AB, Chang ST, Lazzeri C, Tagarakis G, Takahashi M S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Kushner FG, Hand M, Smith SC, King SB, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205-2241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 958] [Cited by in F6Publishing: 811] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 2. | Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3153] [Cited by in F6Publishing: 2951] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 3. | Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Carroll JD. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 934] [Cited by in F6Publishing: 973] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 4. | Spaulding C, Teiger E, Commeau P, Varenne O, Bramucci E, Slama M, Beatt K, Tirouvanziam A, Polonski L, Stella PR. Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with BallOON angioplasty). JACC Cardiovasc Interv. 2011;4:14-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360:1946-1959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 358] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 6. | Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 7. | Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, Ngu Blackett K, Lisheng L. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 8. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM. Third universal definition of myocardial infarction. Circulation. 2012;126:2020-2035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2200] [Cited by in F6Publishing: 2331] [Article Influence: 194.3] [Reference Citation Analysis (0)] |
| 9. | Holmes DR, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, Williams DO, Kimura T, Moliterno DJ. Stent thrombosis. J Am Coll Cardiol. 2010;56:1357-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Cheneau E, Leborgne L, Mintz GS, Kotani J, Pichard AD, Satler LF, Canos D, Castagna M, Weissman NJ, Waksman R. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003;108:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 343] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP, Chauhan MS, Rodriguez O, Kuntz RE. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103:1967-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 570] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 12. | Honda Y, Fitzgerald PJ. Stent thrombosis: an issue revisited in a changing world. Circulation. 2003;108:2-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J Am Coll Cardiol. 1997;29:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 230] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Wenaweser P, Dörffler-Melly J, Imboden K, Windecker S, Togni M, Meier B, Haeberli A, Hess OM. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Onuma Y, Thuesen L, van Geuns RJ, van der Ent M, Desch S, Fajadet J, Christiansen E, Smits P, Holm NR, Regar E. Randomized study to assess the effect of thrombus aspiration on flow area in patients with ST-elevation myocardial infarction: an optical frequency domain imaging study--TROFI trial. Eur Heart J. 2013;34:1050-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2103] [Cited by in F6Publishing: 2056] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 17. | Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 500] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4265] [Cited by in F6Publishing: 4496] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 19. | Pasceri V, Patti G, Speciale G, Pristipino C, Richichi G, Di Sciascio G. Meta-analysis of clinical trials on use of drug-eluting stents for treatment of acute myocardial infarction. Am Heart J. 2007;153:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Valgimigli M, Campo G, Arcozzi C, Malagutti P, Carletti R, Ferrari F, Barbieri D, Parrinello G, Percoco G, Ferrari R. Two-year clinical follow-up after sirolimus-eluting versus bare-metal stent implantation assisted by systematic glycoprotein IIb/IIIa Inhibitor Infusion in patients with myocardial infarction: results from the STRATEGY study. J Am Coll Cardiol. 2007;50:138-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Menichelli M, Parma A, Pucci E, Fiorilli R, De Felice F, Nazzaro M, Giulivi A, Alborino D, Azzellino A, Violini R. Randomized trial of Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction (SESAMI). J Am Coll Cardiol. 2007;49:1924-1930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Musto C, Fiorilli R, De Felice F, Patti G, Nazzaro MS, Scappaticci M, Bernardi L, Violini R. Long-term outcome of sirolimus-eluting vs bare-metal stent in the setting of acute myocardial infarction: 5-year results of the SESAMI trial. Int J Cardiol. 2013;166:399-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Díaz de la Llera LS, Ballesteros S, Nevado J, Fernández M, Villa M, Sánchez A, Retegui G, García D, Martínez A. Sirolimus-eluting stents compared with standard stents in the treatment of patients with primary angioplasty. Am Heart J. 2007;154:164.e1-164.e6. [PubMed] [Cited in This Article: ] |
