Published online Nov 26, 2020. doi: 10.4330/wjc.v12.i11.584
Peer-review started: August 28, 2020
First decision: October 5, 2020
Revised: October 12, 2020
Accepted: November 6, 2020
Article in press: November 6, 2020
Published online: November 26, 2020
Immune checkpoint inhibitors (ICIs) are novel therapeutic agents used for various types of cancer. ICIs have revolutionized cancer treatment and improved clinical outcomes among cancer patients. However, immune-related adverse effects of ICI therapy are common. Cardiovascular immune-related adverse events (irAEs) are rare but potentially life-threatening complications.
To estimate the incidence of cardiovascular irAEs among patients undergoing ICI therapy for various malignancies.
We conducted this systematic review and meta-analysis by searching PubMed, Cochrane CENTRAL, Web of Science, and SCOPUS databases for relevant interventional trials reporting cardiovascular irAEs. We performed a single-arm meta-analysis using OpenMeta [Analyst] software of the following outcomes: Myocarditis, pericardial effusion, heart failure, cardiomyopathy, atrial fibrillation, myocardial infarction, and cardiac arrest. We assessed the heterogeneity using the I2 test and managed to solve it with Cochrane’s leave-one-out method. The risk of bias was performed with the Cochrane’s risk of bias tool.
A total of 26 studies were included. The incidence of irAEs follows: Myocarditis: 0.5% [95% confidence interval (CI): 0.1%-0.9%]; Pericardial effusion: 0.5% (95%CI: 0.1%-1.0%); Heart failure: 0.3% (95%CI: 0.0%-0.5%); Cardiomyopathy: 0.3% (95%CI: -0.1%-0.6%); atrial fibrillation: 4.6% (95%CI: 1.0%-14.1%); Myocardial infarction: 0.4% (95%CI: 0.0%-0.7%); and Cardiac arrest: 0.4% (95%CI: 0.1%-0.8%).
The most common cardiovascular irAEs were atrial fibrillation, myocarditis, and pericardial effusion. Although rare, data from post market surveillance will provide estimates of the long-term prevalence and prognosis in patients with ICI-associated cardiovascular complications.
Core Tip: Cardiovascular immune-related adverse events (irAEs) are rare but potentially life-threatening complications that can occur in patients receiving immune checkpoint inhibitor (ICI) therapy. The most common ICI-associated adverse events are atrial fibrillation, myocarditis, and pericardial effusion. Risk factors for cardiovascular irAEs include treatment with combination immunotherapy, male sex, and a history of cardiac disease. Ongoing post-market surveillance is imperative to characterize long-term risks and improve outcomes among patients receiving ICIs.
- Citation: Nso N, Antwi-Amoabeng D, Beutler BD, Ulanja MB, Ghuman J, Hanfy A, Nimo-Boampong J, Atanga S, Doshi R, Enoru S, Gullapalli N. Cardiac adverse events of immune checkpoint inhibitors in oncology patients: A systematic review and meta-analysis. World J Cardiol 2020; 12(11): 584-598
- URL: https://www.wjgnet.com/1949-8462/full/v12/i11/584.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i11.584
Immune checkpoint inhibitors (ICIs) have demonstrated remarkable efficacy in various malignancies, including lung cancer, melanoma, Hodgkin’s lymphoma, bladder cancer, and microsatellite instability[1]. ICIs exert their effects through blocking inhibitory receptors on tumor cells [programmed cell death 1 Ligand-1 (PD-L1)][2,3] or T-lymphocytes [programmed cell death protein-1 (PD-1) or cytotoxic T lymphocyte-associated protein-4 (CTLA-4)][4,5]. The blockade of these receptors activates the effector T cells to target neoplastic cells[2]. Many studies have demonstrated significant survival benefits of ICIs[6-8] and over 1200 trials are currently ongoing[9].
The mechanism of action of ICIs involves non-specific activation of the immune system[10]. Consequently, autoimmune inflammatory reactions frequently occur; this can ultimately lead to a broad spectrum of immune-related adverse events (irAEs) affecting both on-target and off-target organs[11]. Reactions involving the skin, gastrointestinal tract, and endocrine system are relatively common among cancer patients on ICIs[12,13]. Approximately 80% of patients treated with agents targeting CTLA-4, 70% of patients treated with anti-PD-1 drugs, and 40% of those treated with anti-PD-L1 agents develop irAEs[13,14]. Severe events are common and up to 40% of patients on ICIs require treatment discontinuation due to irAEs[10].
Cardiovascular irAEs are rare, but potentially life-threatening[15]. Although the initial trials on ICIs did not assess myocardial activity, growing evidence from case reports, case series, and cohort studies have raised awareness of unexpected cardiac toxicities associated with ICI therapy[16-18]. Dual therapy appears to markedly increase the risk of cardiovascular irAEs; using the Bristol-Myers Squibb safety database, the estimated rate of myocarditis in patients receiving combination immunotherapy (ipilimumab plus nivolumab) was 0.27% as compared to 0.06% in those receiving nivolumab monotherapy[18].
Data on other ICI-related cardiac toxicities are scarce. This study aims to provide high-class evidence on the incidence of ICI-related cardiovascular adverse events through a systematic review and meta-analysis.
This systematic review and meta-analysis complies with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement[19] and Cochrane’s Handbook of Systematic Reviews of Interventions[20].
Our analysis included interventional trials involving patients receiving an ICI in which an adverse cardiovascular event was reported. We excluded the following: Non-randomized trials, trials involving concurrent use of other anticancer interventions, animal studies, non-clinical studies, reviews, and meta-analyses. We also excluded studies without accessible data, conference abstracts, and studies for which there was no English language translation.
We searched PubMed, Cochrane CENTRAL, SCOPUS, and Web of Science databases for possible included articles according to our eligibility criteria from May 1st, 2020 through May 15th, 2020. We retrieved articles using a combination of the following keywords: “cardiotoxicity”, “adverse”, “events”, “myocard*”, “pericard*”, “neoplasm”, “cancer”, and “immune checkpoint inhibitor.”
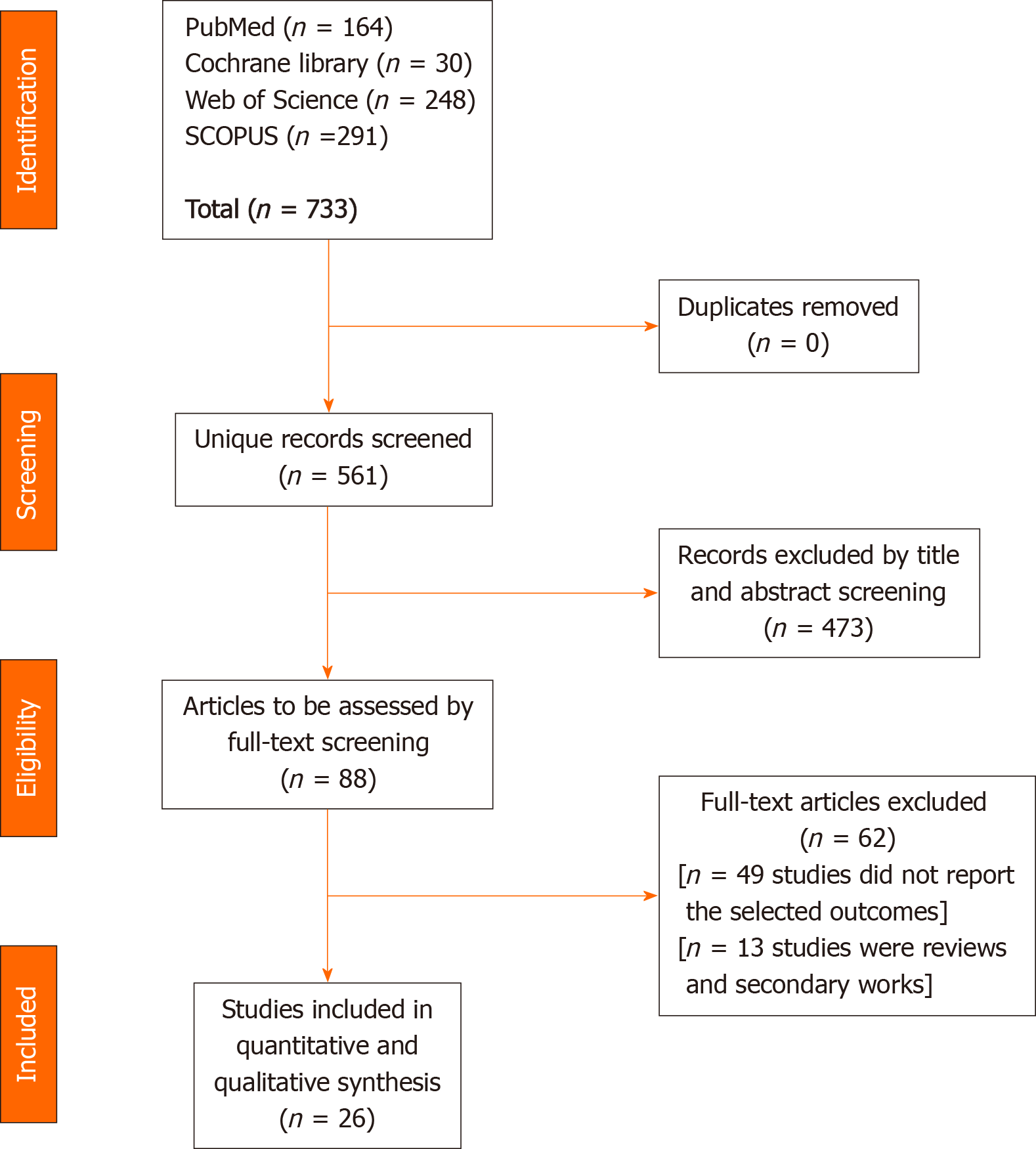
Screening of results: We performed the screening of retrieved studies through two stages. The first stage involved the inclusion and exclusion of studies based on title and abstract review. Selected studies underwent full-text screening against the inclusion criteria. Studies that had a mismatch with a single inclusion criterion were excluded. We conducted another search through the references of the included trials to ensure that no trials were inadvertently excluded. We considered studies which included multiple treatment arms as separate studies based on the adverse event reporting and refer to them as first author last name, year of publication followed by a, b, or c in the forest plot diagrams and Table 1[3,6-8,21-42]. Figure 1 shows a PRISMA flow chart of the literature search.
| Study | n | ICI | Cancer type | Males | Median age (range), yr | Median follow-up (range), mo | Race, Asian | Race, Black | Tobacco users |
| Antonia et al[8], 2016a | 98 | Nivolumab | Small cell carcinoma of the lung | 61 (62) | 63 (57-68) | 10.07 (NR) | NR | 3 (3) | 95 (97) |
| Antonia et al[8], 2016b | 61 | Nivolumab plus ipilimumab | Small cell carcinoma of the lung | 35 (57) | 66 (58-71) | 12.03 (9.10-15.67) | NR | 1 (2) | 57 (93) |
| Antonia et al[8], 2016c | 54 | Nivolumab plus ipilimumab | Small cell carcinoma of the lung | 32 (59) | 61 (56-65) | 8.68 (8.27-9.6) | NR | 0 | 48 (89) |
| Antonia et al[21], 2017 | 476 | Durvalumab | Stage III non-small cell lung cancer | 334 (70.2) | 64 (NR) | 14.5 (0.2-29.9) | 120 (25.2) | 12 (2.5) | 433 (91) |
| Balar et al[22], 2017 | 370 | Pembrolizumab plus cisplatin | Advanced, unresectable metastatic urothelial cancer | 286 (77) | 74 (34-94) | 5 (30-8.6) | NR | NR | NR |
| Barlesi et al[23], 2018 | 393 | Avelumab | Advanced non-small-cell lung cancer | 269 (68) | 64 (59-70) | 18.9 (IQR 13.2-23) | 102 (26) | 5 (1) | 324 (82) |
| Bott et al[24], 2018 | 21 | Nivolumab | Resectable non–small cell lung cancer | 10 (48) | 67 (55-84) | 1.1 (0.57-1.13) | NR | NR | 18 (86) |
| Brohl et al[25], 2016 | 31 | Ipilimumab plus peginterferon | Unresectable melanoma | 18 (58.1) | 65 (38-83) | 35.8 (19.7-50.2) | NR | NR | NR |
| Cho et al[26], 2018 | 33 | Pembrolizumab | Relapsed thymic epithelial tumor | 21 (63.6) | 57 (26-78) | 14.9 (IQR 6.25-20.7) | NR | NR | NR |
| Choueiri et al[3], 2018 | 55 | Avelumab plus axitinib | Advanced clear cell renal cell carcinoma | 42 (76) | 60 (55–68) | 13 (9.35-14.02) | 6 (11) | 3 (6) | NR |
| Chung et al[27], 2019 | 11 | p53MVA vaccine combined with pembrolizumab | Advanced breast, pancreatic, hepatocellular, or head and neck cancer | NR | NR | 16.26 (15.42-17.27) | NR | NR | NR |
| Dudnik et al[28], 2018 | 260 | Nivolumab | Non-small cell lung cancer | 176 (68) | 67 (41-99) | 8.4 (2-16.8) | NR | NR | 197 (76) |
| Eggermont et al[29], 2015 | 475 | Ipilimumab | High-risk stage III melanoma | 296 (62) | 51 (20-84) | 7.5 (7-11.4) | NR | NR | NR |
| Giaccone et al[30], 2018 | 40 | Pembrolizumab | Thymic carcinoma | 28 (70) | 57 (25-80) | 8.4 (2-16.8) | 4 (10) | 2 (5) | NR |
| Herbst et al[31], 2020 | 26 | Ramucirumab plus pembrolizumab | Advanced non-small-cell lung cancer | 21 (78) | 65 (56-72) | 33.3 (IQR 27.7-39.2) | NR | 1 (4) | 26 (96) |
| Hodi et al[32], 2018 | 313 | Nivolumab plus ipilimumab | Advanced melanoma | NR | NR | 20 (IQR 14-26) | NR | NR | NR |
| Juergens et al[7], 2020 | 136 | Durvalumab with or without tremelimumab and platinum-doublet | Lung cancer (unspecified) | 67 (49) | 61.9 (30.1-83.2) | 32.8 (IQR 28.1-33.6) | 8 (6) | 1 (1) | NR |
| Loi et al[33], 2019 | 58 | Pembrolizumab plus trastuzumab | Lung cancer (unspecified) | 0 | 52 (43-92) | 46.9 (48-NR) | NR | NR | NR |
| Maio et al[34], 2017 | 382 | Tremelimumab | Malignant mesothelioma | 283 (74) | 66 (60-72) | 19.61 (0.23-26.48) | 7 (2) | 3 (< 1%) | NR |
| Mateos et al[35], 2019 | 125 | Pembrolizumab plus pomalidomide and dexamethasone | Multiple myeloma | 77 (62) | 65 (60-72) | 25.7 (IQR 25.6-25.8) | NR | NR | NR |
| Motzer et al[36], 2019 | 550 | Nivolumab plus ipilimumab | Advanced renal cell carcinoma | NR | NR | 2 (1-3) | NR | NR | NR |
| Sarocchi et al[37], 2018 | 59 | Nivolumab | Advanced non-small cell lung cancer | 41 (NR) | 69 (44-81) | 8.1 (IQR 4.5-10.9) | NR | NR | 51 (86) |
| Scherpereel et al[6], 2019 | 63 | Nivolumab or nivolumab plus ipilimumab | Relapsed malignant pleural mesothelioma | 47 (75) | 72.3 (32.5-87) | 32.4 (IQR 13.4-36.3) | NR | NR | 34 (54) |
| Tawbi et al[38], 2018 | 94 | Nivolumab plus ipilimumab | Melanoma with brain metastases | 65 (69) | 59 (22-81) | NR | NR | NR | NR |
| Ueno et al[39], 2019a | 30 | Nivolumab alone | Unresectable or recurrent biliary tract cancer | NR | NR | 20.1 (IQR 19.6-20.3) | NR | NR | NR |
| Ueno et al[39], 2019b | 30 | Nivolumab in combination with cisplatin | Unresectable or recurrent biliary tract cancer | NR | NR | 14 (6-NR) | NR | NR | NR |
| Usmani et al[40], 2019a | 151 | Pembrolizumab | Multiple myeloma | 70 (46) | 74 (70-79) | 5.1 (IQR 3.4-7) | NR | NR | NR |
| Usmani et al[40], 2019b | 150 | Lenalidomide | Multiple myeloma | 71 (47) | 74 (70-78 | 8.2 (IQR 7-14) | NR | NR | NR |
| Wrangle et al[41], 2018 | 21 | ALT-803, an IL-15 superagonist, in combination with nivolumab | Metastatic non-small cell lung | 15 (71) | 55 (46-67) | 6.6 (IQR 3.4-9.6) | NR | NR | 12 (57) |
| Yang et al[42], 2018a | 42 | Preoperative chemotherapy | Non-small cell lung cancer | 21 (50) | NR | 6.6 (IQR 3.4-9.6) | NR | 7 (17) | NR |
| Yang et al[42], 2018b | 13 | Ipilimumab | Non-small cell lung cancer | 5 (38) | NR | 6.9 (IQR 5.5-12.0) | NR | 3 (23) | NR |
Data extraction: We used a data extraction form specifically designed for this study. Three main categories of data were extracted. The first category included baseline data about the study participants, such as patients’ age, gender, cancer type, and drug administered and dose. The second category included different outcome endpoints for analysis (any reported cardiovascular adverse event). The third category involved data used to assess the risk of bias among the included studies.
