INTRODUCTION
One of the most important challenges for the cardiologists is the assessment of myocardial function and deformation. Echocardiography represents the most common approach for assessing cardiac function, but is limited by operator-dependency, time-consumption and relatively low sensitivity in detecting subtle abnormalities in myocardial contraction. The early assessment of cardiac involvement is crucial to address therapeutic strategies and to improve the quality of life and the survival of patients in different clinical settings, such as rheumatoid arthritis (RA)[1], renal transplant recipients[2], and systemic inflammatory diseases[3].
Until now, visual assessment of wall motion and thickening according to the recommendations of the American Society of Echocardiography[4] has been the most used method to study left ventricular (LV) mechanics, but it allows only a subjective evaluation of myocardial function and requires long-term training.
Furthermore, the visual assessment of LV ejection fraction, which relies mainly on myocardial radial performance with little consideration of longitudinal deformation, is limited by high inter- and intra-observer variability, and provides a subjective evaluation of endocardial thickening and excursion. Thus, we can only obtain a qualitative assessment of LV function and it is mandatory to highlight that, in different clinical conditions, subtle myocardial function impairment could be present despite normal ejection fraction.
At the same time, LV ejection fraction evaluation using Simpson’s biplane method of discs shows some limitations: it is semi-quantitative, operator-dependent and requires an optimal visualization of LV apex and endocardial border.
Recently, to overcome standard echocardiographic limitations regarding the study of LV function, tissue Doppler imaging (TDI) has been introduced into clinical practice. It appears more sensitive than ejection fraction for detecting subtle abnormalities in LV myocardial function, but it is limited to longitudinal and radial deformation measurements[5]. TDI has been considered a reliable tool to point out subclinical cardiac involvement in systemic sclerosis patients who show normal standard echo parameters[6]; moreover, Birdane et al [7] have demonstrated that RA patients have significant impairment of left and right ventricular TDI parameters compared to healthy controls. However, this method is limited by angle-dependency: whereas myocardium deforms simultaneously in three dimensions, and only deformation along the ultrasound beam can be derived from velocities with TDI[8]. In fact, at insonation angle greater than 20 degrees, Doppler-derived deformation is significantly underestimated[9]. TDI is also not able to differentiate active and passive myocardial motion[9] and high temporal resolution images are required for TDI acquisitions.
Speckle tracking echocardiography (STE) is an emerging algorithm that is able to analyze echocardiographic imaging, which provides an objective and reproducible quantification of global and regional myocardial function.
STE AND MYOCARDIAL STRAIN
STE is a new technique of two-dimensional echo image analysis that allows the study of regional myocardial deformation[10] expressed by a dimensionless parameter, the strain (ε), defined by the Lagrangian formula as the percent change from the original dimension[11]. On the other hand, displacement reflects myocardial motion: over a defined period of time, if all parts of a myocardial segment have the same motion, the segment will change position (displacement) but not shape (deformation), whereas when different parts of a segment have different motion, there is overall deformation of the segment. Thus, the study of both ε and displacement allows one to discriminate between passive movement and active contraction of each myocardial segment[12].
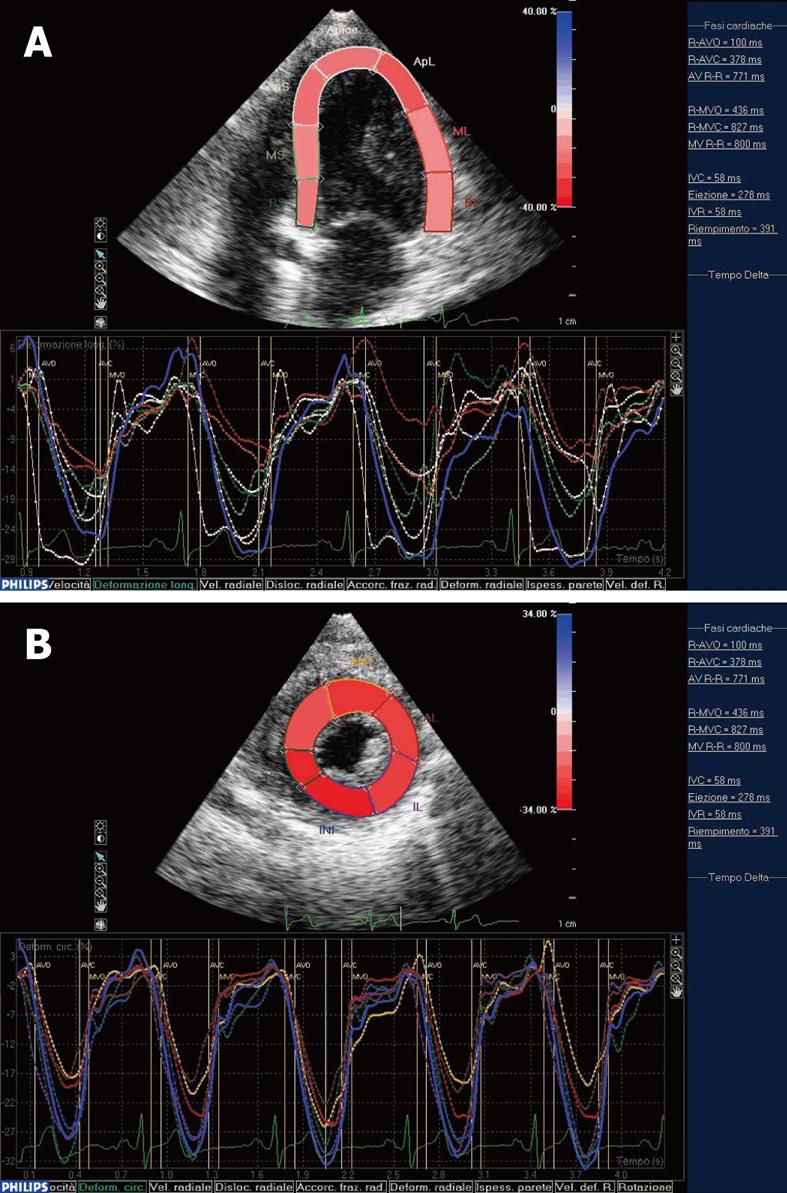
As described for the first time by Heimdal et al [13], deformation of a tissue occurs over time during the cardiac cycle and the rate of this deformation, the strain rate (SR), is equivalent to the velocity gradient. Myocardial ε can be determined both by TDI and STE. Different from TDI, STE is an angle-independent technique that may allow an accurate assessment of segmental myocardial deformation by grey-scale based imaging analysis frame by frame. Moreover, the lack of angle-dependency is of great advantage because myocardial ε could be tracked in two-dimensional echo imaging, along the direction of the wall and not along the ultrasound beam[8]. This means that we can analyze myocardial ε along three spatial axes according to the cardiac muscle physiology. In fact, after electro-mechanical activation, systolic myocardial deformation occurs in three spatial dimensions: a longitudinal and circumferential shortening and a radial thickening. Thus, longitudinal (Figure 1A) and circumferential deformations (Figure 1B) result in a negative ε, while radial thickening consists of a positive ε[14]. In clinical practice, we can track longitudinal epsilon in a four-chamber view with the ultrasound beam along the major LV axis, while circumferential ε can be detected in the short axis view. Radial ε can be evaluated in both acoustic windows.
Figure 1 Systolic myocardial deformation after electro-mechanical activation.
A: LV longitudinal strain from the apical four-chamber view: time-strain curves show a negative end-systolic strain representing myocardial shortening during systole; B: LV circumferential strain from the short axis view: time-strain curves show a negative end-systolic strain representing myocardial shortening during systole. End-systole has been identified by the AVC. At this point, we could observe the negative peak of the time-strain curves corresponding to each myocardial segment.
When myocardial deformation is graphically represented as time-strain curves, cardiac cycle phases can be recognized as follows: from the original length, during systole, we observe a negative wave that reaches its peak at the aortic valve closure (AVC), which represents the maximal longitudinal myocardial shortening during contraction. In diastole, strain values progressively increase towards the original length. Recently, the usefulness of STE has been reported to detect early systolic function abnormalities in patients with hypertrophic cardiomyopathy[15] and to quantify LV dys-synchrony[16].
Regarding technical issues, STE needs high quality grey-scale images with an optimal frame rate between 50 and 70 frames/s. Amundsen et al[17] have demonstrated that STE can quantify regional myocardial deformation independently of insonation angle and thus simultaneously assess systolic long-axis and short-axis ε. Moreover, the same authors have confirmed the accuracy of STE by using sonomicrometry and cardiac magnetic resonance (CMR) imaging as reference methods.
The major clinical applications of STE are represented by the quantitative assessment of regional myocardial function in ischemic disease. Bjork Ingul et al[18] have described the incremental prognostic value of strain imaging in association with wall motion analysis during dobutamine stress echocardiography. However, this study highlighted the TDI pitfalls that can be overcome by the implementation of STE. As detected by Choi et al[19], LV peak systolic longitudinal strain by STE might be a sensitive screening for severe coronary artery disease in the absence of regional wall motion abnormalities at rest.
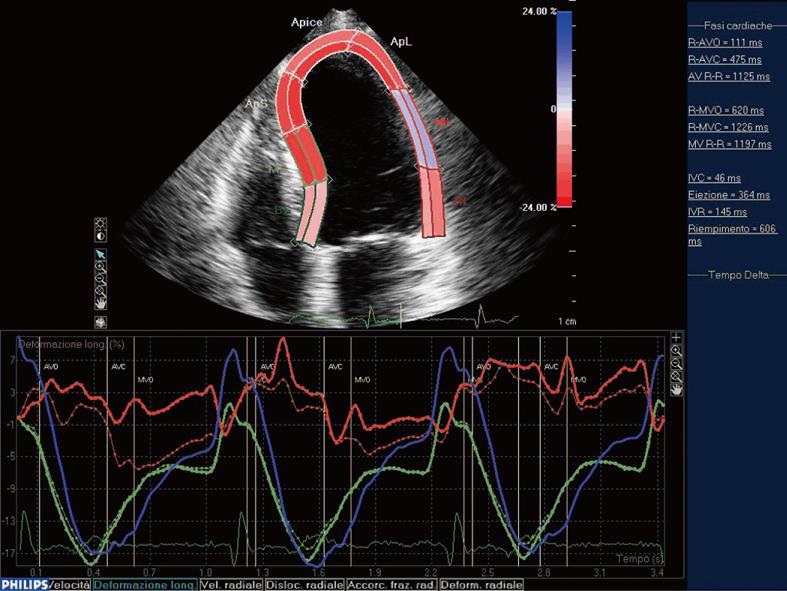
Leitman et al[10] have observed that the major difference between normal and ischemic myocardium was the lower peak systolic strain in the hypokinetic segments. Furthermore, STE is able to assess the infarct size adding important diagnostic and prognostic information[20]. The study of both epicardial and endocardial border and the comparison between them could provide further information about the transmurality of myocardial infarction (Figure 2).
Figure 2 Comparison between epicardial (continous line) and endocardial border (discontinuous line) in lateral myocardial infarction.
In the mid-septal segment (green line) the endocardial and epicardial curves are indistinguishable and result in an end-systolic shortening, while in the ischemic mid-lateral segment (red line) the epicardial curve separates from the endocardial one. Moreover, in this segment, we can observe the lack of myocardial deformation after the electromechanical activation.
Another interesting challenge for cardiologists is the differentiation between hypertrophic cardiomyopathy and athlete’s heart. Even in this field, STE seems to provide interesting advantages: Richand et al[15], comparing global and regional myocardial deformation in professional soccer players, control subjects and patients with hypertrophic cardiomyopathy have suggested that STE analysis can be considered a highly reproducible method to differentiate “physiological” from pathological hypertrophy. A noteworthy application of STE is the evaluation of LV mechanical dys-synchrony. Becker et al[21] have demonstrated the usefulness of the assessment of circumferential strain in the detailed analysis of the myocardial contraction sequence: the optimal LV lead position results in a greater improvement of LV function and in more reverse LV remodeling.
STE appears to be a reliable tool to detect early subtle cardiac involvement in different clinical settings such as connective tissue diseases[22,23].
Beyond longitudinal and circumferential shortening and radial thickening, LV torsion has been recently evaluated[24]. It results from the oblique alignment of the longitudinal fibers arranged in opposite directions between subendocardial and subepicardial layers[25]. Until now, CMR with tissue tagging has been used as the gold standard to evaluate LV torsion considering the difference between basal and apical rotation[26]. Echocardiography is an alternative noninvasive method and the recent introduction of STE draws new attention to LV torsion. Notomi et al[27] have demonstrated that STE correlated with CMR assessment of torsion in 13 normal subjects, while Zhang et al[28] have shown the potential of STE to study the different contribution to LV torsion of the subendocardial and subepicardial myocardium layer. However, further studies are necessary to validated STE in comparison with CMR for the study of LV torsion.
With standard echocardiography, the quantification of regional and global function of the right ventricle is limited by the complex geometry of the chamber. Recently, STE has been used to assess right ventricular free wall longitudinal myocardial deformation in normal subjects[29], and seems to be able to evaluate quantitatively regional and global systolic function of the right ventricle in patients with pulmonary arterial hypertension[30]. However, the reliability of STE for the study of the right ventricle function should be validate in larger studies.
CONCLUSION
STE is a reliable and feasible tool to evaluate myocardial ε, which describes the myocardial deformation throughout the cardiac cycle. It seems to overcome the subjective and semi-quantitative study of LV function by visual assessment of wall motion and ejection fraction and appears more trustworthy than TDI. In fact, the lack of angle-dependency allows a global insight in LV myocardial mechanics that investigate circumferential, radial and longitudinal fibers function. Moreover, it is able to provide important information about LV torsion.
Beyond its potential usefulness in the assessment of myocardial viability and follow-up of the ischemic cardiac disease, evidence has underlined the sensitivity of STE to detect early preclinical cardiac involvement in asymptomatic patients affected by connective tissue diseases and data are available about its role in the study of LV dys-synchrony.
In conclusion, STE analysis seems to provide important information regarding the assessment of myocardial function, already applicable primarily in research, but also in clinical settings. The application of STE has been implemented in several experimental and clinical studies, some of which have been cited representatively in this editorial. However, further studies need to be done to introduce this new technique widely in clinical practice.