| 24. | van der Hoeven BL, Liem SS, Jukema JW, Suraphakdee N, Putter H, Dijkstra J, Atsma DE, Bootsma M, Zeppenfeld K, Oemrawsingh PV. Sirolimus-eluting stents versus bare-metal stents in patients with ST-segment elevation myocardial infarction: 9-month angiographic and intravascular ultrasound results and 12-month clinical outcome results from the MISSION! Intervention Study. J Am Coll Cardiol. 2008;51:618-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Valgimigli M, Campo G, Percoco G, Bolognese L, Vassanelli C, Colangelo S, de Cesare N, Rodriguez AE, Ferrario M, Moreno R. Comparison of angioplasty with infusion of tirofiban or abciximab and with implantation of sirolimus-eluting or uncoated stents for acute myocardial infarction: the MULTISTRATEGY randomized trial. JAMA. 2008;299:1788-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Tierala I. Helsinki Area Acute Myocardial Infarction Treatment Re-Evaluation-Should the Patients Get a Drug-Eluting or Normal Stent (HAAMU-STENT) study. 2014; Available from: http:// www.tctmd.com/searchresults.aspx?sstring=HAAMU&stype=Slide%20Presentations. [Cited in This Article: ] |
| 27. | Chechi T, Vittori G, Biondi Zoccai GG, Vecchio S, Falchetti E, Spaziani G, Baldereschi G, Giglioli C, Valente S, Margheri M. Single-center randomized evaluation of paclitaxel-eluting versus conventional stent in acute myocardial infarction (SELECTION). J Interv Cardiol. 2007;20:282-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Dirksen MT, Vink MA, Suttorp MJ, Tijssen JG, Patterson MS, Slagboom T, Kiemeneij F, Laarman GJ. Two year follow-up after primary PCI with a paclitaxel-eluting stent versus a bare-metal stent for acute ST-elevation myocardial infarction (the PASSION trial): a follow-up study. EuroIntervention. 2008;4:64-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377:2193-2204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 30. | Sánchez PL, Gimeno F, Ancillo P, Sanz JJ, Alonso-Briales JH, Bosa F, Santos I, Sanchis J, Bethencourt A, López-Messa J. Role of the paclitaxel-eluting stent and tirofiban in patients with ST-elevation myocardial infarction undergoing postfibrinolysis angioplasty: the GRACIA-3 randomized clinical trial. Circ Cardiovasc Interv. 2010;3:297-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Hong MK, Rhee KS, Chae JK, Ko JK. A randomized comparison of sirolimus- versus Paclitaxel-eluting stent implantation in patients with diabetes mellitus. J Am Coll Cardiol. 2008;52:727-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Juwana YB, Suryapranata H, Ottervanger JP, De Luca G, van’t Hof AW, Dambrink JH, de Boer MJ, Gosselink AT, Hoorntje JC. Comparison of rapamycin- and paclitaxel-eluting stents in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;104:205-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Di Lorenzo E, Sauro R, Varricchio A, Carbone G, Cortese G, Capasso M, Lanzillo T, Manganelli F, Mariello C, Siano F. Long-Term outcome of drug-eluting stents compared with bare metal stents in ST-segment elevation myocardial infarction: results of the paclitaxel- or sirolimus-eluting stent versus bare metal stent in Primary Angioplasty (PASEO) Randomized Trial. Circulation. 2009;120:964-972. [PubMed] [Cited in This Article: ] |
| 34. | Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440-1455; discussion 1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 574] [Cited by in F6Publishing: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 35. | Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol. 2005;45:2088-2092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 487] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 36. | Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Jüni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol. 2008;52:1134-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 37. | Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 713] [Cited by in F6Publishing: 696] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 38. | Lee CW, Park DW, Lee SH, Kim YH, Hong MK, Kim JJ, Park SW, Yun SC, Seong IW, Lee JH. Comparison of the efficacy and safety of zotarolimus-, sirolimus-, and paclitaxel-eluting stents in patients with ST-elevation myocardial infarction. Am J Cardiol. 2009;104:1370-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Kang WC, Ahn T, Lee K, Han SH, Shin EK, Jeong MH, Yoon JH, Park JS, Bae JH, Hur SH. Comparison of zotarolimus-eluting stents versus sirolimus-eluting stents versus paclitaxel-eluting stents for primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction: results from the Korean Multicentre Endeavor (KOMER) acute myocardial infarction (AMI) trial. EuroIntervention. 2011;7:936-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 41. | Sabaté M, Brugaletta S, Cequier A, Iñiguez A, Serra A, Hernádez-Antolín R, Mainar V, Valgimigli M, Tespili M, den Heijer P. The EXAMINATION trial (Everolimus-Eluting Stents Versus Bare-Metal Stents in ST-Segment Elevation Myocardial Infarction): 2-year results from a multicenter randomized controlled trial. JACC Cardiovasc Interv. 2014;7:64-71. [PubMed] [Cited in This Article: ] |
