Published online Oct 26, 2010. doi: 10.4330/wjc.v2.i10.325
Revised: August 19, 2010
Accepted: August 26, 2010
Published online: October 26, 2010
Ischemia/reperfusion (I/R) injury is an inflammatory condition that is characterized by innate immunity and an adaptive immune response. This review is focused on the acute inflammatory response in I/R injury, and also the adaptive immunological mechanisms in chronic ischemic disease that lead to increased vulnerability during acute events, in relation to the cell types that have been shown to mediate innate immunity to an adaptive immune response in I/R, specifically myocardial infarction. Novel aspects are also highlighted in respect to the mechanisms within the cardiovascular system and cardiovascular risk factors that may be involved in the inflammatory response accompanying myocardial infarction. Experimental myocardial I/R has suggested that immune cells may mediate reperfusion injury. Specifically, monocytes, macrophages, T-cells, mast cells, platelets and endothelial cells are discussed with reference to the complement cascade, toll-like receptors, cytokines, oxidative stress, renin-angiotensin system, and in reference to the microvascular system in the signaling mechanisms of I/R. Finally, the findings of the data summarized in this review are most important for possible translation into clinical cardiology practice and possible avenues for drug development.
- Citation: Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World J Cardiol 2010; 2(10): 325-332
- URL: https://www.wjgnet.com/1949-8462/full/v2/i10/325.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i10.325
In this special issue of the World Journal of Cardiology we have assembled a cosmopolitan group of expert faculty to address the role of inflammation in cardiovascular disease.
Inflammation plays a critical role in the pathophysiology of ischemia/reperfusion (I/R) injury as evidenced by the experimental and clinical studies published during the past 20 years. Several clinical syndromes are secondary to I/R injury: myocardial injury, stroke, organ transplantation, limb ischemia and multiple organ system dysfunction. The mechanisms involved are multifaceted and complex; however, several recent studies[1-7] provide evidence that immune cells are involved in I/R injury, as well as wound healing.
I/R causes local cellular hypoxia that is accompanied by inflammatory responses that lead to the recruitment of leukocytes and subsequent peri-infarct damage, healing, and scar formation[8-10]. Restoration of blood flow to the ischemic tissue may paradoxically exacerbate tissue injury. Elucidating the mechanisms of inflammation and their relationship to myocardial disease is of growing importance to basic and clinical cardiovascular scientists[6,11-13]. Ischemic myocardial injury results in decreased oxygen tension with loss of oxidative phosphorylation and subsequent decreased generation of high energy phosphates (ATP); all leading to failure of the sodium pump, loss of potassium, influx of sodium and water, and cellular swelling. Ischemia leads to anaerobic metabolism, ATP depletion and accumulation of byproducts, like lactic acid, within seconds of ischemia. This leads to loss of contractility, and within minutes, reversible ultrastructural cardiomyocyte changes appear, including cellular and mitochondrial swelling and glycogen depletion. After 20-40 min of sustained ischemia, irreversible cardiomyocyte injury develops and is seen in disruption of sarcolemma and the presence of small amorphous densities in the mitochondria[12].
Classically, ischemia has been shown to lead to endothelial dysfunction with an increase in permeability, increased expression of adhesion molecules, and recruitment of leukocytes. The activation of the innate immune response is considered an acute reaction to I/R, with several molecular mechanisms establishing links between this innate immunity and adaptive immunity. This review will focus on the cellular mediators of the molecular mechanisms involved in I/R with specific reference to the cardiovascular system.
Several studies have shown increases in myocardial cytokines, in response to experimental I/R, and in human patients subsequent to myocardial infarction (MI), coronary bypass grafting, and chronic heart failure[3]. Other humoral mediators of I/R include oxygen and nitrogen free radicals. All of these factors combined reflect the body’s nonspecific innate immunity and regulate locomotion and trafficking of leukocytes in basal and inflammatory processes, such as I/R[10-12,14-16]. Although the heart’s macrophages are a rich source of several inflammatory cytokines[3,12,17,18], other cell types affect inflammation in experimental models of I/R[2,19-21] and may serve as potential candidate sources of cytokines following MI. Polymorphonuclear cells (PMNs) are the major leukocytes that are found in I/R injury with subsequent neutrophil accumulation and microvascular plugging and parenchymal damage leading to tissue destruction and necrosis. Monocytes and macrophages will infiltrate the tissue at later time points during I/R injury; however, the direct role of these cells in injury vs repair mechanisms is still being investigated.