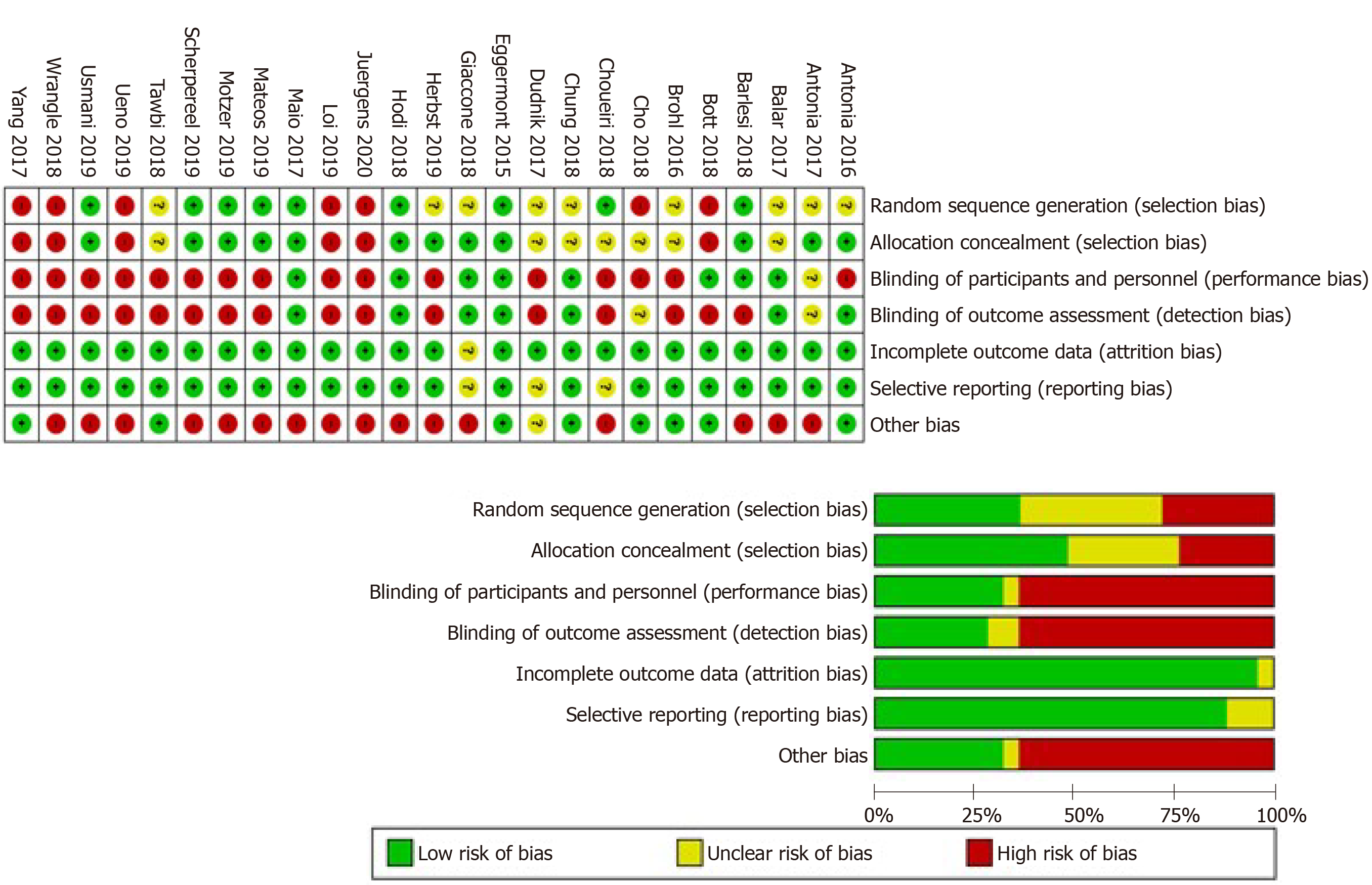
Quality and risk of bias assessment: This systematic review and meta-analysis were conducted in accordance with the principles of the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). We included clinical trials only to ensure high-quality evidence. For assessment of the risk of bias, we used the Cochrane’s Risk of Bias tool[43].
The extracted data were restricted to dichotomous outcomes, as all the data for the analysis are adverse events expressed as events/total. Using the OpenMeta[Analyst] Software, the intended scores were pooled as risk ratios (RR), and the presence of heterogeneity was assessed using two main tests[44], the I-square test (I2) and the P value of the Chi-square test. The analysis is said to be heterogeneous if values of I2 > 50% and P < 0.1 were present, according to the Cochrane Handbook[20]. We performed the analysis of homogeneous data under a fixed-effects model, while heterogeneous data were analyzed under the random-effects model.
We present the analysis of 4622 cancer patients from 26 studies. Figure 1 presents a flow diagram of the number of studies at each stage of the study selection process. Males were slightly overrepresented as compared to females [2420 (52.4%) vs 2202 (47.6%)]. The mean age was 63.7 years. Further details pertaining to study characteristics, cancer type, ICI administered, and demographic data are illustrated in Table 1.
The overall risk of bias was high among the included studies. Studies reported various data regarding randomization of patients, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, attrition bias, and selective reporting. The risk of bias status is summarized in Figure 2.
