Published online Dec 26, 2014. doi: 10.4330/wjc.v6.i12.1245
Revised: July 8, 2014
Accepted: October 14, 2014
Published online: December 26, 2014
Cardiomyopathy is a disease of myocardium categorized into three major forms, hypertrophic (HCM), dilated (DCM) and restrictive cardiomyopathy (RCM), which has recently been demonstrated to be a monogenic disease due to mutations in various proteins expressed in cardiomyocytes. Mutations in HCM and RCM typically increase the myofilament sensitivity to cytoplasmic Ca2+, leading to systolic hyperfunction and diastolic dysfunction. In contrast, mutations in DCM typically decrease the myofilament sensitivity to cytoplasmic Ca2+ and/or force generation/transmission, leading to systolic dysfunction. Creation of genetically-manipulated transgenic and knock-in animals expressing mutant proteins exogenously and endogenously, respectively, in their hearts provides valuable animal models to discover the molecular and cellular mechanisms for pathogenesis and promising therapeutic strategy in vivo. Recently, cardiomyocytes have been differentiated from patient’s induced pluripotent stem cells as a model of inherited cardiomyopathies in vitro. In this review, we provide overview of experimental models of cardiomyopathies with a focus on revealed molecular and cellular pathogenic mechanisms and potential therapeutics.
Core tip: Current experimental models of inherited cardiomyopathies (hypertrophic cardiomyopathy, dilated cardiomyopathy and restricted cardiomyopathy), including genetically-manipulated mouse models (transgenic and knock-in mice) and patient’s induced pluripotent stem cell-derived cardiomyocyte models, are summarized and discussed with a focus on revealed molecular pathogenic mechanisms and potential drug therapeutics.
- Citation: Nonaka M, Morimoto S. Experimental models of inherited cardiomyopathy and its therapeutics. World J Cardiol 2014; 6(12): 1245-1251
- URL: https://www.wjgnet.com/1949-8462/full/v6/i12/1245.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i12.1245
Cardiomyopathies are categorized, based on ventricular morphology and function, into three major forms, hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM)[1]. HCM is characterized by increased left ventricular (LV) wall thickness, cardiomyocyte disarray, increased myocardial fibrosis and impaired LV diastolic function with normal or increased LV systolic function[2-4]. DCM is characterized by LV dilatation and systolic dysfunction, frequently resulting in heart failure, arrhythmias and sudden death, with heart transplantation being the most effective treatment for survival at end stage because of no effective therapeutic drugs[5]. RCM is an uncommon form of cardiomyopathy, characterized by restrictive filling of LV and/or right ventricle despite normal or near-normal wall thickness and systolic function[6,7].
Following the uncovering of a gene mutation in β-myosin heavy chain (β-MyHC) of familial HCM patients at 1990[8], a large number of mutations in the genes encoding sarcomere proteins in cardiac muscle have been found to cause HCM, DCM and RCM[9]. Many animal models have been created to discover the functional consequences of these mutations and molecular mechanisms for the pathogenesis of cardiomyopathies in vivo, which should be critical for advancement of diagnosis and therapy. Recently, premature cardiomyocytes have been created from induced pluripotent stem cells (iPSC) of patients with inherited cardiomyopathies as a novel disease model in vitro. This review summarizes the recent advances in our understanding about molecular pathogenic mechanisms and potential therapeutic strategy brought about from these experimental models.
HCM, characterized by unexplained LV wall thickening and diastolic dysfunction, has an overall prevalence of 200 per 100000 individuals[10]. It is known that LV systolic function is not impaired but rather increased in HCM patients[2]. Structural remodeling involving hypertrophic growth of LV is believed to be caused by enhanced protein synthesis in cardiomyocytes leading to hyperplasia of myofibrils and thus cardiomyocyte enlargement. The purpose of current therapy for HCM is to improve diastolic dysfunction indirectly through suppressing systolic function using β-blockers, Ca2+ channel blockers or Na+ channel blockers[11-13].
Human HCM is a monogenic disorder, which is caused by several hundred distinct mutations in many genes found in patients and families with HCM[14,15]. The causal genes for HCM include those encoding cardiac myosin-binding protein C (MYBPC3), β-MyHC (MYH7), cardiac troponin C (TNNC1), cardiac troponin I (TNNI3), cardiac troponin T (TNNT2), cardiac actin (ACTC), α-tropomyosin (TPM1), regulatory myosin light chain, essential myosin light chain and titin/connectin. Mutations in these genes account for approximately 65% of all HCM cases[16], indicating that HCM is a disease of sarcomeric protein genes. The total number of mutations in each genes increase depending on the gene size, so that any one of mutations in two large genes encoding MYH7 and MYBPC3 are identified in about 50% of cases while mutations in other genes only account for less than 20% of cases[16].
Soon after discovery of these mutations in sarcomeric proteins, extensive studies have been started to understand the pathogenic mechanisms by exploring the effects of mutations on the in vitro sarcomeric function as well as the in vivo global structure and function of the heart using genetically modified animal models. In vitro studies revealed that HCM-linked mutations in thin filament-associated regulatory proteins, including TNNT2, consistently increase the myofilament sensitivity to cytoplasmic Ca2+ and thus probably impair diastolic function through a malfunction in the troponin-tropomyosin regulatory system[17-26]. Animal models of human HCM with mutations in cardiac troponin T[17,19,20,22,24], TNNI3[21,23] and TPM1[18,25,26] demonstrated that increased cardiac myofilament Ca2+ sensitivity is a root cause that initiates molecular cascades involving pathological cardiac remodeling in HCM. These findings indicate that reversal of the increased myofilament Ca2+ sensitivity toward normal levels is a promising definitive therapeutic strategy for HCM. At present, however, there exists no drugs that decrease the myofilament Ca2+ sensitivity through directly acting on the thin filament regulatory system, making it worthwhile to develop novel drugs “Ca2+ desensitizers”. Epigallocatechin gallate, a major polyphenol in green tea, is a potential lead compound for Ca2+ desensitizers, which has been demonstrated to decrease the myofilament Ca2+ sensitivity in membrane-permeabilized cardiac muscle fibers through binding to a C-terminal lobe region of TNNC1[27]. Poor absorption from the intestine and permeability into cells, however, may be serious problems to be solved. Another potential lead compound is blebbistatin, which has also been demonstrated to decrease the myofilament Ca2+ sensitivity in membrane-permeabilized cardiac muscle fibers through inhibiting the interaction between actin and myosin and prevent arrhythmia induced by Ca2+ sensitizer[28]. Crossing transgenic mice harboring HCM-linked sarcomeric mutation with transgenic mice harboring DCM-linked sarcomeric mutation conferring decreased myofilament Ca2+ sensitivity was found to normalize overall myofilament Ca2+ sensitivity and prevent cardiac deterioration[29,30], supporting the idea that Ca2+ desensitizer might be beneficial for HCM patients affected by mutations in sarcomeric protein genes.
HCM-causing mutations that increase the myofilament sensitivity to cytoplasmic Ca2+ also alter the regulation of intracellular Ca2+ level, which could activate hypertrophic response and failure in the myocardium[31]. Cardiomyocytes isolated from experimental mouse models of HCM show abnormal intracellular Ca2+ handling, including increased diastolic Ca2+ associated with decreased Ca2+ store in the sarcoplasmic reticulum (SR), and dysregulation of intracellular Ca2+ precede hypertrophic remodeling of the heart[32,33]. The voltage-dependent L-type Ca2+ channel inhibitor, diltiazem, restored the normal intracellular Ca2+ handling and suppressed cardiac hypertrophy in young mice with HCM-causing myosin R403Q mutation[33], indicating that pharmacologic interventions targeting early key intracellular events caused by abnormal intracellular Ca2+ regulation could prevent disease development.
DCM is characterized by progressive LV dilatation and systolic dysfunction, being the most common indication for cardiac transplantation[5]. Many mutations in various genes encoding sarcomeric proteins, cytoskeletal proteins, nuclear envelope proteins and sarcolemmal membrane proteins have been shown to be linked to approximately 25%-30% of the DCM cases[34-39]. Cardiomyocyte hypertrophy and fibrosis, but not cardiomyocyte disarray, are commonly observed as in the case of HCM[36]. DCM is frequently accompanying with abnormal cardiac conduction system, arrhythmias and sudden death probably due to pathophysiological myocardial remodeling and severe fibrosis. Underlying molecular mechanisms include diminished force generation/transmission, altered energy metabolism, and impaired intracellular calcium handling in cardiomyocytes[3]. The purpose of current standard therapy for DCM is to prevent the progression of myocardial remodeling and systolic dysfunction by a combination of cardioprotective drugs, including β-adrenergic receptor blockers, vasodilators (angiotensin converting enzyme inhibitors or angiotensin II receptor blockers), aldosterone antagonists and diuretics[40].
In contrast to HCM-causing mutations, DCM-causing mutations in TPM1[41] and TNNT2 consistently decrease the myofilament sensitivity to cytoplasmic Ca2+ and thus impair systolic function through a malfunction in the troponin-tropomyosin regulatory system[42,43]. A mouse model of DCM caused by the deletion mutation ΔK210 in TNNT2 demonstrated that lessened cardiac myofilament Ca2+ sensitivity is a root cause that initiates molecular cascades involving pathological cardiac remodeling in DCM[44]. This mouse model developed an early-onset severe LV dilation with high incidence of sudden death despite showing no heart failure symptoms, resembling the phenotypes of a human family of DCM patients with this mutation[35]. These findings indicate that reversal of the decreased myofilament Ca2+ sensitivity toward normal levels is a promising definitive therapeutic strategy for DCM linked to sarcomeric regulatory protein gene mutations. Early intervention with a Ca2+ sensitizer, pimobendan, had remarkable effects of preventing cardiac remodeling, systolic dysfunction and sudden death in this DCM model mouse[44]. However, it remains to be determined whether pimobendan has also therapeutic effects on DCM mice with this mutation after developing decompensated, end-stage heart failure. It may be worth noting that combination therapy with pimobendan and β-blocker has provided beneficial effects in DCM patients with severe heart failure[45,46].
Cardiomyocyte contraction is evoked by Ca2+, which is rapidly released into cytoplasm from SR upon sarcolemmal depolarization. Cytoplasmic Ca2+ is rapidly returned to a low level during diastole by reuptake into SR through SR Ca2+pump (SERCA2a). Myocardial expression of SERCA2a is down-regulated in the patients with end-stage congestive heart failure[47,48], resulting in a decrease in the rate of Ca2+ reuptake by SR[49-51]. Myocardial expression of SERCA2a was also confirmed to be markedly decreased in a mouse model of DCM[52]. In a pressure-overload heart failure model of rats, transfection of adenovirus expression vector carrying SERCA2a cDNA into the heart normalized the hemodynamic parameters, including LV end-systolic pressure, maximum rates of LV pressure increase and decrease, and isovolumic relaxation rate[53]. Another study using a pressure-overload model of rats demonstrated that adenoviral transfection of SERCA2a during heart failure reversed the LV dilation and improved the myocardial energy metabolism and survival[54]. SERCA2a gene transfer also improved the contractile function of cardiomyocytes taken from patients with heart failure by increasing the rates of contraction and relaxation, decreasing and increasing the cytoplasmic Ca2+ at diastole and systole, respectively, and normalizing the frequency dependence of force generation[55]. Taken together, these studies suggest that enhancement of SERCA2a expression in cardiomyocytes may serve as potential therapeutic strategy for DCM patients.
RCM is characterized by increased stiffness of ventricular chambers, with wall thickness and systolic function usually being within normal limits. The reduction in myocardial compliance results in an abnormally large increase in early diastolic ventricular pressure against small increment in volume and an abrupt termination of filling. Most individuals with RCM develop heart failure and die within a few years[56]. Several reports suggest clinical and genetic overlaps between RCM and HCM[56-58]. RCM is rare, and its genetic etiology has just started to be explored. To date, RCM-linked mutations are found in sarcomere protein genes, including TNNI3, TNNT2, MYH7 and ACTC[58-61].
Like sarcomeric gene mutations in other types of cardiomyopathy, RCM-causing sarcomeric gene mutations alter myofilament sensitivity to cytoplasmic Ca2+ through a malfunction in the troponin-tropomyosin regulatory system. Membrane-permeabilized cardiac muscle fibers prepared from transgenic mouse model of RCM are more sensitive to Ca2+ and show more force at low Ca2+ levels than those from transgenic mice overexpressing wild-type proteins[62]. This is consistent with the findings from earlier in vitro studies in which recombinant RCM-causing mutant proteins are exchanged into membrane-permeabilized cardiac muscle fibers[63-65]. Kobayashi et al[66] demonstrated that the increase in myofilament Ca2+ sensitivity was caused by increased affinity of troponin C for Ca2+ in the thin filament. Thus, the myofilament hypersensitivity to cytoplasmic Ca2+ is a common feature that RCM-causing mutations share with HCM-causing mutations. In vitro experiments using membrane-permeabilized cardiac muscle fibers reconstituted with recombinant mutant proteins revealed that RCM-causing mutations give much greater Ca2+ sensitivity to the myofilament compared with HCM-causing mutations[62,63]. Consistent with these in vitro reconstitution experiments, membrane-permeabilized cardiac muscle fibers prepared from transgenic mice expressing RCM-causing TNNI3 R145W mutant showed a much larger increase in the Ca2+ sensitivity of ATPase activity and force generation compared with those from transgenic mice expressing HCM-causing TNNI3 R145G mutant[62,67]. Crossing transgenic mice expressing RCM-causing TNNI3 R193H mutant with transgenic mice expressing N-terminal truncated TNNI3, known to decrease myofilament Ca2+ sensitivity, corrected the impaired relaxation in R193H RCM transgenic mice[68], supporting the idea that myofilament Ca2+ desensitizer could also be beneficial to treat RCM caused by sarcomeric protein gene mutations. Design of new compounds that exert lusitropic action on the heart directly through decreasing the myofilament Ca2+ sensitivity is an innovative and exciting challenge to overcome RCM as well as HCM.
Although the contribution of gene-manipulated animal models to the understanding of inherited cardiomyopathies in in vivo system has been enormous, small animals have significantly different intrinsic properties in the heart from human, including faster heart rate, shorter plateau phase in the action potential of ventricles, and much higher ratio of α/β-MyHC isoforms in ventricles. Intact cardiomyocytes are difficult to obtain from healthy parson and even from cardiomyopathy patients. The iPSC technology may offer a unique opportunity for creating disease-specific models directly from human patients with monogenic disease to investigate underlying mechanisms and carry out drug screening in human cardiomyocytes, though only in vitro[69,70]. Premature but self-beating cells like cardiomyocytes have been shown to be differentiated from human iPSC[71,72]. Patient-specific iPSC-derived cardiomyocytes have been created for HCM-causing missense mutation R663H in MYH7[73]. These iPSC-derived cardiomyocytes developed cellular hypertrophy and arrhythmia at the single cell level accompanying irregular Ca2+ cycling and elevation in resting cytoplasmic Ca2+ level. Further, pharmacological inhibition of Ca2+ entry with L-type Ca2+ channel blockers verapamil, nifedipine and diltiazem prevented development of cellular hypertrophy and electrophysiological abnormality. It is somewhat surprising that these numerous aspects of HCM phenotype can be reproduced in an in vitro cultured system without any neurohormornal stimulation, since these phenotypes are thought to develop as a long-term consequence of adaptation or compensation in vivo to an abnormal contractile function conferred by the mutation in a motor protein encoded in MYH7. The results of this study on patient-specific iPSC-derived cardiomyocytes, however, clearly show that iPSC-derived cardiomyocytes are a useful platform to elucidate molecular and cellular pathogenic mechanisms underlying inherited HCM and to identify novel therapies for this disease.
iPSC-derived cardiomyocytes from a three-generation family of DCM patients affected by a missense mutation R173W in TNNT2 have been shown to exhibit a lessened force generation capability, one of the common root causes for DCM, with impaired Ca2+ handling and abnormal distribution of Z-band α-actinin but no abnormalities in electrophysiological properties and cell size[74]. β1-selective adrenergic receptor blocker metoprolol improved the sarcomeric disorganization judged by α-actinin distribution, and over-expression of SERCA2a improved contractile function and Ca2+ handling. These findings demonstrated that cardiomyocytes differentiated from iPSCs of DCM patients recapitulated the disease phenotype to some extent and could be used as an in vitro experimental model to explore molecular and cellular pathogenic mechanisms underlying inherited DCM and to carry out drug screening for this disease.
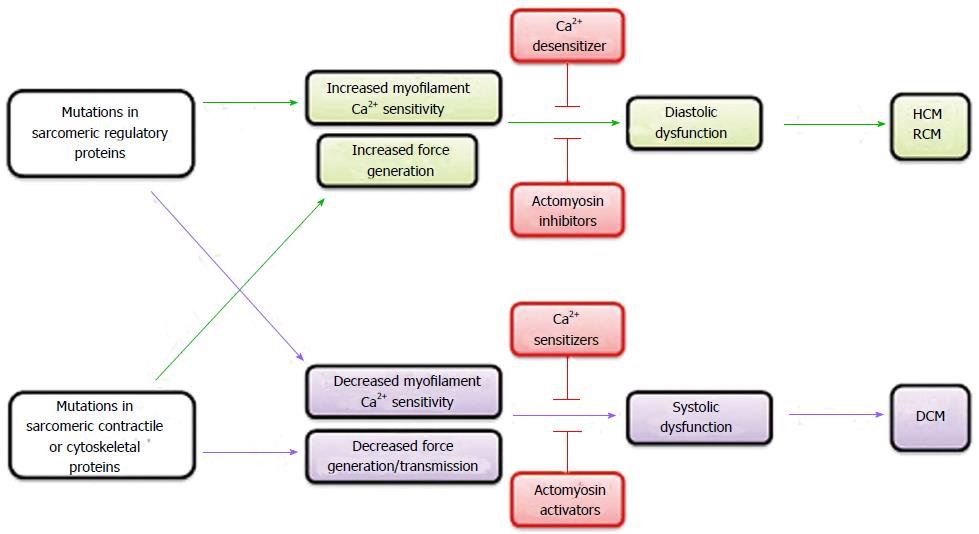
Abnormal sensitivity to cytoplasmic Ca2+ or force generation/transmission of cardiac myofilament, which is incurred as a direct functional consequence of mutations in genes encoding proteins in cardiomyocytes, is the primary root cause that initiates subsequent molecular and cellular events leading to pathological remodeling in inherited cardiomyopathies. HCM/RCM-causing mutations usually heighten the myofilament sensitivity to cytoplasmic Ca2+ or force generation, whereas DCM-causing mutations lessen the myofilament sensitivity to cytoplasmic Ca2+ or force generation/transmission. Therefore, reversal of the altered myofilament Ca2+ sensitivity or force generation/transmission capability toward normal levels should be a promising definitive therapeutic strategy to prevent or even reverse the progression of the disease in inherited cardiomyopathies (Figure 1). Further studies using gene-manipulated animal models and patient’s iPSC-derived cardiomyocytes briefly summarized in this review are important to develop novel therapeutic drugs for inherited cardiomyopathy patients.
P- Reviewer: Hwang KC S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Callis TE, Jensen BC, Weck KE, Willis MS. Evolving molecular diagnostics for familial cardiomyopathies: at the heart of it all. Expert Rev Mol Diagn. 2010;10:329-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1394] [Cited by in F6Publishing: 1377] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 3. | Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77:659-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res. 2011;108:743-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 642] [Cited by in F6Publishing: 610] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med. 1997;336:267-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 331] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Rivenes SM, Kearney DL, Smith EO, Towbin JA, Denfield SW. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2000;102:876-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 963] [Cited by in F6Publishing: 896] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 9. | Lu QW, Wu XY, Morimoto S. Inherited cardiomyopathies caused by troponin mutations. J Geriatr Cardiol. 2013;10:91-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 36] [Reference Citation Analysis (0)] |
| 10. | Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1766] [Cited by in F6Publishing: 1735] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 11. | Harrison Dc, Braunwald E, Glick G, Mason Dt, Chidsey Ca, Ross J. Effects Of Beta Adrenergic Blockade On The Circulation With Particular Reference To Observations In Patients With Hypertrophic Subaortic Stenosis. Circulation. 1964;29:84-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Rosing DR, Kent KM, Maron BJ, Epstein SE. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. II. Effects on exercise capacity and symptomatic status. Circulation. 1979;60:1208-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 153] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:1251-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881-1891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 361] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 15. | Marian AJ. Genetic determinants of cardiac hypertrophy. Curr Opin Cardiol. 2008;23:199-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Morimoto S, Yanaga F, Minakami R, Ohtsuki I. Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am J Physiol. 1998;275:C200-C207. [PubMed] [Cited in This Article: ] |
| 18. | Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, Wolska B, Evans C, Solaro RJ, Wieczorek DF. Mouse model of a familial hypertrophic cardiomyopathy mutation in alpha-tropomyosin manifests cardiac dysfunction. Circ Res. 1999;85:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Nakaura H, Morimoto S, Yanaga F, Nakata M, Nishi H, Imaizumi T, Ohtsuki I. Functional changes in troponin T by a splice donor site mutation that causes hypertrophic cardiomyopathy. Am J Physiol. 1999;277:C225-C232. [PubMed] [Cited in This Article: ] |
| 20. | Nakaura H, Yanaga F, Ohtsuki I, Morimoto S. Effects of missense mutations Phe110Ile and Glu244Asp in human cardiac troponin T on force generation in skinned cardiac muscle fibers. J Biochem. 1999;126:457-460. [PubMed] [Cited in This Article: ] |
| 21. | James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, Robbins J. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ Res. 2000;87:805-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Szczesna D, Zhang R, Zhao J, Jones M, Guzman G, Potter JD. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. J Biol Chem. 2000;275:624-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Elliott K, Watkins H, Redwood CS. Altered regulatory properties of human cardiac troponin I mutants that cause hypertrophic cardiomyopathy. J Biol Chem. 2000;275:22069-22074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Chandra M, Rundell VL, Tardiff JC, Leinwand LA, De Tombe PP, Solaro RJ. Ca(2+) activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol. 2001;280:H705-H713. [PubMed] [Cited in This Article: ] |
| 25. | Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro RJ, Wieczorek DF. A familial hypertrophic cardiomyopathy alpha-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815-1828. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Wolska BM, Wieczorek DM. The role of tropomyosin in the regulation of myocardial contraction and relaxation. Pflugers Arch. 2003;446:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Tadano N, Du CK, Yumoto F, Morimoto S, Ohta M, Xie MF, Nagata K, Zhan DY, Lu QW, Miwa Y. Biological actions of green tea catechins on cardiac troponin C. Br J Pharmacol. 2010;161:1034-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893-3903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga GM, Solaro RJ, Liggett SB, Wieczorek DF. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol. 2007;293:H949-H958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Davis J, Metzger JM. Combinatorial effects of double cardiomyopathy mutant alleles in rodent myocytes: a predictive cellular model of myofilament dysregulation in disease. PLoS One. 2010;5:e9140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106:2342-2347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 322] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 32. | Guinto PJ, Haim TE, Dowell-Martino CC, Sibinga N, Tardiff JC. Temporal and mutation-specific alterations in Ca2+ homeostasis differentially determine the progression of cTnT-related cardiomyopathies in murine models. Am J Physiol Heart Circ Physiol. 2009;297:H614-H626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, Reiken S, Mende U, Marks AR, Kass DA. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Hershberger RE, Morales A, Siegfried JD. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genet Med. 2010;12:655-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 35. | Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688-1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 491] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 36. | Schönberger J, Seidman CE. Many roads lead to a broken heart: the genetics of dilated cardiomyopathy. Am J Hum Genet. 2001;69:249-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Fatkin D, Otway R, Richmond Z. Genetics of dilated cardiomyopathy. Heart Fail Clin. 2010;6:129-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 384] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 39. | Judge DP. Use of genetics in the clinical evaluation of cardiomyopathy. JAMA. 2009;302:2471-2476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Luk A, Ahn E, Soor GS, Butany J. Dilated cardiomyopathy: a review. J Clin Pathol. 2009;62:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101:205-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:913-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Lu QW, Morimoto S, Harada K, Du CK, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Ohtsuki I. Cardiac troponin T mutation R141W found in dilated cardiomyopathy stabilizes the troponin T-tropomyosin interaction and causes a Ca2+ desensitization. J Mol Cell Cardiol. 2003;35:1421-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Yoshikawa T, Baba A, Suzuki M, Yokozuka H, Okada Y, Nagami K, Takahashi T, Mitamura H, Ogawa S. Effectiveness of carvedilol alone versus carvedilol + pimobendan for severe congestive heart failure. For the Keio Interhospital Cardiology Study (KICS) Group. Am J Cardiol. 2000;85:1495-1497; A7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Murai K, Seino Y, Kimata N, Inami T, Murakami D, Abe J, Yodogawa K, Maruyama M, Takano M, Ohba T. Efficacy and limitations of oral inotropic agents for the treatment of chronic heart failure. Int Heart J. 2013;54:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol. 2001;33:1345-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | DiPaola NR, Sweet WE, Stull LB, Francis GS, Schomisch Moravec C. Beta-adrenergic receptors and calcium cycling proteins in non-failing, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol. 2001;33:1283-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Schmidt U, Hajjar RJ, Kim CS, Lebeche D, Doye AA, Gwathmey JK. Human heart failure: cAMP stimulation of SR Ca(2+)-ATPase activity and phosphorylation level of phospholamban. Am J Physiol. 1999;277:H474-H480. [PubMed] [Cited in This Article: ] |
| 50. | Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 281] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 51. | Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994;74:555-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 245] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Li L, Morimoto S, Take S, Zhan DY, Du CK, Wang YY, Fan XL, Yoshihara T, Takahashi-Yanaga F, Katafuchi T. Role of brain serotonin dysfunction in the pathophysiology of congestive heart failure. J Mol Cell Cardiol. 2012;53:760-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 415] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 54. | del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | del MonteF SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308-2311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 346] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 56. | Sen-Chowdhry S, Syrris P, McKenna WJ. Genetics of restrictive cardiomyopathy. Heart Fail Clin. 2010;6:179-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Karam S, Raboisson MJ, Ducreux C, Chalabreysse L, Millat G, Bozio A, Bouvagnet P. A de novo mutation of the beta cardiac myosin heavy chain gene in an infantile restrictive cardiomyopathy. Congenit Heart Dis. 2008;3:138-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Kubo T, Gimeno JR, Bahl A, Steffensen U, Steffensen M, Osman E, Thaman R, Mogensen J, Elliott PM, Doi Y. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J Am Coll Cardiol. 2007;49:2419-2426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 59. | Kaski JP, Syrris P, Burch M, Tomé-Esteban MT, Fenton M, Christiansen M, Andersen PS, Sebire N, Ashworth M, Deanfield JE. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart. 2008;94:1478-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Mogensen J, Kubo T, Duque M, Uribe W, Shaw A, Murphy R, Gimeno JR, Elliott P, McKenna WJ. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest. 2003;111:209-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Peddy SB, Vricella LA, Crosson JE, Oswald GL, Cohn RD, Cameron DE, Valle D, Loeys BL. Infantile restrictive cardiomyopathy resulting from a mutation in the cardiac troponin T gene. Pediatrics. 2006;117:1830-1833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Wen Y, Xu Y, Wang Y, Pinto JR, Potter JD, Kerrick WG. Functional effects of a restrictive-cardiomyopathy-linked cardiac troponin I mutation (R145W) in transgenic mice. J Mol Biol. 2009;392:1158-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Yumoto F, Lu QW, Morimoto S, Tanaka H, Kono N, Nagata K, Ojima T, Takahashi-Yanaga F, Miwa Y, Sasaguri T. Drastic Ca2+ sensitization of myofilament associated with a small structural change in troponin I in inherited restrictive cardiomyopathy. Biochem Biophys Res Commun. 2005;338:1519-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Pinto JR, Parvatiyar MS, Jones MA, Liang J, Potter JD. A troponin T mutation that causes infantile restrictive cardiomyopathy increases Ca2+ sensitivity of force development and impairs the inhibitory properties of troponin. J Biol Chem. 2008;283:2156-2166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 65. | Gomes AV, Liang J, Potter JD. Mutations in human cardiac troponin I that are associated with restrictive cardiomyopathy affect basal ATPase activity and the calcium sensitivity of force development. J Biol Chem. 2005;280:30909-30915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Kobayashi T, Solaro RJ. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem. 2006;281:13471-13477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Wen Y, Pinto JR, Gomes AV, Xu Y, Wang Y, Wang Y, Potter JD, Kerrick WG. Functional consequences of the human cardiac troponin I hypertrophic cardiomyopathy mutation R145G in transgenic mice. J Biol Chem. 2008;283:20484-20494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Li Y, Charles PY, Nan C, Pinto JR, Wang Y, Liang J, Wu G, Tian J, Feng HZ, Potter JD. Correcting diastolic dysfunction by Ca2+ desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol. 2010;49:402-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7589] [Cited by in F6Publishing: 6999] [Article Influence: 411.7] [Reference Citation Analysis (0)] |
| 70. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14327] [Cited by in F6Publishing: 13579] [Article Influence: 848.7] [Reference Citation Analysis (0)] |
| 71. | Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30-e41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 936] [Cited by in F6Publishing: 946] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 72. | Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 73. | Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 475] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 74. | Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |