Published online Oct 26, 2011. doi: 10.4330/wjc.v3.i10.322
Revised: July 8, 2011
Accepted: July 15, 2011
Published online: October 26, 2011
AIM: To characterize hydraulic right ventricle (RV) afterload by pulmonary arterial pressure waveform analysis in an acute pulmonary hypertension (PH) model.
METHODS: Pulmonary artery (PA) flow and pressure were recorded in six anesthetized sheep. Acute isobaric PH was induced by phenylephrine (active) and PA mechanical constriction (passive). We estimated the amplitude of the forward and reflected pressure waves according to the inflection point. In most cases the inflection pressure was smooth, thus the inflection point was defined as the time at which the first derivative of pulmonary arterial pressure reached its first minimum. We calculated the input and characteristic (ZC, time-domain Li method) impedances, the capacitance index (stroke volume/pulse pressure), the augmentation index (AI) (reflected pressure/pulse pressure), the fractional pulse pressure (pulse pressure/mean pressure) and the wasted energy generated by the RV due to wave reflection during ejection (EW).
RESULTS: Pulse pressure, fractional pulse pressure, AI and ZC increased and capacitance index decreased during passive PH with respect to control (P < 0.05). In contrast, ZC and the capacitance index did not change and EW and the AI decreased during active PH. Pulse pressure correlated with EW and ZC and the AI was correlated with EW (r > 0.6, P < 0.05).
CONCLUSION: PA pressure waveform analysis allows the quantification of the dynamic RV afterload. Prospective clinical studies will be necessary to validate this time-domain approach to evaluate the dynamic RV afterload in chronic PH.
- Citation: Grignola JC, Domingo E, Devera L, Ginés F. Assessment of right ventricular afterload by pressure waveform analysis in acute pulmonary hypertension. World J Cardiol 2011; 3(10): 322-328
- URL: https://www.wjgnet.com/1949-8462/full/v3/i10/322.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i10.322
Clinical signs of right ventricle (RV) failure are often not clearly related to the progression of pulmonary hypertension (PH), as assessed by pulmonary vascular resistance. Adaptation of the RV to acute and/or chronic PH depends on both the stationary and the pulsatile components of afterload[1,2]. Due to the low resistance and high compliance of the healthy pulmonary vascular tree, it is very important to consider the pulsatile component of hydraulic load. Vascular smooth muscle (VSM) activation improves the buffering function of conduit central vessels, attenuating the pulsatile component of afterload and improving RV-pulmonary artery (PA) coupling in acute PH[3].
The morphology and amplitude of the PA pressure wave (Pp, pulse pressure) results from the interaction between the RV and the PA system. The Pp consists of a forward wave (inflection pressure-diastolic pressure, Pi-Pd) generated by RV ejection, and the arrival of a reflected wave (systolic pressure-Pi, Ps-Pi) from the periphery. Pi is the PA pressure upon the return of the reflected wave (which coincides with maximal pulmonary flow - PF), and the reflected wave/Pp ratio is the augmentation index (AI)[4]. The time to the inflection point (Ti) quantifies the timing of the pressure wave reflection. The forward wave depends on the elastic properties of the main PA and its branches, while the reflected wave depends on those of the entire arterial tree, as well as the pulse wave velocity and the distance from the main reflection sites.
In healthy young individuals, AI is low and weakly contributes to Pp. The reflected pressure wave is rather diffuse and maintains a relatively high central arterial pressure in early diastole, thus reducing pulsatile afterload. Increased proximal arterial stiffness determines the increase and narrowing of the reflected pressure wave, contributing significantly to Pp (high AI value) rather than increasing early diastolic pressure[5]. PA stiffening is also responsible for increasing the characteristic impedance (ZC), thus the forward pressure-wave amplitude increases and also pulse wave velocity is increased, resulting in premature return of the reflected wave during systole and augmentation of central pulse pressure without affecting mean arterial pressure[5].
The capacitance index (Cp), a measure of global pulsatile vascular load, has proved to be an independent predictor of mortality in patients with idiopathic PH[6]. Although both the fractional pulse pressure (Ppf = Pp/mean arterial pressure) and the AI enable a differential diagnosis between idiopathic PH and chronic thromboembolic PH[7], a complete dynamic afterload evaluation in the time domain has not been evaluated in PH.
The aims of the present study were to characterize the stationary (pulmonary vascular resistance, input impedance) and pulsatile (Cp, AI, ZC) components of the hydraulic RV afterload in the time domain (using the PA pressure waveform) in an acute PH ovine model.
Six Merino sheep weighting 25-30 kg were anesthetized with intravenous sodium pentobarbital (35 mg/kg bolus and 3 mg/kg per hour infusion), fentanyl (2 µg/kg bolus and 1 µg/kg per hour infusion) and atracurium (1 mg/kg per hour infusion). The animals underwent tracheostomy and were ventilated using a ventilator (Dräger SIMV Polyred 201, Spain) with a 40% inspired oxygen fraction. Oxygen and carbon dioxide partial pressures were monitored (Radiometer BMS 3 MK2, Copenhagen, Denmark). Both breathing rate and tidal volume were adjusted to maintain arterial pCO2 between 35 mmHg and 45 mmHg, pH between 7.35 and 7.4 and arterial pO2 greater than 80 mmHg. Deep periodic inhalations and transitory increases of 5-10 cm H2O in end-expiratory positive pressure were performed to reduce the occurrence of atelectasis. The right saphenous vein was catheterized to allow administration of saline, anesthetic drugs and phenylephrine. A fluid column catheter was placed from the right femoral artery to the abdominal aorta to monitor systemic arterial pressure and obtain blood samples for gases analysis. A thoracotomy was performed at the 4th left intercostal space. After opening the pericardium, the PA and its main branches were exposed. A transonic perivascular flow probe was placed around the PA (16 mm or 20 mm, Transonic Systems, Ithaca, NY, United States), 2 cm from the pulmonary valve. A solid-state pressure microtransducer (model P7, 1200 Hz, Konigsberg Instruments, Inc., Pasadena, CA, United States), previously calibrated using a mercury manometer, was inserted distal to the flow probe through a small incision in the PA[3,8]. As a result, both the flow and instantaneous pressure signals were recorded simultaneously and in the same location of the PA. An occluder band was placed around each PA branch to induce acute increments in PA pressure. Pulmonary flow was measured using a Doppler flow meter (T-106; Transonic Systems, Ithaca, NY, United States) with a low-pass 100 Hz filter.
After surgical instrumentation, 30 min were allowed for stabilization of the recordings. All signals were acquired during three stable hemodynamic states according to the following sequence: (1) control steady state at normal pressure: stable basal state without PA occlusion nor phenylephrine administration; (2) active PH: VSM was activated by iv phenylephrine infusion (5 µg/kg·per min, Sigma, St. Louis, MO, United States), achieving stabilization of pulmonary pressure and flow 15-20 min after infusion; and (3) passive PH: the PA branch occluders were compressed in order to obtain a mechanical high pressure non-active state for 10 min. Pressure levels were established to ensure isobaric pulmonary conditions between this maneuver and phenylephrine.
Between steps 2 and 3, 20 min were allowed to re-establish control state pressure and flow values. The similar level of mean arterial pressure in both PH states enabled an isobaric analysis. Upon completion of the experimental protocol, the animals were sacrificed using a pentobarbital overdose followed by i.v. potassium chloride. The present protocol was approved by the Honorary Commission for Animal Experimentation of the Universidad de la República (Uruguay), in accordance with the principles outlined in the international guides for the care and use of laboratory animals published by the National Institutes of Health (NIH Publication Nº 86-23, revised 1995).
All invasive signals were monitored in real time and digitized on-line using a 200 Hz A/D converter, and software developed in our laboratory (SAMAY MD16), as previously described[3,8]. Approximately 10-15 consecutive beats were analyzed in each stable hemodynamic state. During data acquisition the ventilator was turned-off. Both direct values and those derived from the signals were processed off-line.
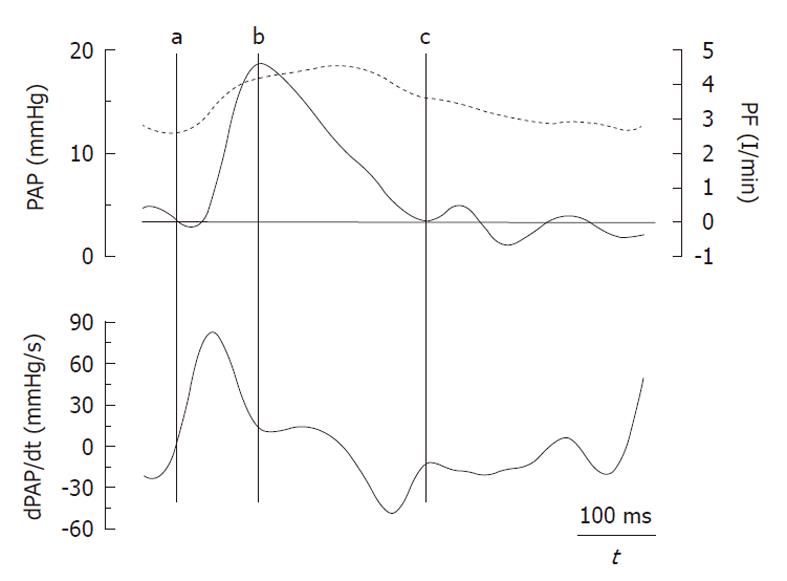
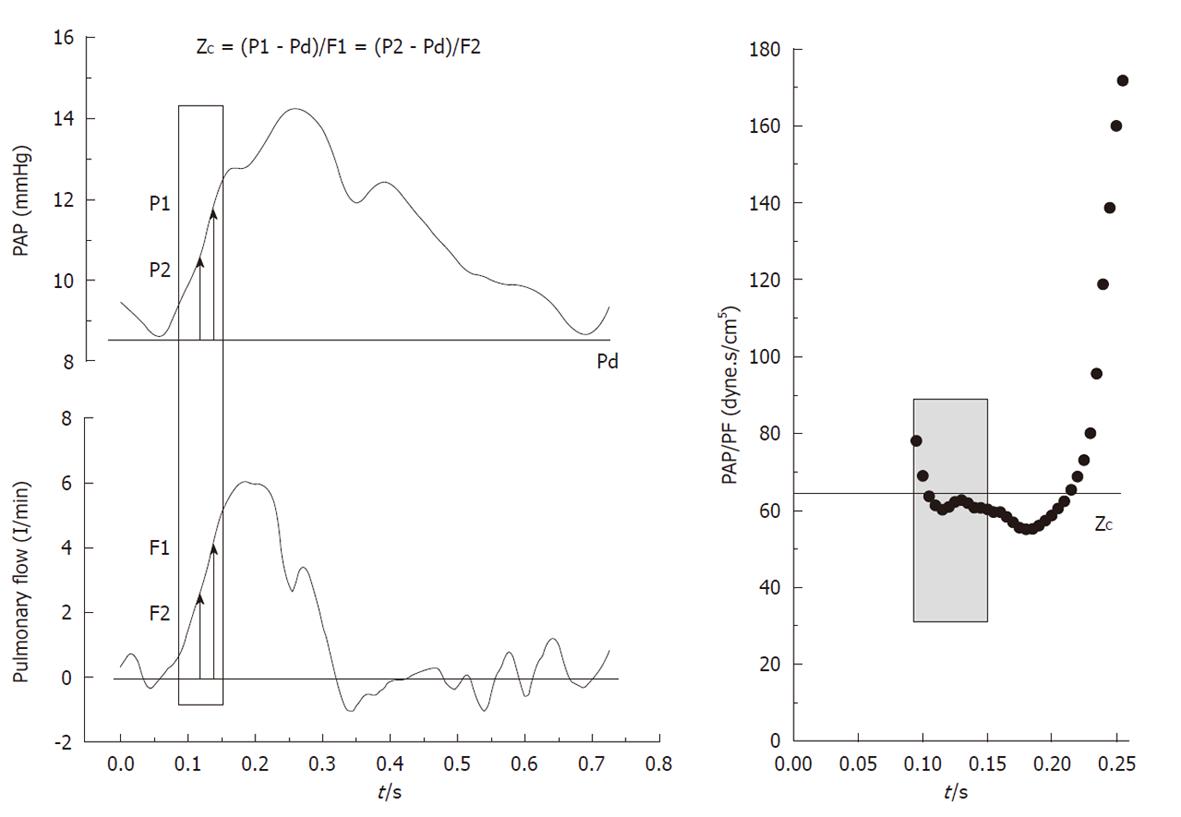
The amplitude of the forward (Pi-Pd) and reflected (Ps-Pi) waves were estimated from the inflection point (Pi, Ti). As it was previously reported by Kelly et al[9] in the systemic circulation, this point was determined when the first derivative of the arterial pressure reached its first minimum, which coincided with the peak of the local arterial flow (Figure 1). The AI was estimated by Ps-Pi/Pp, and the return time of the reflected wave was estimated by Ti. The energy lost by the RV due to the wave reflected during ejection (extra workload, EW) was quantified using [(Ts-Ti)(Ps-Pi)π/2], where (Ts-Ti) and (Ps-Pi) correspond to the systolic duration and magnitude of the reflected wave, respectively[4]. Input impedance was estimated by the ratio between mean pulmonary AP and PF. ZC was calculated using the instantaneous quotient of pressure and PF above the diastolic value in the first 60 ms (Li method), assuming a lack of wave reflection in early ejection (Figure 2, shaded area)[10]. Pulmonary Cp was estimated as the ratio between stroke volume and Pp[11,12].
Data were expressed as mean ± SD. Friedman’s and Wilcoxon’s tests were used, as well as simple linear regression analysis, with P values < 0.05 considered significant.
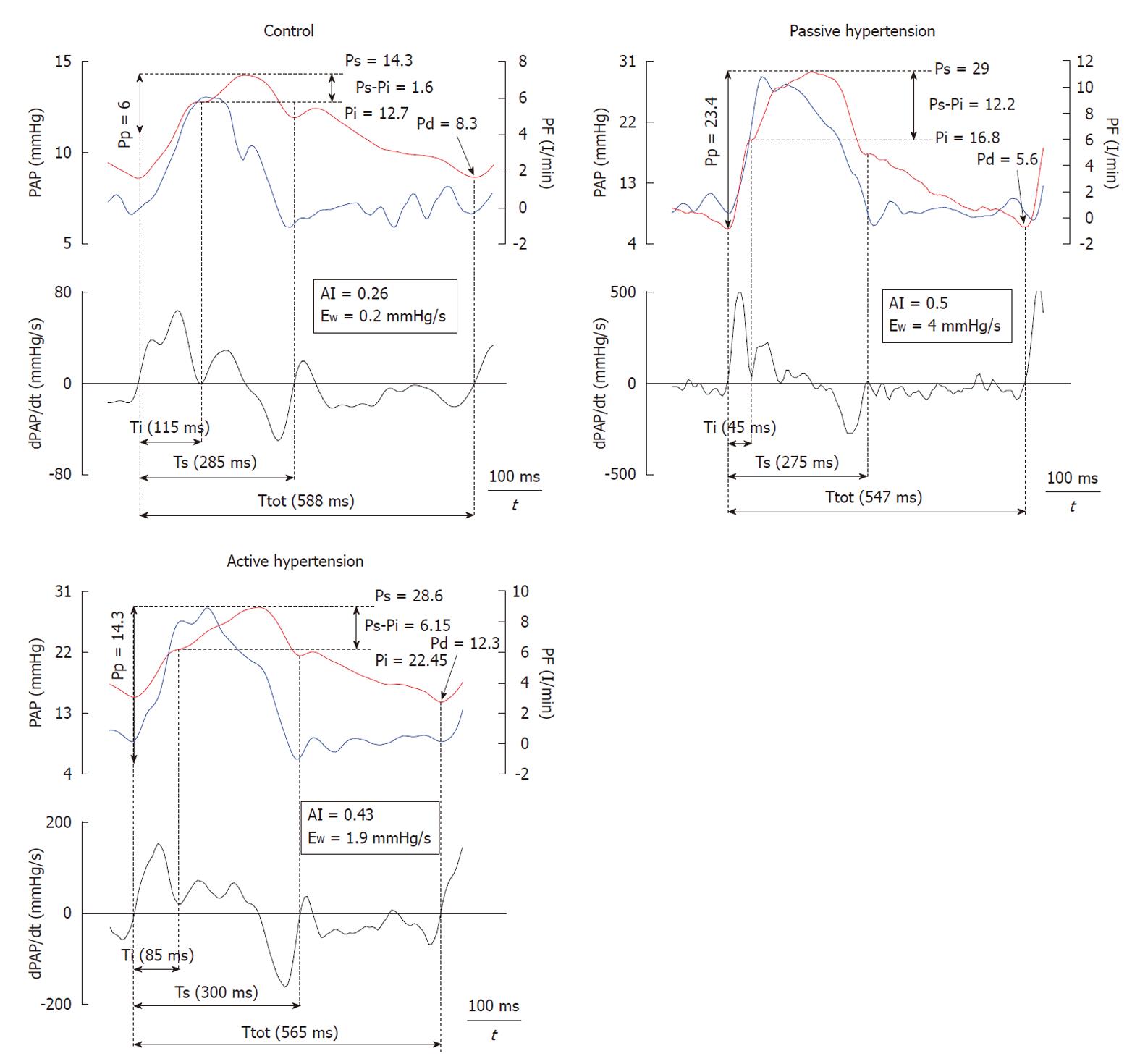
The grouped values of the three experimental situations are presented in Table 1. Heart rate and PF were similar in all three experimental conditions. During both passive and active PH, mean PA pressure increased in a similar fashion, allowing isobaric analysis. During passive PH, both Pp and ZC increased, whereas Cp decreased with respect to control (P < 0.05). While Pp increased during phenylephrine infusion, this increase was significantly lower than in passive PH, with preservation of ZC and Cp.
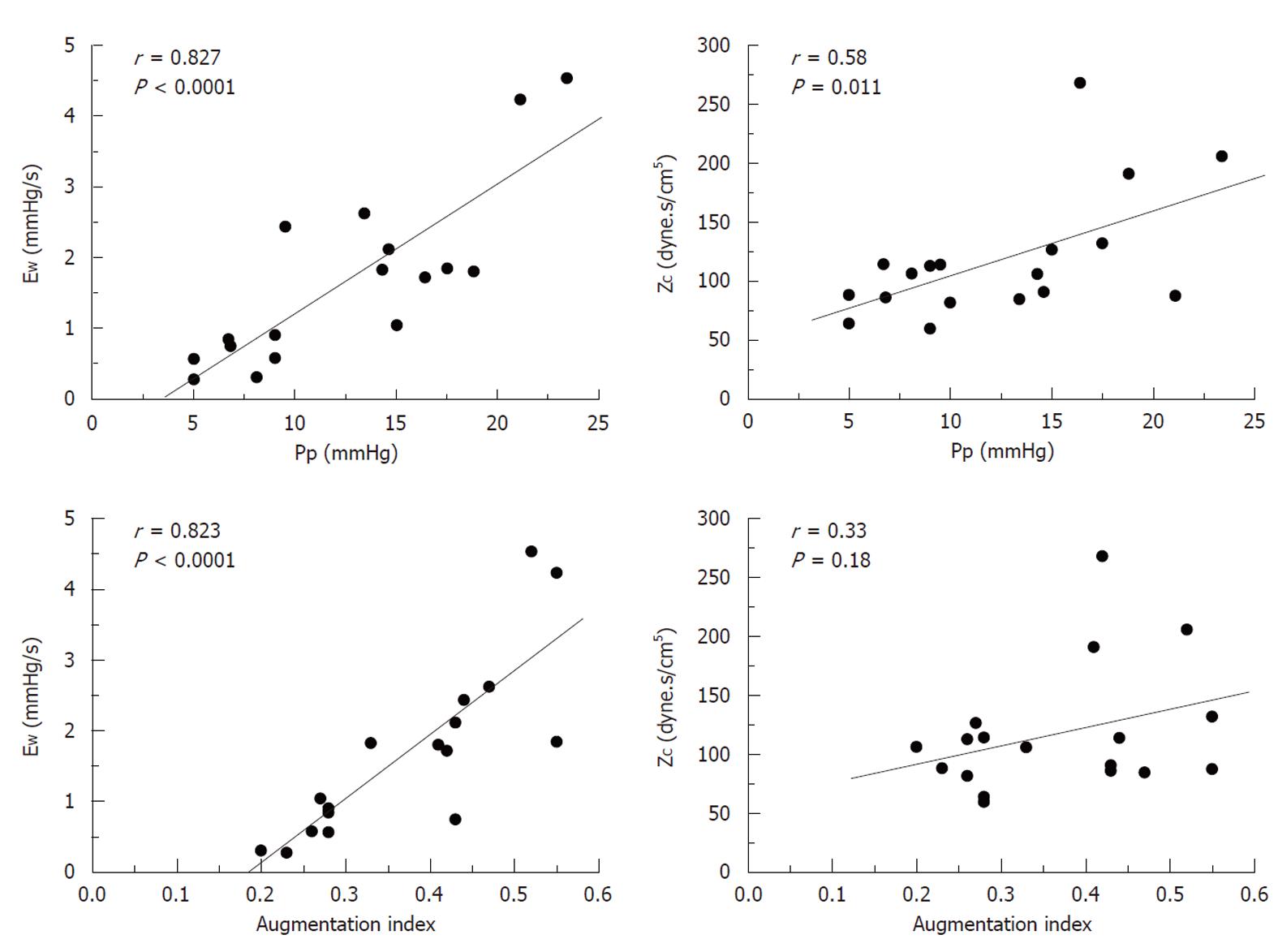
Figure 3 shows the recordings of the pressure wave and PF, in addition to the first derivative of the PA pressure during each experimental condition. It shows a good correlation between the time at the first minimum of the derivative of PA pressure and the time at the peak PF. Mean value, and values for each experimental condition, of the various calculated indices are presented in Table 2. Both the forward wave (Pi-Pd) and the reflected wave (Ps-Pi, EW), Ppf and AI showed a significant increase during passive PH, while phenylephrine caused an isobaric reduction in the magnitude of the reflected wave and the AI (P < 0.05). Pi increased significantly during PH. While Ti decreased during PH states, this reduction was significantly greater during passive PH. Figure 4 shows the correlations of Pp and AI with pulsatile load (EW; ZC) during the 3 experimental conditions. Finally, ZC was correlated with the forward wave (Pi-Pd) (r = 0.62, P < 0.05).
| CTL | PPH | APH | |
| Ppf (Pp/Pm) | 0.53 ± 0.17 | 0.83 ± 0.25a | 0.56 ± 0.17c |
| Pi-Pd (mmHg) | 5.9 ± 1.8 | 9.1 ± 1.9a | 7.4 ± 2.0ac |
| Ps-Pi (mmHg) | 1.8 ± 0.5 | 8.7 ± 3.0a | 4.3 ± 1.7ac |
| (Ps-Pi)/(Pi-Pd) | 0.31 ± 0.08 | 0.95 ± 0.24a | 0.6 ± 0.21ac |
| AI [(Ps-Pi)/Pp] | 0.25 ± 0.03 | 0.48 ± 0.06a | 0.37 ± 0.08ac |
| Ti (ms) | 104 ± 21 | 50 ± 13a | 70 ± 17ac |
| Pi (mmHg) | 16.2 ± 3.3 | 22.9 ± 4.4a | 22.1 ± 6.0a |
| EW (mmHg/s) | 0.6 ± 0.2 | 3 ± 1.1a | 1.6 ± 0.8ac |
Although the estimation of arterial impedance (frequency domain) enables a complete description of hydraulic RV afterload, it is complex to obtain and to interpret[12,13]. On the other hand, we have shown here that the analysis of PA pressure wave morphology and amplitude allows both components (stationary and pulsatile) of RV afterload to be quantified, as well as differentiating local PA stiffness (proximal pulsatile load) from the magnitude and return time of the reflected wave (distal pulsatile load). Due to the isobaric condition, it was possible to show that during active PH, the RV pulsatile load was attenuated, by means of preserving proximal PA stiffness and decreasing the magnitude of the reflected wave.
The central PA pressure wave is composed of a forward traveling wave generated by the RV and a later arriving reflected wave returning from the periphery. Adjustments in heart rate and ejection systolic time on the one hand (ventricular factor) and in arterial stiffness (pulse wave velocity) and vasomotor tone (vascular factor) on the other, determine the synchronization between forward and reflected wave, as well as the proportion of the forward wave that is reflected, which in turns enables modulation of ventriculo-arterial coupling[14]. In addition, in order to properly match the pulse wave velocity and pulmonary length, the reflected pressure wave must return to the proximal PA during diastole rather than systole. Proper matching occurs when time required for wave to travel to the periphery and back equals the systolic ejection period. Therefore, correct coupling between pulse wave velocity and the distance from the reflection sites occurs when the time required for the pulse wave to travel to and back from the periphery is similar to systolic time, so the Ti/systolic time quotient should be close to 0.5[15]. Under normal conditions, the Pi was practically similar to the systolic arterial pressure with a Ps-Pi of 1.8 ± 0.5 mmHg and the Ti/systolic time quotient was 0.4 ± 0.09. Thus, the augmentation pressure is low and weakly contributes to Pp.
Though Pp increased significantly during both forms of acute PH, phenylephrine caused a decrease in Pp with respect to passive PH (P < 0.05). Whereas mean arterial pressure (stationary component of afterload) depends on cardiac output and peripheral vascular resistance, the determining factors of Pp (ejection volume, arterial stiffness and reflected wave) interact in a complex manner[15]. The passive increase in Pp during PH was due both to an increase in amplitude of the reflected wave (Ps-Pi, EW) and to an increase in ZC, which was accompanied by a decrease in Cp and an increase in Ppf (P < 0.05). During active PH, ZC was unchanged and the increase in the peripheral pulsatile component (due to a lower increase in the reflected wave) was significantly lower than in the passive condition (Ps-Pi, EW), which explains the preservation of Ppf and Cp. In this respect, our work showed that the reduction in ZC during active PH, despite a reduction in PA diameter secondary to phenylephrine-induced isobaric vasoconstriction, is due to a simultaneous reduction in the parietal elastic index with respect to passive PH, with preservation of the conduit function (to conduct blood) and an improvement in the buffer function (to buffer cardiac pulsatility). This is likely due to the activation of VSM, preventing recruitment of collagen fibers[8].
Figure 4 shows how Pp depends on the peripheral (EW) and central (ZC) components of pulsatile afterload (P < 0.05). Unlike Pp, AI was only correlated with EW. This concurs with previous studies sustaining that ZC depends on the geometry (radius and thickness) and on the viscoelastic properties of the vascular wall, whereas AI is determined by the amplitude and timing of the reflected wave, in addition to the duration of ventricular ejection[4]. Therefore, an increase in Pp together with an increase in AI would involve a greater reflected wave (EW) with or without an increase in ZC, while an increase in Pp with unchanged AI would mainly involve ZC (PA stiffness). Although obtaining PF simultaneously enables estimation of ZC and EW, Pi could also be determined with the first derivative of PA pressure and the Pi-Pd values correlated with ZC, allowing it to be applied clinically in hemodynamic studies.
In clinical settings, PH vasculopathy affects either distal resistive and/or proximal elastic vessels, with different dynamic afterload and different RV adaptation. Mitchell et al[16] have noninvasively assessed the components of pulsatile hemodynamic load (ZC, AI and Cp) in compensated patients with chronic heart failure. They emphasized that the increased pulsatile load represents an important therapeutic target in chronic heart failure. Although the current results cannot be extrapolated to chronic pulmonary vascular disease, they provide some facts about the change of pulmonary afterload during passive and active PH and the relation with group 4 (chronic thromboembolic PH, particularly with proximal disease) and group 1 (idiopathic PAH, with diffuse remodeling) of the DanaPoint classification. In agreement with this, Nakayama et al[17] have shown that both Ppf, or pulsatility index, and the AI enable a differential diagnosis between idiopathic PH and chronic thromboembolic PH. Also, Castelain et al[7], through the comparison of idiopathic PAH and chronic thromboembolic PH patients with similar mean pulmonary pressure and Pp, showed the presence of an early, and greater magnitude reflected wave in patients with chronic thromboembolic PH. We have recently shown that the isobaric steady component analysis differentiated the pulsatile component between idiopathic PAH and proximal operable chronic thromboembolic PH[18]. The higher dynamic RV afterload in chronic thromboembolic PH patients would be related to different vascular wall remodeling.
Instantaneous PA flow velocities can be measured by transthoracic pulsed Doppler echocardiography. Therefore, performing instantaneous PA pressure and flow measurements during a routine right heart catheterization with Doppler echocardiography in patients with PAH would allow estimation of the time-domain parameters[13,16].
A number of limitations of our study need to be emphasized. The experimental model was designed to apply a time domain approach for evaluating the dynamic RV afterload, particularly pulsatile hemodynamics. Although the results of the present study apply to acute PH situations and therefore should not be extrapolated to chronic hypertensive vascular disease, they provide a methodological basis for estimating the whole pulsatile load of RV. Our aim was not to mimic any specific chronic group of the DanaPoint PH classification. However, during phenylephrine infusion both large and small pulmonary vessels contracted, thus, the pressure increase recorded in the main PA was due to increased pulse wave velocity and wave reflection, while during “passive” PH, PA pressure was increased by mechanical obstruction of the proximal PA, current elements in idiopathic PAH and chronic (proximal) thromboembolic PH.
In conclusion, time-domain analysis of the central PA pressure waveform allows global estimation of the RV dynamic afterload, as well as the analysis of the determining factors in ventriculo-arterial coupling. This may constitute a valid substitute for pulmonary vascular impedance (frequency-domain analysis). Given that RV failure is the chief cause of death in PH patients, this approach may be useful in the diagnosis, follow-up, medical therapeutic effects and prognosis of the various forms of chronic PH. Prospective clinical studies will be necessary to validate this approach to evaluate the dynamic RV afterload in chronic PH.
This study was presented in part in the XVI World Congress of Cardiology. Circulation 117(19): 218, P1199, 2008
The morphology of the pressure wave contour in any point along the pulmonary arterial vascular tree represents the sum of the forward and reflected waves at that point and depends on three factors: the amplitude and duration of ventricular ejection, the amplitude of the reflected wave, and the pulse wave velocity. These three key parameters are influenced by several important factors including the stiffness (elastic properties and vasomotor tone) of the pulmonary arterial wall, the distance of the wave reflection sites, the heart rate and the pulmonary vascular resistance. Pulmonary artery (PA) stiffening alters right ventricular systolic pressure in two ways: first, by causing a greater rise of pressure at the time of peak pulmonary flow, and second, by increasing pulse wave velocity in the PA and so causing the reflected wave from peripheral sites to return early and boost pressure in late systole. Wave reflection is an integral part of the central pressure waveform that increases right ventricular load by increasing pulmonary arterial pressure in late systole.
The important focus in the current study was to characterize the hydraulic right ventricular afterload, considering the central pulmonary arterial pressure waveform analysis in an acute pulmonary hypertension (PH) experimental model. This time domain approach should analyze the steady and pulsatile components of the right ventricular afterload by several indexes and has the advantage of assessing the global arterial mechanics concomitantly.
A comprehensive analysis of the central pulmonary arterial pressure waveform has been carried out in an acute PH model, during both passive (pulmonary arterial occlusion) and active (phenylephrine infusion) PH. This may constitute a valid alternative to pulmonary vascular impedance (frequency-domain analysis) in evaluation of dynamic right ventricular afterload in PH.
Performing simultaneous pulmonary arterial pressure and flow velocity measurements in patients with PH undergoing routine right heart catheterization with Doppler echocardiography, should allow estimation of the different time-domain indexes. We believe that the time-domain study of the central pulmonary arterial pressure waveform and flow in patients with chronic PH may be useful for analyzing direct and long-term effects of specific drugs designed for treatment of pulmonary arterial hypertension.
Overall quality of manuscript is good and it is possible to accept for publication with minor revisions.
Peer reviewers: Maurizio Turiel, Professor, Cardiology Unit, IRCCS Galeazzi Orthopedic Institute, Università di Milano, Via R. Galeazzi 4 - 20161 Milan, Italy; Yves D Durandy, MD, Perfusion and Intensive Care, Pediatric Cardiac Surgery, Institut Hospitalier J. Cartier, Avenue du Noyer Lambert, Massy 91300, France; Dirk Skowasch, MD, Department of Cardiology, University of Bonn, Sigmund-Freud-Str. 25, 53105 Bonn, Germany
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
| 1. | Wauthy P, Pagnamenta A, Vassalli F, Naeije R, Brimioulle S. Right ventricular adaptation to pulmonary hypertension: an interspecies comparison. Am J Physiol Heart Circ Physiol. 2004;286:H1441-H1447. [PubMed] [Cited in This Article: ] |
| 2. | Greyson CR. The right ventricle and pulmonary circulation: basic concepts. Rev Esp Cardiol. 2010;63:81-95. [PubMed] [Cited in This Article: ] |
| 3. | Grignola JC, Ginés F, Bia D, Armentano R. Improved right ventricular-vascular coupling during active pulmonary hypertension. Int J Cardiol. 2007;115:171-182. [PubMed] [Cited in This Article: ] |
| 4. | Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543-551. [PubMed] [Cited in This Article: ] |
| 5. | Chemla D, Plamann K, Nitenberg A. Towards new indices of arterial stiffness using systolic pulse contour analysis: a theoretical point of view. J Cardiovasc Pharmacol. 2008;51:111-117. [PubMed] [Cited in This Article: ] |
| 6. | Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799-803. [PubMed] [Cited in This Article: ] |
| 7. | Castelain V, Hervé P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol. 2001;37:1085-1092. [PubMed] [Cited in This Article: ] |
| 8. | Santana DB, Barra JG, Grignola JC, Ginés FF, Armentano RL. Pulmonary artery smooth muscle activation attenuates arterial dysfunction during acute pulmonary hypertension. J Appl Physiol. 2005;98:605-613. [PubMed] [Cited in This Article: ] |
| 9. | Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652-1659. [PubMed] [Cited in This Article: ] |
| 10. | Li JKJ. The arterial circulation. Physical principles and clinical applications. Towota, NJ: Humana Press 2000; 69. [Cited in This Article: ] |
| 11. | Chemla D, Hébert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500-H505. [PubMed] [Cited in This Article: ] |
| 12. | Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992-1007. [PubMed] [Cited in This Article: ] |
| 13. | Huez S, Brimioulle S, Naeije R, Vachiéry JL. Feasibility of routine pulmonary arterial impedance measurements in pulmonary hypertension. Chest. 2004;125:2121-2128. [PubMed] [Cited in This Article: ] |
| 14. | Agabiti-Rosei E, Mancia G, O'Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154-160. [PubMed] [Cited in This Article: ] |
| 15. | Mitchell GF, Pfeffer MA. Evaluation and management of patients with uncontrolled systolic hypertension: is another new paradigm really needed? Am Heart J. 2005;149:776-784. [PubMed] [Cited in This Article: ] |
| 16. | Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433-1439. [PubMed] [Cited in This Article: ] |
| 17. | Nakayama Y, Nakanishi N, Hayashi T, Nagaya N, Sakamaki F, Satoh N, Ohya H, Kyotani S. Pulmonary artery reflection for differentially diagnosing primary pulmonary hypertension and chronic pulmonary thromboembolism. J Am Coll Cardiol. 2001;38:214-218. [PubMed] [Cited in This Article: ] |
| 18. | Grignola JC, Ruiz-Cano MJ, Escribano P, Gomez-Sanchez MA, Tello De Meneses R, Delgado J, Jimenez Lopez-Guarch C, Saenz De La Calzada C. Isobaric analysis of pulmonary arterial compliance of idiopathic and chronic thromboembolic pulmonary hypertension: correlation with right ventricular remodeling. Eur Heart J. 2009;30 Suppl:108. [Cited in This Article: ] |