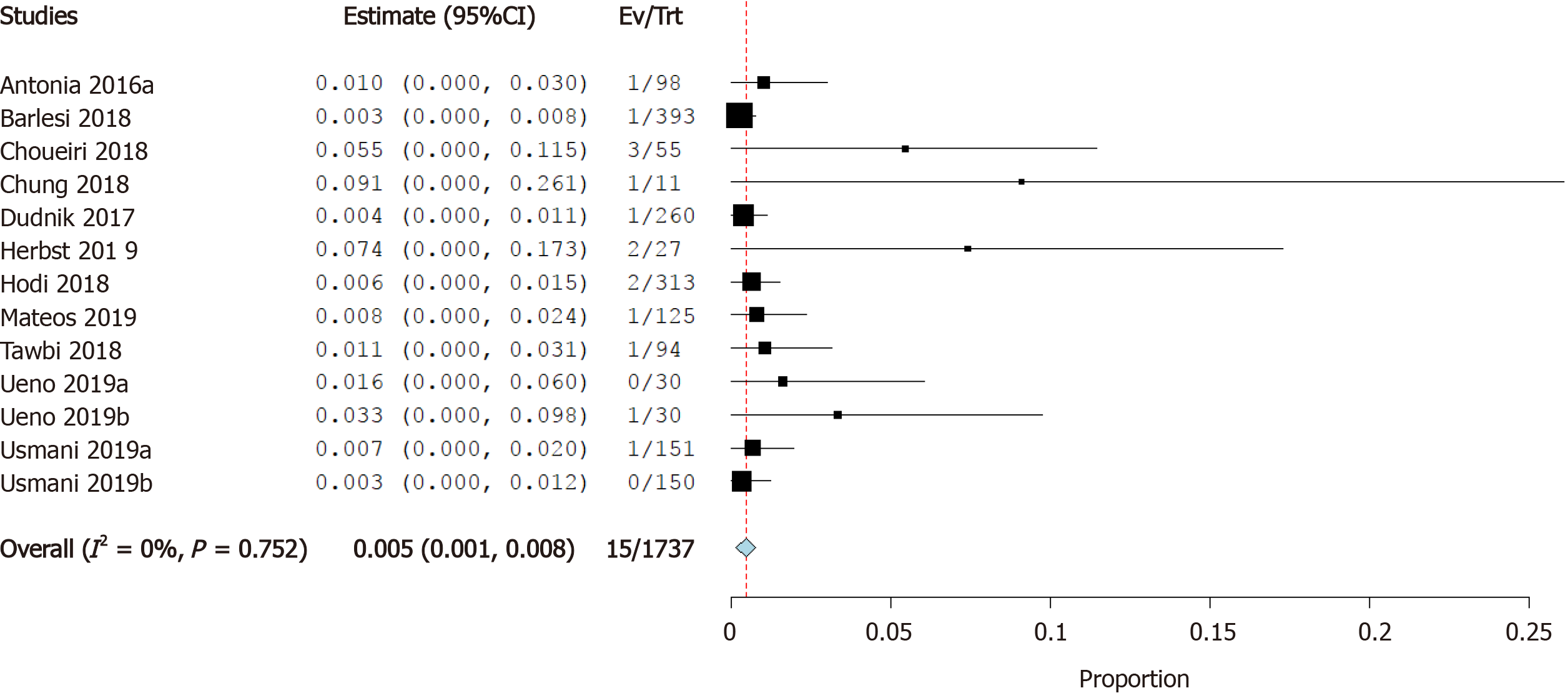
Incidence of myocarditis: Twelve studies reported the incidence of myocarditis as a cardiovascular irAE. The overall effect estimate showed that the incidence of myocarditis was 0.5%; the analysis was significant (95%CI: 0.1%-0.9%) and homogeneous (I2= 0%, P = 0.5) (Figure 3).
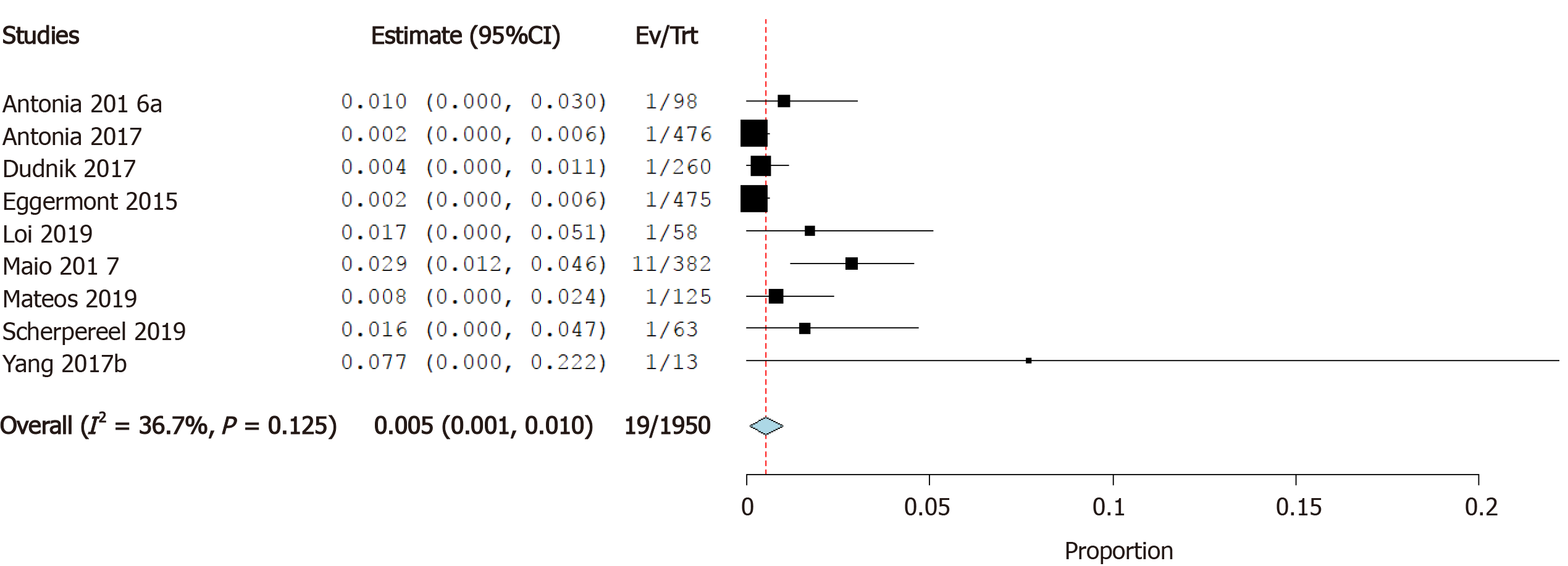
Incidence of pericardial effusion: Nine studies reported the incidence of pericardial effusion as a cardiovascular irAE. The overall effect estimate showed that the incidence of pericardial effusion was 0.5%; the analysis was significant (95%CI: 0.1%-1.0%) and homogeneous (I2= 36.7%, P = 0.1) (Figure 4).
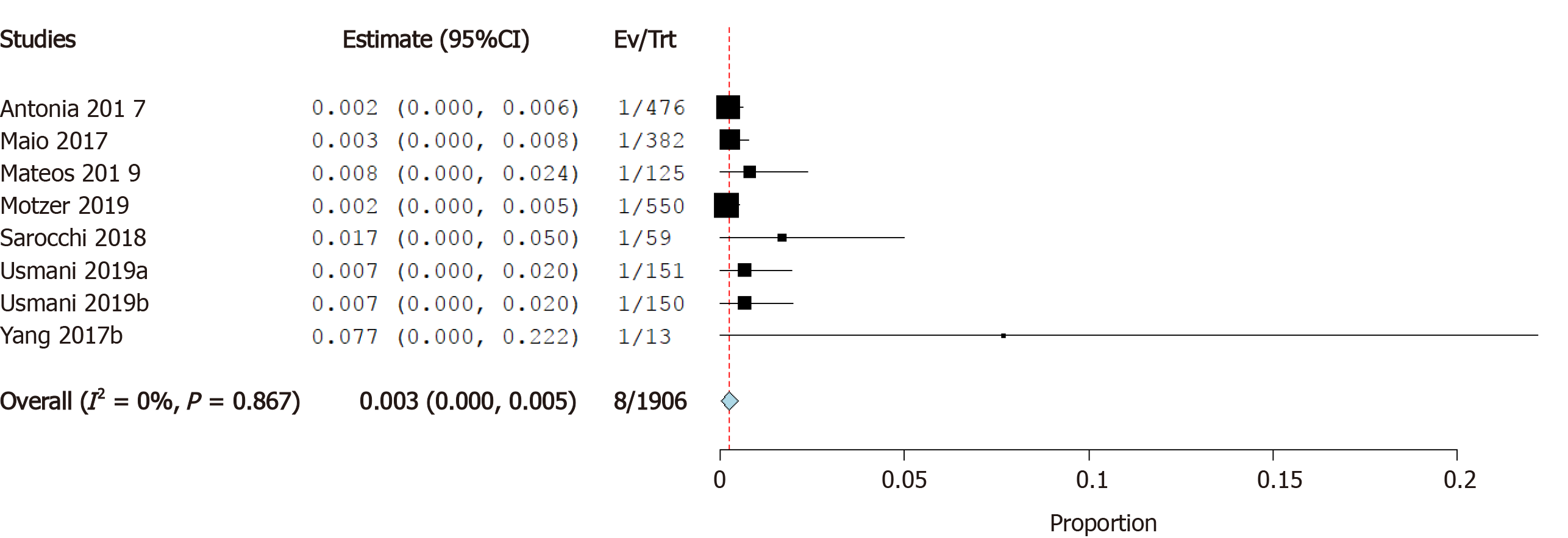
Incidence of heart failure: Seven studies reported the incidence of heart failure as a cardiovascular irAE. The overall effect estimate showed that the incidence of heart failure was 0.3%; the analysis was homogeneous (I2= 0%, P = 0.1) but not significant (95%CI: 0.0%-0.5%) (Figure 5).