Complement activation following myocardial ischemia was first described by Hill and Ward, with subsequent evidence suggesting that myocardial cell necrosis results in the release of subcellular membrane constituents that are abundant in the mitochondria and capable of triggering the complement cascade (C1, C4, C2 and C3)[12,22-24]. Indeed, mRNA and proteins for all the components of the classical complement pathway are upregulated in areas of myocardial infarction (MI)[25,26]. Furthermore, postischemic cardiac lymph is shown to possess leukocyte chemotactic activity, with neutralizing antibodies to C5a added in vitro completely abolishing the chemotactic activity[27,28]. Several animal studies point to a possible beneficial role of complement depletion in the treatment of postischemic myocardial injury[29-34]. Unfortunately, clinical studies focused on complement depletion in humans have not proven effective in the setting of MI[35].
The toll-like receptors (TLRs) are emerging as the primary, non-antigen-specific defense innate immune mechanism, and represent a family of receptors that serve to recognize molecular patterns associated with pathogens and, upon binding of their ligands, induce activation of several kinases and nuclear factor (NF)-κB. To date, 13 members of the TLR family have been identified in mammals; however, their role in cardiac pathology remains poorly understood[11,36]. TLR2, -3, -4, and -6 are expressed in cardiac myocytes[36]. TLR4 is expressed in the heart and is markedly induced in mouse and rat infarcts and in samples obtained from cardiomyopathic hearts with confined intense staining predominantly localized to cardiac myocytes vs the diffuse staining typified in healthy myocytes[36,37]. TLR4 deficient mice have decreased infarct size and suppressed inflammation, and exhibit attenuated adverse remodeling following MI, identifying TLR4 as a key component of the innate immune response in the infarcted heart[11,38,39]. In contrast, TLR2 null animals had similar infarct size and comparable inflammatory leukocyte infiltration with their wildtype littermates, but exhibited decreased fibrosis in the non-infarcted area and attenuated post-infarction ventricular remodeling[40]. These findings suggest that TLR2 signaling may not critically affect the inflammatory response but may modulate fibrous tissue deposition. A role for TLRs in the activation of inflammatory cells after cardiac injury is now clearly established and warrants further elucidation.
In patients who develop atherosclerosis or experience MI, several immune cell types are prevalent in clinically relevant inflammatory conditions, not only in the acute inflammatory response to I/R injury, but also to adaptive immunological mechanisms in chronic ischemic disease that leads to increased vulnerability during acute events[41]. This review will remain focused on monocytes, macrophages, T cells, mast cells, platelets and endothelial cells, as these are the most clinically relevant immune cell types in myocardial I/R, with specific focus on MI, classically regarded as an acute inflammatory response, as well as atherosclerosis, classically regarded as a chronic adaptive immune response.
After MI, monocytes, via CCR2 receptor for monocyte chemotactic protein 1, extravasate into the injured tissue and can give rise to inflammatory dendritic cells or macrophages that accumulate at the target sites of injury[42-46]. Alternatively, atherosclerosis is considered a chronic inflammatory state with distinct and possibly very different inflammatory pathways. The primary immune cell type involved in atherosclerosis is the macrophage. Many studies have supported a role for chemokine-mediated monocyte infiltration to atheromatous lesions[43,47-51], however distinct subsets of monocytes have been shown to infiltrate during 1-4 d post MI (inflammatory phase) (Ly-6Chigh monocytes) vs 4-8 d post-MI (reparative phase) (Ly-6Clow monocytes), thus linking the acute inflammatory responses to the adaptive immune responses seen in chronic atheromatous conditions[43]. The most recent evidence to directly relate atherosclerosis to immune cell recruitment following MI, found that hypercholesterolemic mice recruit more Ly-6Chigh monocytes in infarcts; and that these monocytes persist longer (prolonged inflammatory phase) and with compromised monocyte response and impaired infarct healing, leading to accelerated left ventricular remodeling[52]. Indeed, monocyte biology requires additional investigation to determine the effects of these parallel but distinct inflammatory phases in the setting of atherosclerosis and MI.
Interestingly, atherosclerotic plaques are also rich in T cells and mast cells[53-57], suggesting these cell types may mediate injury and/or repair. Several groups have shown that T cells and mast cells are capable of producing cytokines[6,7,58-61] and that both are involved in inflammation in the heart[5,8,11,12,19]. Indeed, it is these inflammatory cells that may bridge the innate immune response to the adaptive immune response in response to I/R. In addition to the innate immune response, T cells have other functions that may contribute to vascular dysfunction, with recent studies pointing toward additional functions with unresolved impact. For example, T cells contain components of the renin angiotensin system (i.e. angiotensin converting enzyme, renin, renin receptor, and angiotensinogen) suggesting T cells may be able to mediate the production of angiotensin[62]. Hoch et al[62] have shown that angiotensin II has direct action on T cells including the production of tumor necrosis factor (TNF)-α. Huang et al[63] masterfully outline several experimental studies with direct and indirect evidence of T cells modulating I/R injury in the kidney, liver or intestine. During myocardial I/R, Yang et al[64] found that myocardial infarct size was smaller in renin angiotensin 1 (RAG1) deficient mice (RAG1-/-) (vs controls) following 45 min of left anterior descending artery occlusion[6]. After adoptive transfer of CD4+ T cells, the infarct size of the reconstituted RAG1-/- mice was significantly greater than the RAG1-/- mice, however CD4+ T cells from interferon-γ-/- mice showed no increased myocardial infarct size. T cell subsets have also been studied in many organ systems and in experimental models of immunodeficiency with multiple cell types affected, e.g. severe combined immunodeficiency (SCID) mice or RAG1-/- knockout mice. Specifically, Yilmaz et al[60,61] have evaluated and found protective roles of lymphocytes in experimental models of ischemic stroke. In the heart, CD4+ T cell depletion in mice, but not CD8+ depletion, showed significantly smaller infarct size vs control mice[64]. Again, with specific attention to T cells and the vulnerable atherosclerotic plaque, Pryshchep et al[65] report that T cells from acute coronary syndrome patients have a defect in phosphorylating Lck at Tyr505, thus failing to deactivate the membrane-proximal Src kinase, and enabling T cells to respond in conditions that otherwise would be ignored by the adaptive immune system. CD4+ T cells from acute coronary syndrome patients produce proinflammatory cytokines and are cytotoxic toward vascular smooth muscle cells and endothelial cells, directly implicating them in vascular injury and plaque destabilization[65-68]. Further complicating the role of T cell signaling in I/R, is the differential information among studies, with some data showing T cells are responsible for injury and some data showing T cells are required for recovery from injury subsequent to I/R. One study shows mycophenolate mofetil, an anti-proliferative immunosuppressive agent, to be protective in I/R injury of cardiac transplantation, and also accompanied by decreased leukocyte infiltration[69].
The recruitment of leukocytes in post-ischemic microvessels is often accompanied by the accumulation of platelets[70]. Platelet accumulation in post-ischemic post-capillary venules is dependent on leukocyte adhesion and required P-selectin. Platelets and leukocytes bind to one another on the vessel wall and potentially interfere with the binding of either platelets or leukocytes to the vessel wall, thereby potentially creating more injury after I/R by allowing the cell-cell complex to produce more superoxide and platelet-activating factor than either cell is capable of producing alone[70-73]. Specifically, mice genetically deficient in CD4+ or CD8+ T cells exhibit a blunted platelet recruitment response to I/R, again, supporting a possible cell-cell interaction that may lead to microvascular dysfunction[59,70].
Like T cells, mast cells are resident perivascular, multifunctional, inflammatory and pro-fibrotic mediators[74-76] and are hypothesized to quickly respond to mechanical stimuli, such as vasoconstriction during ischemia or vasodilation during reperfusion. Several studies have implicated mast cells as possible mediators of cardiac injury[77-79]. Frangogiannis et al[79] found that resident cardiac mast cells rapidly degranulate following infarction releasing large amounts of histamine and TNF-α, with similar findings from other groups[74,75,80-82]. Mast cell degranulation is likely an early source of preformed histamine and TNF-α, modulating the inflammatory response. Later, there is more of an interaction of cells, cytokines, growth factors and extracellular matrix proteins mediating myocardial repair. TNF-α of mast cell origin may be a crucial factor in upregulating IL-6 in infiltrating cells and initiating the cytokine cascade responsible for myocyte ICAM-1 induction and subsequent neutrophil-induced injury seen in I/R[79], thus providing a mechanistic link among cytokine-producing cell types in myocardial I/R. Histamine may induce surface expression of P-selectin in endothelial cells by facilitating recruitment of rolling leukocytes[83].
Although many mast cell-derived mediators are capable of modulating cellular events critical to the healing infarct, the role of mast cells and their secretory products in cardiac injury and repair remain poorly understood. Experiments in a canine model of reperfused infarction demonstrated that mast cell stabilization using lodoxamide significantly reduced infarct size[84]. An increase in mast cell numbers was noted in the healing myocardium and immature mast cell progenitors were found in the infarcted area. Although the contribution of mast cell proliferation cannot be ruled out, chemotaxis of circulating mast cell precursors in the healing myocardium secondary to stem cell factor (SCF) may be the predominant mechanism responsible for mast cell accumulation in the ischemic heart. Frangogiannis et al[76,77] hypothesize that the role of SCF in infarct healing may not be limited to its effects on mast cells and further suggest that SCF may promote recruitment and homing of primitive bone marrow-derived cells delivered into the infarct, with further differentiation into cardiomyocytes and vascular cells[85,86].
Additionally, Ayach et al[87] found that c-kit mast cell deficient mice are protected from ventricular dilation and hypertrophy, and maintenance of cardiac function is preserved in these mast cell deficient mice with phenotypic rescue of cardiac repair after MI by bone marrow transplantation of wild-type hematopoietic stem/progenitor cells. Microarray analysis revealed the activation of natural killer (NK) cell-mediated mobilization after MI in rescued hearts. Nevertheless, the specific mediators responsible for the injurious effects of mast cell activation in the infarct were not investigated. In addition, elucidation of the role of mast cell-derived cytokines and growth factors in MI is difficult because many of these secretory products are produced by other cell types involved in cardiac repair. On the other hand, the proteases chymase and tryptase are specific mast cell products and may play unique roles in infarct healing. Chymase inhibition in a rat model of non-reperfused MI attenuated left ventricular interstitial fibrosis and diastolic dysfunction without affecting the dilative pattern of cardiac remodeling[88]. Tryptase stimulates granulocyte recruitment, upregulates cytokine and chemokine synthesis, induces fibroblast proliferation and chemotaxis, and upregulates type I collagen production[89-93]. Furthermore, mast cells are important sources of transforming growth factor-α, bFGF, and vascular endothelial growth factor (VEGF), factors that can regulate fibroblast growth, modulate extracellular matrix metabolism and stimulate angiogenesis[94-97]. Histamine has been shown to stimulate fibroblast growth and collagen synthesis in vitro[98]. Mast cells may also influence healing and tissue remodeling by expressing gelatinases A and B, both implicated in extracellular matrix metabolism[99,100].
Similar to T cells, mast cells may also produce renin following ischemia; however, this has been challenged by subsequent groups[101,102]. Cardiac mast cell-derived renin has been shown to promote local angiotensin formation and norepinephrine release, and induce arrhythmia in excised heart preparations of I/R[102]. Mast cell degranulation has also been proposed as a mechanism for the anti-arrhythmic effect of endothelin-1[103].
Endothelial cells comprise the wall of blood vessels and show activation following I/R and assume an inflammatory phenotype following activation, characterized by the enhanced production of ROS, inflammatory cytokines, and expression of adhesion molecules that bind leukocytes and platelets via activation of NF-κB[104]. Blood cell-derived superoxide via NADPH oxidase is most likely produced by leukocytes, as this is the blood cell type with the highest capacity to generate superoxide[105]. The superoxide generated can thereby contribute to endothelial barrier dysfunction and impaired vascular permeability subsequent to I/R. Indeed, anti-endothelial cell antibodies have been detected in a variety of disorders connected with endothelial damage, including patients with myocardial infarction[106].
Recent work suggests a role for stem cells from systemic circulation and local tissue. These stem cells may allow for regeneration of endothelial cells and are common in transplant recipients with allograft rejection or I/R injury[107]. This regenerative mechanism following ischemia is dependent on stem cell mobilization and homing with several mediators of this process, namely stromal-cell-derived factor 1 (SDF-1), which homes stem cells to bone marrow, found to be upregulated following MI and focal cerebral ischemia[108]. Moreover, endothelial nitric oxide synthase (eNOS) also allows for mobilization of stem cells, with eNOS deficient mice showing reduced VEGF-dependent mobilization of endothelial progenitor cells and possibly impaired regeneration processes with reduced systemic NO bioactivity[8,109].
Current therapeutic strategies are aimed at inhibiting oxygen radicals and inflammatory cytokines for the treatment of I/R injury, despite our limited understanding of the specific mechanisms responsible for repair. Study of the innate immune system and the activation of the adaptive immune system, with respect to which individual systems and cell types are mobilized and responsible for I/R injury vs repair, will likely uncover new mechanisms with results still requiring transference into clinical practice. The specific organs and tissues affected by I/R may then be targeted with specific therapies tailored to each individual system and cell type, with specific attention to the differences between immune systems of humans and experimental animal models.
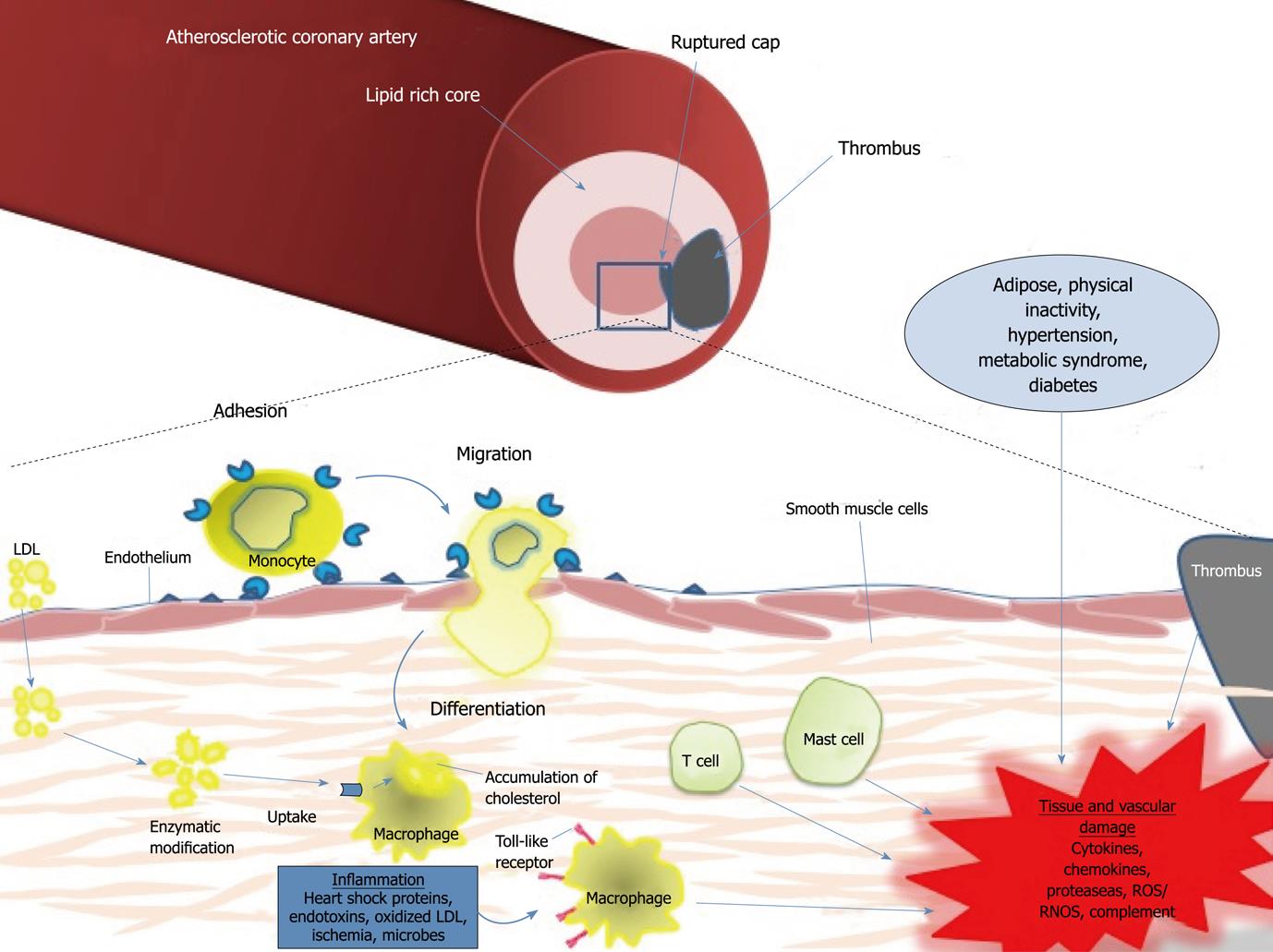
Epidemiologic evidence from humans supports a clear relationship between cardiovascular risk factors and I/R injury. Hypertension, hypercholesterolemia, diabetes mellitus, obesity, and cigarette smoke all have the potential to individually or synergistically increase the sensitivity of the tissues to I/R injury, possibly through the induction of pro-inflammatory, pro-oxidative, and pro-thrombotic environments[70]. Recent research supports the role of inflammation as a key player in coronary artery disease and manifestations of atherosclerosis (Figure 1). Immune cells dominate early atherosclerotic lesions, with effector molecules accelerating the progression of lesions, and activation of inflammation eliciting acute coronary syndrome[110]. Pro-inflammatory cytokines stimulate the expression of leukocyte adhesion molecules on the endothelial surface that promote the binding of monocytes to their surface, with recruitment to the atheroma, and entrance to the arterial intima. Maturation of monocytes into macrophages within the arterial wall, along with recruitment of T-cells and mast cells, induce the expression of scavenger receptors (TLRs) that permit lipid accumulation, foam cell formation, and apoptosis, and thrombogenic microparticles that allow for plaque rupture[111]. Drugs that target immune cells may provide a novel therapeutic strategy for rescuing tissues and vessels from I/R injury in several clinically relevant syndromes, specifically MI.
Peer reviewer: Tienush Rassaf, MD, Professor, University Hospital Düsseldorf, Department of Cardiology, Pulmonology, Angiology, Moorenstr 5, 40225 Düsseldorf, Germany
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619-628. [Cited in This Article: ] |
| 2. | Walsh SK, Kane KA, Wainwright CL. Mast cells, peptides and cardioprotection - an unlikely marriage? Auton Autacoid Pharmacol. 2009;29:73-84. [Cited in This Article: ] |
| 3. | Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577-R595. [Cited in This Article: ] |
| 4. | Balakumar P, Singh AP, Ganti SS, Krishan P, Ramasamy S, Singh M. Resident cardiac mast cells: are they the major culprit in the pathogenesis of cardiac hypertrophy? Basic Clin Pharmacol Toxicol. 2008;102:5-9. [Cited in This Article: ] |
| 5. | Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008;45:56-61. [Cited in This Article: ] |
| 6. | Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando). 2009;23:1-10. [Cited in This Article: ] |
| 7. | Lahat N, Rahat MA, Ballan M, Weiss-Cerem L, Engelmayer M, Bitterman H. Hypoxia reduces CD80 expression on monocytes but enhances their LPS-stimulated TNF-alpha secretion. J Leukoc Biol. 2003;74:197-205. [Cited in This Article: ] |
| 8. | Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652-658. [Cited in This Article: ] |
| 9. | Eppihimer MJ, Granger DN. Ischemia/reperfusion-induced leukocyte-endothelial interactions in postcapillary venules. Shock. 1997;8:16-25. [Cited in This Article: ] |
| 10. | Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation--the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41-59. [Cited in This Article: ] |
| 11. | Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88-111. [Cited in This Article: ] |
| 12. | Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31-47. [Cited in This Article: ] |
| 13. | Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047-1060. [Cited in This Article: ] |
| 14. | Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189-194. [Cited in This Article: ] |
| 15. | Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160-166. [Cited in This Article: ] |
| 16. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [Cited in This Article: ] |
| 17. | Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, Potter BJ, Zhang C. Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H2242-H2249. [Cited in This Article: ] |
| 18. | Meldrum DR, Cain BS, Cleveland JC Jr, Meng X, Ayala A, Banerjee A, Harken AH. Adenosine decreases post-ischaemic cardiac TNF-alpha production: anti-inflammatory implications for preconditioning and transplantation. Immunology. 1997;92:472-477. [Cited in This Article: ] |
| 19. | Sun Y. Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J Mol Cell Cardiol. 2010;48:483-489. [Cited in This Article: ] |
| 20. | Martin M, Mory C, Prescher A, Wittekind C, Fiedler M, Uhlmann D. Protective effects of early CD4(+) T cell reduction in hepatic ischemia/reperfusion injury. J Gastrointest Surg. 2010;14:511-519. [Cited in This Article: ] |
| 21. | Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710-718. [Cited in This Article: ] |
| 22. | Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885-900. [Cited in This Article: ] |
| 23. | Pinckard RN, Olson MS, Giclas PC, Terry R, Boyer JT, O'Rourke RA. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975;56:740-750. [Cited in This Article: ] |
| 24. | Rossen RD, Michael LH, Hawkins HK, Youker K, Dreyer WJ, Baughn RE, Entman ML. Cardiolipin-protein complexes and initiation of complement activation after coronary artery occlusion. Circ Res. 1994;75:546-555. [Cited in This Article: ] |
| 25. | Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259-2267. [Cited in This Article: ] |
| 26. | Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: upregulation by ischemia and reperfusion. Circ Res. 1998;82:1224-1230. [Cited in This Article: ] |
| 27. | Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, LaRosa GJ, Hawkins HK, Smith CW, Michael LH. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684-692. [Cited in This Article: ] |
| 28. | Dreyer WJ, Michael LH, Nguyen T, Smith CW, Anderson DC, Entman ML, Rossen RD. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ Res. 1992;71:1518-1524. [Cited in This Article: ] |
| 29. | Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978;61:661-670. [Cited in This Article: ] |
| 30. | Czermak BJ, Lentsch AB, Bless NM, Schmal H, Friedl HP, Ward PA. Role of complement in in vitro and in vivo lung inflammatory reactions. J Leukoc Biol. 1998;64:40-48. [Cited in This Article: ] |
| 31. | Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733-1740. [Cited in This Article: ] |
| 32. | Weisman HF, Bartow T, Leppo MK, Marsh HC Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146-151. [Cited in This Article: ] |
| 33. | Lucchesi BR, Kilgore KS. Complement inhibitors in myocardial ischemia/reperfusion injury. Immunopharmacology. 1997;38:27-42. [Cited in This Article: ] |
| 34. | Kilgore KS, Friedrichs GS, Homeister JW, Lucchesi BR. The complement system in myocardial ischaemia/reperfusion injury. Cardiovasc Res. 1994;28:437-444. [Cited in This Article: ] |
| 35. | Testa L, Van Gaal WJ, Bhindi R, Biondi-Zoccai GG, Abbate A, Agostoni P, Porto I, Andreotti F, Crea F, Banning AP. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J Thorac Cardiovasc Surg. 2008;136:884-893. [Cited in This Article: ] |
| 36. | Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474-481. [Cited in This Article: ] |
| 37. | Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271-280. [Cited in This Article: ] |
| 38. | Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784-789. [Cited in This Article: ] |
| 39. | Riad A, Jäger S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954-6961. [Cited in This Article: ] |
| 40. | Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905-2910. [Cited in This Article: ] |
| 41. | Loppnow H, Werdan K, Buerke M. Vascular cells contribute to atherosclerosis by cytokine- and innate-immunity-related inflammatory mechanisms. Innate Immun. 2008;14:63-87. [Cited in This Article: ] |
| 42. | Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59-70. [Cited in This Article: ] |
| 43. | Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437-2445. [Cited in This Article: ] |
| 44. | Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000-2007. [Cited in This Article: ] |
| 45. | Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583-597. [Cited in This Article: ] |
| 46. | Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502-512. [Cited in This Article: ] |
| 47. | Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2-/- mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642-1648. [Cited in This Article: ] |
| 48. | Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333-340. [Cited in This Article: ] |
| 49. | Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275-281. [Cited in This Article: ] |
| 50. | Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649-1657. [Cited in This Article: ] |
| 51. | Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894-897. [Cited in This Article: ] |
| 52. | Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629-1638. [Cited in This Article: ] |
| 53. | Lindstedt KA, Mäyränpää MI, Kovanen PT. Mast cells in vulnerable atherosclerotic plaques--a view to a kill. J Cell Mol Med. 2007;11:739-758. [Cited in This Article: ] |
| 54. | Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802-815. [Cited in This Article: ] |
| 55. | Vanderlaan PA, Reardon CA. Thematic review series: the immune system and atherogenesis. The unusual suspects:an overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005;46:829-838. [Cited in This Article: ] |
| 56. | Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084-1088. [Cited in This Article: ] |
| 57. | Jeziorska M, Woolley DE. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J Pathol. 1999;188:189-196. [Cited in This Article: ] |
| 58. | Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041-1047. [Cited in This Article: ] |
| 59. | Osman M, Russell J, Granger DN. Lymphocyte-derived interferon-gamma mediates ischemia-reperfusion-induced leukocyte and platelet adhesion in intestinal microcirculation. Am J Physiol Gastrointest Liver Physiol. 2009;296:G659-G663. [Cited in This Article: ] |
| 60. | Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105-2112. [Cited in This Article: ] |
| 61. | Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12:193-204. [Cited in This Article: ] |
| 62. | Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208-R216. [Cited in This Article: ] |
| 63. | Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4-11. [Cited in This Article: ] |
| 64. | Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056-2064. [Cited in This Article: ] |
| 65. | Pryshchep S, Goronzy JJ, Parashar S, Weyand CM. Insufficient deactivation of the protein tyrosine kinase lck amplifies T-cell responsiveness in acute coronary syndrome. Circ Res. 2010;106:769-778. [Cited in This Article: ] |
| 66. | Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239-250. [Cited in This Article: ] |
| 67. | Pryshchep S, Sato K, Goronzy JJ, Weyand CM. T cell recognition and killing of vascular smooth muscle cells in acute coronary syndrome. Circ Res. 2006;98:1168-1176. [Cited in This Article: ] |
| 68. | Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570-575. [Cited in This Article: ] |
| 69. | Ysebaert DK, De Greef KE, Vercauteren SR, Verhulst A, Kockx M, Verpooten GA, De Broe ME. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Kidney Int. 2003;64:864-873. [Cited in This Article: ] |
| 70. | Rodrigues SF, Granger DN. Role of blood cells in ischaemia-reperfusion induced endothelial barrier failure. Cardiovasc Res. 2010;87:291-299. [Cited in This Article: ] |
| 71. | Suzuki K, Sugimura K, Hasegawa K, Yoshida K, Suzuki A, Ishizuka K, Ohtsuka K, Honma T, Narisawa R, Asakura H. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301-1306. [Cited in This Article: ] |
| 72. | Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275-285. [Cited in This Article: ] |
| 73. | Herd CM, Page CP. Pulmonary immune cells in health and disease: platelets. Eur Respir J. 1994;7:1145-1160. [Cited in This Article: ] |
| 74. | Aller MA, Arias JL, Arias J. The mast cell integrates the splanchnic and systemic inflammatory response in portal hypertension. J Transl Med. 2007;5:44. [Cited in This Article: ] |
| 75. | Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749-786. [Cited in This Article: ] |
| 76. | Frangogiannis NG, Burns AR, Michael LH, Entman ML. Histochemical and morphological characteristics of canine cardiac mast cells. Histochem J. 1999;31:221-229. [Cited in This Article: ] |
| 77. | Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687-698. [Cited in This Article: ] |
| 78. | Ito BR, Engler RL, del Balzo U. Role of cardiac mast cells in complement C5a-induced myocardial ischemia. Am J Physiol. 1993;264:H1346-H1354. [Cited in This Article: ] |
| 79. | Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699-710. [Cited in This Article: ] |
| 80. | Galli SJ, Nakae S. Mast cells to the defense. Nat Immunol. 2003;4:1160-1162. [Cited in This Article: ] |
| 81. | Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7-19. [Cited in This Article: ] |
| 82. | Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174:103-107. [Cited in This Article: ] |
| 83. | Asako H, Kurose I, Wolf R, DeFrees S, Zheng ZL, Phillips ML, Paulson JC, Granger DN. Role of H1 receptors and P-selectin in histamine-induced leukocyte rolling and adhesion in postcapillary venules. J Clin Invest. 1994;93:1508-1515. [Cited in This Article: ] |
| 84. | Jolly SR, Abrams GD, Romson JL, Bailie MB, Lucchesi BR. Effects of lodoxamide on ischemic reperfused myocardium. J Cardiovasc Pharmacol. 1982;4:441-448. [Cited in This Article: ] |
| 85. | Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7 Suppl 3:86-88. [Cited in This Article: ] |
| 86. | Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344-10349. [Cited in This Article: ] |
| 87. | Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304-2309. [Cited in This Article: ] |
| 88. | Kanemitsu H, Takai S, Tsuneyoshi H, Nishina T, Yoshikawa K, Miyazaki M, Ikeda T, Komeda M. Chymase inhibition prevents cardiac fibrosis and dysfunction after myocardial infarction in rats. Hypertens Res. 2006;29:57-64. [Cited in This Article: ] |
| 89. | Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, Caughey GH, Entman ML, Frangogiannis NG. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102-111. [Cited in This Article: ] |
| 90. | He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216-6225. [Cited in This Article: ] |
| 91. | Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161:1939-1946. [Cited in This Article: ] |
| 92. | Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for cultured fibroblasts. J Clin Invest. 1991;88:493-499. [Cited in This Article: ] |
| 93. | Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren S, Schwartz LB, Korn JH. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997;158:2310-2317. [Cited in This Article: ] |
| 94. | Pennington DW, Lopez AR, Thomas PS, Peck C, Gold WM. Dog mastocytoma cells produce transforming growth factor beta 1. J Clin Invest. 1992;90:35-41. [Cited in This Article: ] |
| 95. | Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564-573. [Cited in This Article: ] |
| 96. | Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J Exp Med. 1998;188:1135-1145. [Cited in This Article: ] |
| 97. | Shiota N, Rysä J, Kovanen PT, Ruskoaho H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1935-1944. [Cited in This Article: ] |
| 98. | Hatamochi A, Fujiwara K, Ueki H. Effects of histamine on collagen synthesis by cultured fibroblasts derived from guinea pig skin. Arch Dermatol Res. 1985;277:60-64. [Cited in This Article: ] |
| 99. | Chancey AL, Brower GL, Janicki JS. Cardiac mast cell-mediated activation of gelatinase and alteration of ventricular diastolic function. Am J Physiol Heart Circ Physiol. 2002;282:H2152-H2158. [Cited in This Article: ] |
| 100. | Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999;162:5528-5535. [Cited in This Article: ] |
| 101. | Krop M, van Veghel R, Garrelds IM, de Bruin RJ, van Gool JM, van den Meiracker AH, Thio M, van Daele PL, Danser AH. Cardiac Renin levels are not influenced by the amount of resident mast cells. Hypertension. 2009;54:315-321. [Cited in This Article: ] |
| 102. | Mackins CJ, Kano S, Seyedi N, Schäfer U, Reid AC, Machida T, Silver RB, Levi R. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063-1070. [Cited in This Article: ] |
| 103. | Walsh SK, Kane KA, Wainwright CL. Mast cell degranulation--a mechanism for the anti-arrhythmic effect of endothelin-1? Br J Pharmacol. 2009;157:716-723. [Cited in This Article: ] |
| 104. | Cooper D, Stokes KY, Tailor A, Granger DN. Oxidative stress promotes blood cell-endothelial cell interactions in the microcirculation. Cardiovasc Toxicol. 2002;2:165-180. [Cited in This Article: ] |
| 105. | Stokes KY, Russell JM, Jennings MH, Alexander JS, Granger DN. Platelet-associated NAD(P)H oxidase contributes to the thrombogenic phenotype induced by hypercholesterolemia. Free Radic Biol Med. 2007;43:22-30. [Cited in This Article: ] |
| 106. | Elsheikh E, Sylvén C, Henareh L. Anti-endothelial cell antibodies are increased in patients with previous myocardial infarction. Scand Cardiovasc J. 2010;Epub ahead of print. [Cited in This Article: ] |
| 107. | Lagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JH. Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet. 2001;357:33-37. [Cited in This Article: ] |
| 108. | Penn MS, Zhang M, Deglurkar I, Topol EJ. Role of stem cell homing in myocardial regeneration. Int J Cardiol. 2004;95 Suppl 1:S23-S25. [Cited in This Article: ] |
| 109. | Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370-6. [Cited in This Article: ] |
| 110. | Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695. [Cited in This Article: ] |
| 111. | Libby P, Crea F. Clinical implications of inflammation for cardiovascular primary prevention. Eur Heart J. 2010;31:777-783. [Cited in This Article: ] |