| 42. | Hofma SH, Brouwer J, Velders MA, van’t Hof AW, Smits PC, Queré M, de Vries CJ, van Boven AJ. Second-generation everolimus-eluting stents versus first-generation sirolimus-eluting stents in acute myocardial infarction. 1-year results of the randomized XAMI (XienceV Stent vs. Cypher Stent in Primary PCI for Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2012;60:381-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Räber L, Kelbæk H, Ostojic M, Baumbach A, Heg D, Tüller D, von Birgelen C, Roffi M, Moschovitis A, Khattab AA. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 44. | Palmerini T, Biondi-Zoccai G, Della Riva D, Mariani A, Sabaté M, Valgimigli M, Frati G, Kedhi E, Smits PC, Kaiser C. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 45. | Sabaté M, Räber L, Heg D, Brugaletta S, Kelbaek H, Cequier A, Ostojic M, Iñiguez A, Tüller D, Serra A. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7:55-63. [PubMed] [Cited in This Article: ] |
| 46. | Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation. 2014;129:211-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 381] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 47. | van Geuns RJ, Tamburino C, Fajadet J, Vrolix M, Witzenbichler B, Eeckhout E, Spaulding C, Reczuch K, La Manna A, Spaargaren R. Self-expanding versus balloon-expandable stents in acute myocardial infarction: results from the APPOSITION II study: self-expanding stents in ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2012;5:1209-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Van Geuns RJ. Randamized comparison between the STENYS Self-Apposing Sirolimus-Eluting Coronary Stent and a balloon-expandable stent in Acute Myocardial Infarction. Presented at TCT. 2013; Available from: http:// www.tctmd.com/show.aspx?id=124277. [Cited in This Article: ] |
| 49. | Stone GW, Abizaid A, Silber S, Dizon JM, Merkely B, Costa RA, Kornowski R, Abizaid A, Wojdyła R, Maehara A. Prospective, Randomized, Multicenter Evaluation of a Polyethylene Terephthalate Micronet Mesh-Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction: The MASTER Trial. J Am Coll Cardiol. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 50. | Massberg S, Byrne RA, Kastrati A, Schulz S, Pache J, Hausleiter J, Ibrahim T, Fusaro M, Ott I, Schömig A. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST 5) trial. Circulation. 2011;124:624-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 51. | Carrié D, Berland J, Verheye S, Hauptmann KE, Vrolix M, Violini R, Dibie A, Berti S, Maupas E, Antoniucci D. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J Am Coll Cardiol. 2012;59:1371-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Tada N, Virmani R, Grant G, Bartlett L, Black A, Clavijo C, Christians U, Betts R, Savage D, Su SH. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv. 2010;3:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Costa JR, Abizaid A, Costa R, Feres F, Tanajura LF, Abizaid A, Maldonado G, Staico R, Siqueira D, Sousa AG. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc Interv. 2009;2:422-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Zhang L, Yuan J, Liu G, Zhong JP, Yin YH, She Q, Su L, Ling ZY, Chen YQ. One-year clinical outcome of a randomized trial of polymer-free paclitaxel-eluting stents versus biodegradable polymer-based rapamycin-eluting stents in patients with coronary heart disease. J Interv Cardiol. 2012;25:604-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Yu M, Xu B, Kandzari DE, Wu Y, Yan H, Chen J, Qian J, Qiao S, Yang Y, Gao RL. First report of a novel polymer-free dual-drug eluting stent in de novo coronary artery disease: results of the first in human BICARE trial. Catheter Cardiovasc Interv. 2014;83:405-411. [PubMed] [Cited in This Article: ] |
| 56. | Legutko J, Zasada W, Kałuża GL, Heba G, Rzeszutko L, Jakala J, Dragan J, Klecha A, Giszterowicz D, Dobrowolski W. A clinical evaluation of the ProNOVA XR polymer-free sirolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions (EURONOVA XR I study). Indian Heart J. 2013;65:388-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Dang Q, Li YJ, Gao L, Jin Z, Gou LX. Six-month angiographic and one-year clinical outcomes of polymer free paclitaxel-eluting stent in patients with ST-segment elevation myocardial infarction: a comparison with permanent polymer sirolimus-eluting stent. Chin Med J (Engl). 2012;125:3393-3397. [PubMed] [Cited in This Article: ] |
| 58. | Gori T, Schulz E, Hink U, Wenzel P, Post F, Jabs A, Münzel T. Early outcome after implantation of Absorb bioresorbable drug-eluting scaffolds in patients with acute coronary syndromes. EuroIntervention. 2014;9:1036-1041. [PubMed] [Cited in This Article: ] |
| 59. | Kajiya T, Liang M, Sharma RK, Lee CH, Chan MY, Tay E, Chan KH, Tan HC, Low AF. Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in patients with ST-segment elevation myocardial infarction (STEMI). EuroIntervention. 2013;9:501-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Brugaletta S, Gomez-Lara J, Bruining N, Radu MD, van Geuns RJ, Thuesen L, McClean D, Koolen J, Windecker S, Whitbourn R. Head to head comparison of optical coherence tomography, intravascular ultrasound echogenicity and virtual histology for the detection of changes in polymeric struts over time: insights from the ABSORB trial. EuroIntervention. 2012;8:352-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Fernández-Rodríguez D, Brugaletta S, Otsuki S, Sabaté M. Acute ABSORB bioresorbable vascular scaffold thrombosis in ST-segment elevation myocardial infarction: to stent or not to stent? EuroIntervention. 2013;Epub ahead of print. [PubMed] [Cited in This Article: ] |