Incidence of cardiomyopathy: Five studies reported the incidence of cardiomyopathy as a cardiovascular irAE. The overall effect estimate showed that the incidence of cardiomyopathy was 0.3%; the analysis was homogeneous (I2= 0%, P = 0.6) but not significant (95%CI: -0.1%-0.6%) (Figure 6).
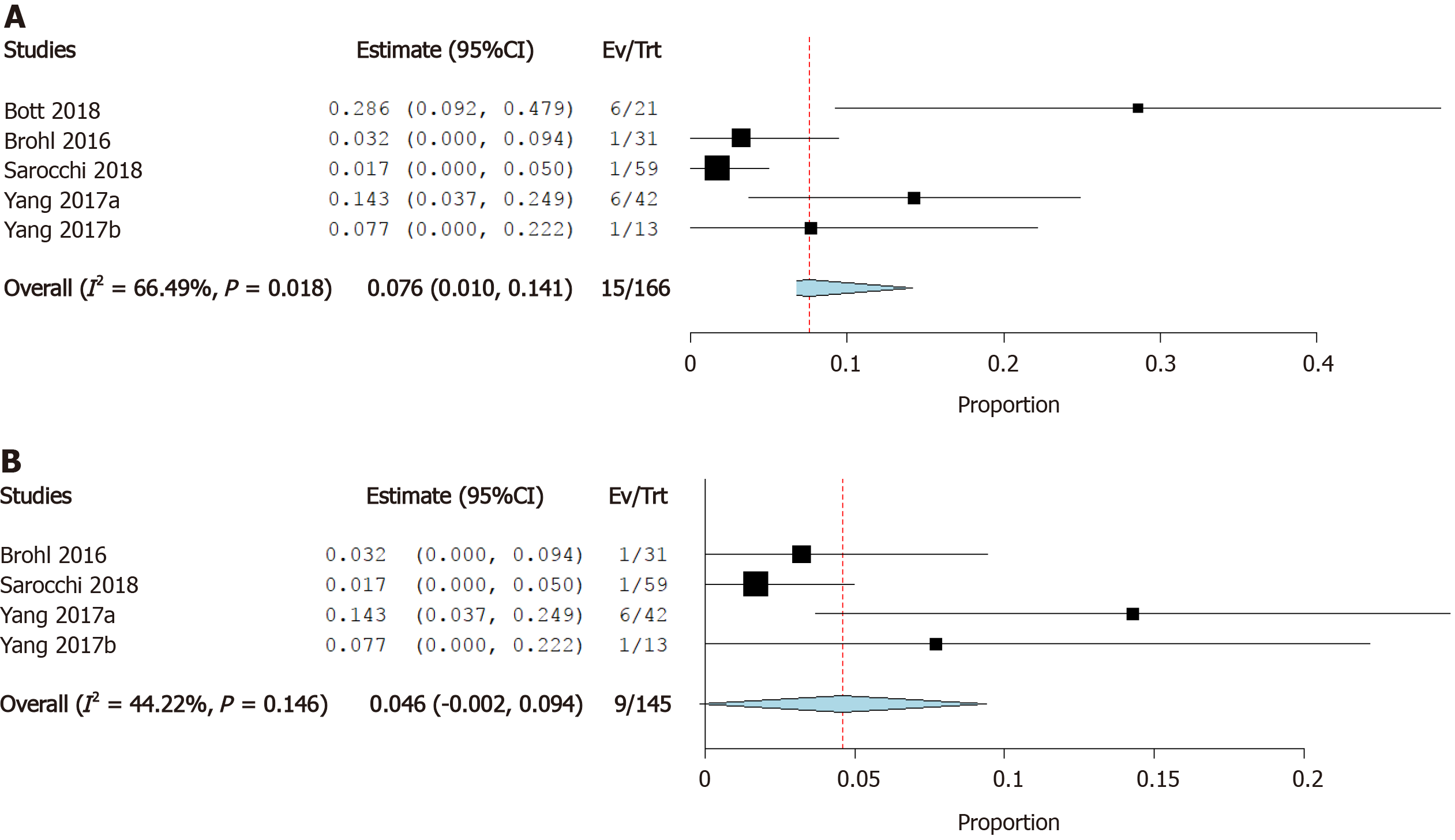
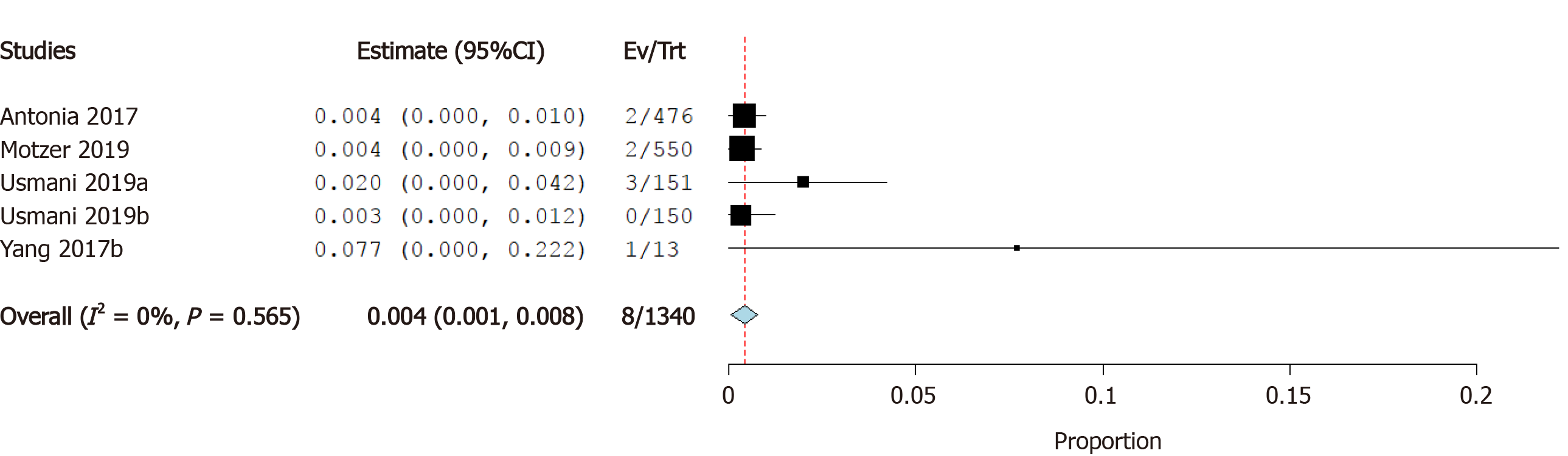
Incidence of atrial fibrillation: Four studies reported the incidence of atrial fibrillation as a cardiovascular irAE. The overall effect estimate showed that the incidence of atrial fibrillation was 7.6%; the analysis was significant (95%CI: 1.0%-14.1%) and heterogeneous (I2= 66%, P = 0.02) (Figure 7A). Using Cochrane’s leave-one-out method, we solved the heterogeneity by excluding one study (Bott et al). Homogeneous results revealed an incidence rate of atrial fibrillation of 4.6%. The results were not significant (95%CI: -0.2%-9.4%) (Figure 7B).
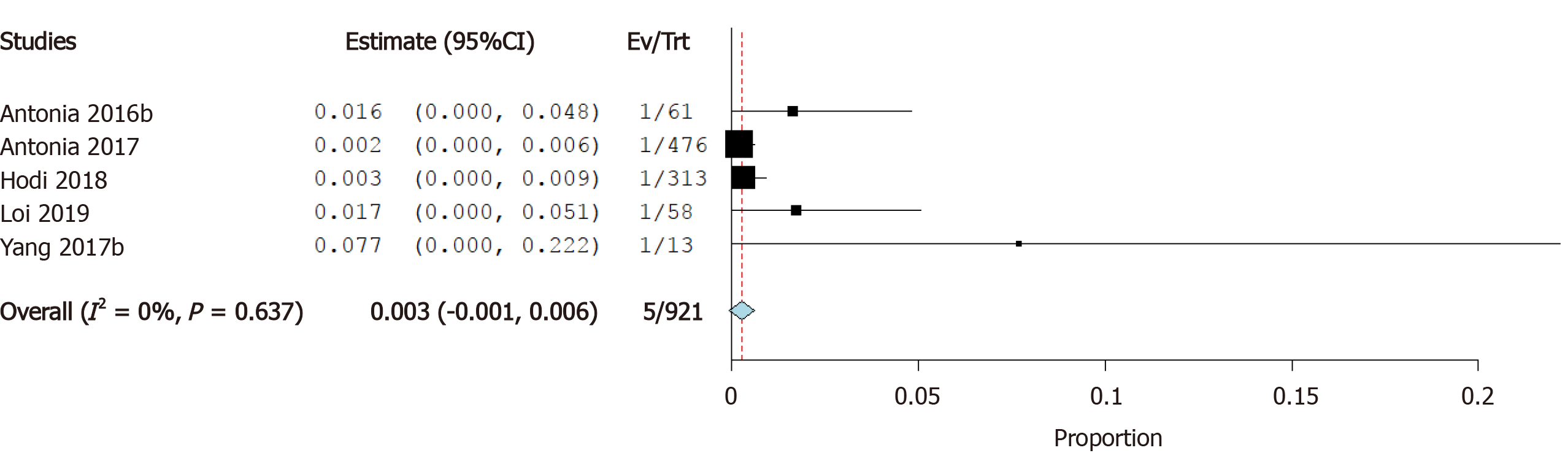
Incidence of myocardial infarction: Six studies reported the incidence of myocardial infarction as a cardiovascular irAE. The overall effect estimate showed that the incidence of MI was 0.4%; the analysis was homogeneous (I2= 0%, P = 0.1) but not significant (95%CI: 0.0%-0.7%) (Figure 8).
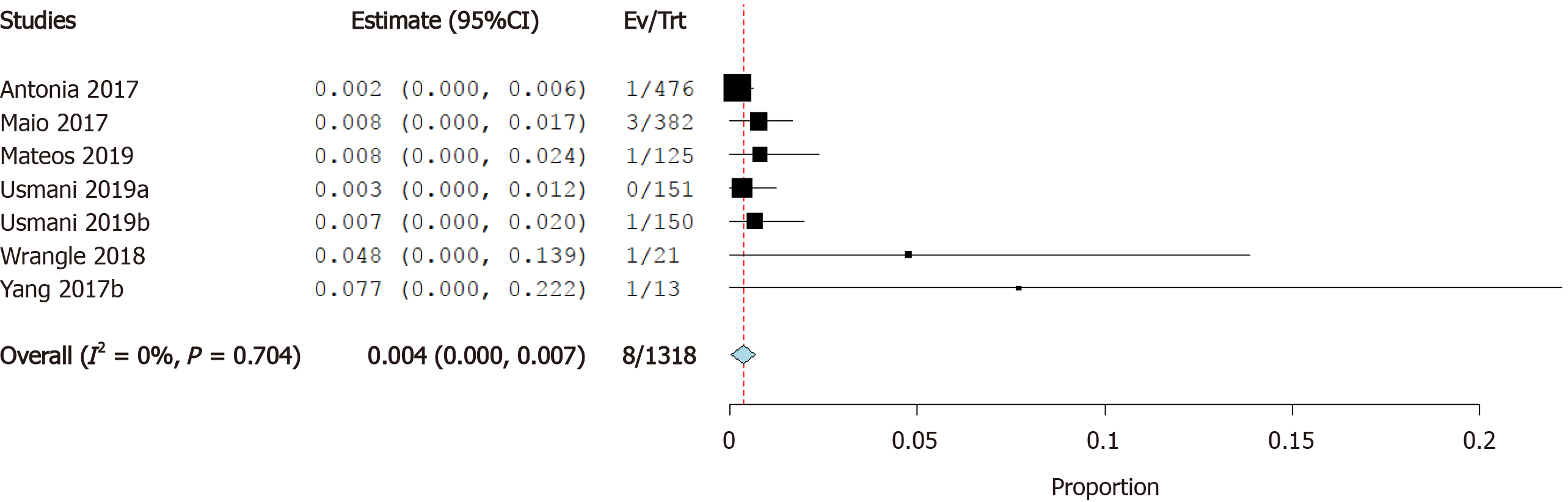
Incidence of cardiac arrest: Four studies reported the incidence of cardiac arrest as a cardiovascular irAE. The overall effect estimate showed that the incidence of cardiac arrest was 0.4%; the analysis was significant (95%CI: 0.1%-0.8%) and homogeneous (I2= 0%, P = 0.6) (Figure 9).
Cardiotoxicity is a rare but potentially fatal adverse effect of ICI therapy. The incidence of cardiovascular irAEs remains to be established[45]. Our meta-analysis of 26 studies including a total of 4622 ICI-treated cancer patients showed that 0.5% of cancer patients treated with ICIs developed myocarditis, 0.3% developed heart failure, and 4.6% developed atrial fibrillation. In addition, pericardial effusion occurred in 0.5% of patients, cardiomyopathy in 0.3% of patients, myocardial infarction in 0.4% of patients, and cardiac arrest in 0.4% of patients. These results are relatively consistent as evidenced by the low level of statistical heterogeneity.
The underlying pathogenesis of cardiovascular irAEs has yet to be fully elucidated. However, several mechanisms have been proposed. The most frequently postulated mechanism underlying myocarditis is that T-lymphocytes could target an antigen common to both neoplastic tissue and the heart. Indeed, in a recent report by Johnson et al[18] the authors described a common high-frequency T-lymphocyte sequence found in both tumor and cardiac muscles. Preclinical studies of mouse models have also shown that PD-1 and CTLA-4 deficiency is associated with myocarditis. The deletion of the PD-1 and CTLA-4 axes induces autoimmune myocarditis, indicating that the PD-1/PD-L1 interaction and CLTA-4 play important roles in protecting against T-lymphocyte-mediated inflammation[46-48]. Injury usually occurs within first three months of initiating ICI; however, late presentation is not uncommon[49,50].
T-lymphocyte-mediated inflammation may also be implicated in the pathogenesis of ICI-related atrial fibrillation. In one recent case report, histopathologic analysis of a patient with atrial fibrillation displayed patchy infiltrations of lymphocytes in the sinoatrial and atrioventricular nodes[18]; this suggests that T-lymphocytes are intricately involved in the development of atrial fibrillation and other ICI-induced conduction disorders. In addition, it has been hypothesized that the increased risk of atrial fibrillation among patients taking ICIs may be attributed to the direct connection between the sinoatrial node and the autonomic nervous system, which make the atria sensitive indicators of any disruptive processes in the body[10].
T-lymphocyte-related inflammatory processes are also suspected in pericardial effusion[51] and myocardial infarction[52]. Lyon et al[52] suggested that the development of ICI-induced myocardial infarction could be due to the activation of an inflammatory reaction that triggers atherosclerotic coronary plaque formation and acute infarction. Conversely, Nykl et al[53] argued that the PD-1 inhibitory effect of ICIs leads to coronary vasospasm and ST-segment elevation. The mechanism by which coronary vasospasm develops is unclear but could be associated with systemic inflammatory response syndrome[43].
The incidence of cardiovascular irAEs is affected by many risk factors. Patients treated with combination therapy were more susceptible to cardiac complications as compared to those treated with ICI monotherapy[49]. In addition, male patients are at higher risk of developing cardiovascular irAEs. A retrospective analysis showed that 77% of cases with ICI-related cardiac toxicity were males[48]. In addition, another multicenter study found that 23 out of 35 irAEs (71%) occurred in male patients[54]. However, data is limited and based on retrospective analyses of a small number of cases (65 cases). Furthermore, concomitant cardiovascular disease is a potential risk factor for cardiovascular irAEs[55].
Cardiovascular irAEs are classified into four grades by the Society for Immunotherapy of Cancer[56]. The management of patients with cardiovascular irAEs differs based on the grade and severity of the symptoms. Grade I is usually asymptomatic and requires neither treatment nor discontinuation of immunotherapy. Grade II is characterized by mild cardiac symptoms that should be controlled by holding cancer immunotherapy and management of the coexisting cardiac disease and its risk factors. Grade III cardiovascular symptoms are significant and require the withdrawal of ICI therapy as well as urgent initiation of high-dose prednisone (1-2 mg/kg). Grade IV cardiovascular irAEs are life-threatening conditions characterized by decompensated cardiac function with moderate-to-severe symptoms; corticosteroid therapy is the first-line treatment. The addition of intravenous immunoglobulins, infliximab, or anti-thymocyte globulin should be considered as second-line treatments for patients with grade IV cardiovascular irAEs[10,56].
Long-term data regarding the prognosis of patients with cardiovascular irAEs are limited. However, the available findings suggest a high fatality rate. In a systematic review that included 99 patients with cardiovascular irAEs, the fatality rate was 35%[50,57]. In addition, observational studies report a 50% rate of major adverse cardiac events in ICI-associated myocarditis, which is significantly higher than that of non-ICI-related myocarditis[58,59].
This study represents an attempt to estimate the overall incidence of cardiovascular irAEs in cancer patients receiving ICI therapy. The quality of the included studies ranged from low to moderate according to the Cochrane Risk of Bias Assessment tool[43]. The main limitation of our analysis is that the included studies were not primarily designed to investigate the incidence of ICI-induced cardiac adverse events. In addition, there was a high risk of bias resulting from the difficulty in blinding and randomization of some studies. The definitions to determine adverse events were slightly different across all studies. We did not consider medication dose, which may influence the severity of adverse effects. Furthermore, although some trials noted an increased risk of cardiovascular irAEs among males, patients receiving multiple ICIs, and patients with pre-existing cardiovascular disease, raw data were not available to perform further subgroup analysis[48,49,54,55]. It is also important to note that malignancy in and of itself is a risk factor for coronary artery disease and other cardiovascular comorbidities and hence it is difficult to differentiate a concomitant cardiovascular irAE[60]. It is therefore reasonable to perform cardiovascular magnetic resonance to distinguish a pre-existing cardiovascular disease from a cardiovascular irAE[58,60]. Nevertheless, we believe this analysis provides a valuable framework for further studies on ICI-associated cardiovascular events.
Cardiovascular irAEs are rare but potentially life-threatening complications that can occur in patients receiving ICI therapy. Our analysis revealed that the most frequent ICI-associated adverse events are atrial fibrillation, myocarditis, and pericardial effusion. Risk factors for cardiovascular irAEs include treatment with combination immunotherapy, male sex, and a history of cardiac disease. Data on the prognosis of cardiac irAEs are limited. Ongoing post-market surveillance is therefore imperative to characterize long-term risks and improve outcomes among patients receiving ICIs.
Immune checkpoint inhibitors (ICIs) are novel antineoplastic agents that are used with increasing frequency throughout the developed world. However, although ICIs have demonstrated remarkable efficacy for the treatment of many malignancies, a range of adverse events have been reported.
Cardiovascular adverse events have been associated with numerous anticancer agents. ICIs have been available for nearly a decade, however, and yet the rate of cardiovascular ICI-related adverse events (irAEs) remains to be definitively established.
We reviewed the medical literature in order to identify, quantify, and characterize the risk of cardiovascular irAEs.
We conducted a systematic review and meta-analysis by searching PubMed, Cochrane CENTRAL, Web of Science, and SCOPUS databases for relevant interventional trials reporting cardiovascular irAEs. We performed a single-arm meta-analysis using OpenMeta [Analyst] software of the following outcomes: Myocarditis, pericardial effusion, heart failure, cardiomyopathy, atrial fibrillation, myocardial infarction, and cardiac arrest. A total of 26 studies were included.
New-onset atrial fibrillation was the most common cardiovascular irAE observed among patients taking ICIs, occurring in 4.6% of individuals included in the analysis. Other relatively common cardiovascular adverse events included pericardial effusion and myocarditis, both of which occurred in 0.5% of patients receiving ICI therapy. The mechanism underlying cardiovascular irAEs remains to be definitively established, but it has been hypothesized that T-lymphocyte-mediated inflammation causes direct myocardial injury and disrupts sinoatrial node activity.
Cardiovascular irAEs—including atrial fibrillation, pericardial effusion, and myocarditis—are uncommon but potentially life-threatening complications of ICI therapy. Mechanisms of pathogenesis and patient- and ICI-associated risk factors warrant further investigation.
Cardiovascular irAEs represent rare but potentially life-threatening complications of ICIs. Data from post-market surveillance will play a vital role in clarifying the risk of cardiovascular irAEs. Based on the available evidence, however, close cardiac monitoring of patients receiving ICIs may be warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang Y S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Hirsch L, Zitvogel L, Eggermont A, Marabelle A. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer. 2019;120:3-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, Heydebreck AV, Chin K, Cuillerot JM, Kelly K. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18:599-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 3. | Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, Powles T, McDermott D, Robbins PB, Chism DD, Cho D, Atkins MB, Gordon MS, Gupta S, Uemura H, Tomita Y, Compagnoni A, Fowst C, di Pietro A, Rini BI. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19:451-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 4. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 5734] [Article Influence: 637.1] [Reference Citation Analysis (0)] |
| 5. | Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2362] [Cited by in F6Publishing: 2504] [Article Influence: 357.7] [Reference Citation Analysis (0)] |
| 6. | Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O, Monnet I, Corre R, Audigier-Valette C, Locatelli-Sanchez M, Molinier O, Guisier F, Urban T, Ligeza-Poisson C, Planchard D, Amour E, Morin F, Moro-Sibilot D, Zalcman G; French Cooperative Thoracic Intergroup. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20:239-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 7. | Juergens RA, Hao D, Ellis PM, Tu D, Mates M, Kollmannsberger C, Bradbury PA, Tehfe M, Wheatley-Price P, Robinson A, Bebb G, Laskin J, Goffin J, Hilton J, Tomiak A, Hotte S, Goss GD, Brown-Walker P, Sun X, Tsao MS, Cabanero M, Gauthier I, Song X, Dennis PA, Seymour LK, Smoragiewicz M, Laurie SA. A phase IB study of durvalumab with or without tremelimumab and platinum-doublet chemotherapy in advanced solid tumours: Canadian Cancer Trials Group Study IND226. Lung Cancer. 2020;143:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 901] [Cited by in F6Publishing: 902] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 9. | Topalian SL. Targeting Immune Checkpoints in Cancer Therapy. JAMA. 2017;318:1647-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2245] [Cited by in F6Publishing: 2239] [Article Influence: 373.2] [Reference Citation Analysis (0)] |
| 11. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2308] [Cited by in F6Publishing: 2613] [Article Influence: 435.5] [Reference Citation Analysis (0)] |
| 12. | Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Chesney J, Robert C, Grossmann K, McDermott D, Walker D, Bhore R, Larkin J, Postow MA. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol. 2017;35:3807-3814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 13. | Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol. 2017;35:785-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 707] [Cited by in F6Publishing: 797] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 14. | Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1248] [Cited by in F6Publishing: 1423] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 15. | Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2:1346-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 547] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 16. | Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015;2015:794842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 18. | Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749-1755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1305] [Cited by in F6Publishing: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 19. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47017] [Cited by in F6Publishing: 43548] [Article Influence: 2903.2] [Reference Citation Analysis (0)] |
| 20. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, UK: John Wiley and Sons, 2019. [Cited in This Article: ] |
| 21. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2750] [Cited by in F6Publishing: 2759] [Article Influence: 394.1] [Reference Citation Analysis (0)] |
| 22. | Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 726] [Cited by in F6Publishing: 896] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 23. | Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19:1468-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 24. | Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, Downey RJ, Brahmer JR, Battafarano R, Bush E, Chaft J, Forde PM, Jones DR, Broderick SR. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2019;158:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 25. | Brohl AS, Khushalani NI, Eroglu Z, Markowitz J, Thapa R, Chen YA, Kudchadkar R, Weber JS. A phase IB study of ipilimumab with peginterferon alfa-2b in patients with unresectable melanoma. J Immunother Cancer. 2016;4:85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol. 2019;37:2162-2170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 27. | Chung V, Kos FJ, Hardwick N, Yuan Y, Chao J, Li D, Waisman J, Li M, Zurcher K, Frankel P, Diamond DJ. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers. Clin Transl Oncol. 2019;21:363-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Dudnik E, Moskovitz M, Daher S, Shamai S, Hanovich E, Grubstein A, Shochat T, Wollner M, Bar J, Merimsky O, Zer A, Goldstein DA, Hammerman A, Cyjon A, Shechtman Y, Abu-Amna M, Flex D, Roisman LC, Peled N; Israel Lung Cancer Group. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: The real-life data. Lung Cancer. 2018;126:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Konto C, Hoos A, de Pril V, Gurunath RK, de Schaetzen G, Suciu S, Testori A. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 863] [Cited by in F6Publishing: 891] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 30. | Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, Manning M, Mogg R, Blumenschein WM, Tan MT, Subramaniam DS, Liu SV, Kaplan IM, McCutcheon JN. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 31. | Herbst RS, Arkenau HT, Bendell J, Arrowsmith E, Wermke M, Soriano A, Penel N, Santana-Davila R, Bischoff H, Chau I, Mi G, Wang H, Rasmussen E, Ferry D, Chao BH, Paz-Ares L. Phase 1 Expansion Cohort of Ramucirumab Plus Pembrolizumab in Advanced Treatment-Naïve Non-Small Cell Lung Cancer. J Thorac Oncol. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 929] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 33. | Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, André F; International Breast Cancer Study Group and the Breast International Group. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019; 20:371-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 294] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 34. | Maio M, Scherpereel A, Calabrò L, Aerts J, Perez SC, Bearz A, Nackaerts K, Fennell DA, Kowalski D, Tsao AS, Taylor P, Grosso F, Antonia SJ, Nowak AK, Taboada M, Puglisi M, Stockman PK, Kindler HL. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18:1261-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 296] [Article Influence: 42.3] [Reference Citation Analysis (2)] |
| 35. | Mateos MV, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, Goldschmidt H, Larocca A, Chanan-Khan A, Sherbenou D, Avivi I, Benyamini N, Iida S, Matsumoto M, Suzuki K, Ribrag V, Usmani SZ, Jagannath S, Ocio EM, Rodriguez-Otero P, San Miguel J, Kher U, Farooqui M, Liao J, Marinello P, Lonial S; KEYNOTE-183 Investigators. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6:e459-e469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 36. | Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, Porta C, George S, Neiman V, Bracarda S, Tykodi SS, Barthélémy P, Leibowitz-Amit R, Plimack ER, Oosting SF, Redman B, Melichar B, Powles T, Nathan P, Oudard S, Pook D, Choueiri TK, Donskov F, Grimm MO, Gurney H, Heng DYC, Kollmannsberger CK, Harrison MR, Tomita Y, Duran I, Grünwald V, McHenry MB, Mekan S, Tannir NM; CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370-1385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 533] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 37. | Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, Rijavec E, Barletta G, Rossi G, Biello F, Ghigliotti G, Canepa M, Mussap M, Brunelli C, Spallarossa P. Serial Troponin for Early Detection of Nivolumab Cardiotoxicity in Advanced Non-Small Cell Lung Cancer Patients. Oncologist. 2018;23:936-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, Reardon DA, Puzanov I, Kudchadkar RR, Thomas RP, Tarhini A, Pavlick AC, Jiang J, Avila A, Demelo S, Margolin K. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379:722-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 801] [Cited by in F6Publishing: 837] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 39. | Ueno M, Ikeda M, Morizane C, Kobayashi S, Ohno I, Kondo S, Okano N, Kimura K, Asada S, Namba Y, Okusaka T, Furuse J. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:611-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 40. | Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, Yimer HA, LeBlanc R, Takezako N, McCroskey RD, Lim ABM, Suzuki K, Kosugi H, Grigoriadis G, Avivi I, Facon T, Jagannath S, Lonial S, Ghori RU, Farooqui MZH, Marinello P, San-Miguel J; KEYNOTE-185 Investigators. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6:e448-e458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 41. | Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, Taffaro-Neskey M, Sherman C, Suriano S, Swiderska-Syn M, Sion A, Harris J, Edwards AR, Rytlewski JA, Sanders CM, Yusko EC, Robinson MD, Krieg C, Redmond WL, Egan JO, Rhode PR, Jeng EK, Rock AD, Wong HC, Rubinstein MP. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19:694-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 281] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 42. | Yang CJ, McSherry F, Mayne NR, Wang X, Berry MF, Tong B, Harpole DH Jr, D'Amico TA, Christensen JD, Ready NE, Klapper JA. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann Thorac Surg. 2018;105:924-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18487] [Cited by in F6Publishing: 21489] [Article Influence: 1653.0] [Reference Citation Analysis (2)] |
| 44. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39087] [Cited by in F6Publishing: 42062] [Article Influence: 2003.0] [Reference Citation Analysis (1)] |
| 45. | Ganatra S, Neilan TG. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist. 2018;23:879-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 46. | Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res. 2007;101:248-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2109] [Cited by in F6Publishing: 2110] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 48. | Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188:4876-4884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 49. | Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755-1764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 648] [Cited by in F6Publishing: 865] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 50. | Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, Lalevee N, Barraud J, Peyrol M, Laine M, Bonello L, Paganelli F, Cohen A, Barlesi F, Ederhy S, Thuny F. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation. 2017;136:2085-2087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 51. | Yang S, Asnani A. Cardiotoxicities associated with immune checkpoint inhibitors. Curr Probl Cancer. 2018;42:422-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447-e458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 53. | Nykl R, Fischer O, Vykoupil K, Taborsky M. A unique reason for coronary spasm causing temporary ST elevation myocardial infarction (inferior STEMI) - systemic inflammatory response syndrome after use of pembrolizumab. Arch Med Sci Atheroscler Dis. 2017;2:e100-e102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Mahrholdt H, Wagner A, Judd RM, Sechtem U. Assessment of myocardial viability by cardiovascular magnetic resonance imaging. Eur Heart J. 2002;23:602-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Tadokoro T, Keshino E, Makiyama A, Sasaguri T, Ohshima K, Katano H, Mohri M. Acute Lymphocytic Myocarditis With Anti-PD-1 Antibody Nivolumab. Circ Heart Fail. 2016;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1337] [Cited by in F6Publishing: 1239] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 57. | Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 667] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 58. | Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J Am Coll Cardiol. 2017;70:1964-1976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 59. | Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, Petrella D, Garascia A, Pedrotti P, Roghi A, Bonacina E, Moreo A, Bottiroli M, Gagliardone MP, Mondino M, Ghio S, Totaro R, Turazza FM, Russo CF, Oliva F, Camici PG, Frigerio M. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation. 2017;136:529-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 60. | Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J Am Heart Assoc. 2020;9:e013757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |