Published online Jun 28, 2014. doi: 10.4329/wjr.v6.i6.223
Revised: April 13, 2014
Accepted: May 16, 2014
Published online: June 28, 2014
The presence of white matter hyperintensities (WMHs) has been commonly associated with poor outcome in subjects with major affective disorders. Unfortunately, WMHs may be frequently confounded by the use of psychoactive medications and duration of illness. Although findings from the current literature are quite conflicting, we proposed that subjects with WMHs may be at higher suicidal risk when compared to other subgroups without. Based on the Fazekas modified scale, the severity of WMHs may serve as a trait marker of disease. Interestingly, the presence of WMHs may represent a neurobiological marker between the underlying vulnerability and clinical presentation of major affective disorders.
Core tip: Understanding neural correlates underlying psychiatric morbidity over time is critical but, to date, structural magnetic resonance imaging studies identified only not stable risk predictors of unfavourable outcome in psychiatric populations. The presence of white matter hyperintensities (WMHs) has been commonly associated with a poor outcome in individuals with major affective disorders. Based on our studies, subjects with WMHs may be considered at higher suicidal risk than those without and the severity of WMHs as assessed by the Fazekas modified scale may serve as a trait marker of disease. WMHs may represent an interesting neurobiological marker between the underlying vulnerability and clinical presentation of major affective disorders.
- Citation: Serafini G, Gonda X, Rihmer Z, Girardi P, Amore M. White matter abnormalities: Insights into the pathophysiology of major affective disorders. World J Radiol 2014; 6(6): 223-229
- URL: https://www.wjgnet.com/1949-8470/full/v6/i6/223.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i6.223
Major affective disorders are chronic and disabling diseases that are associated with significant functional impairment. Patients with major affective disorders commonly experience long-term negative outcomes, frequent relapses, incomplete recovery between episodes, residual symptoms, persistent psychosocial impairment and high suicide risk[1,2]. Among all major affective disorders, bipolar disorder (BD), including both BD type I and type II, is a serious mental illness that affects approximately 1%-3% of the adult population. However, if subthreshold cases are also considered, the lifetime prevalence of bipolar disorders is around 6%[3]. White matter hyperintensities (WMHs) are, no doubt, the most common neuroimaging finding in patients with BD, regardless of age[4]. However, WMHs are also frequent in other major affective disorders (for details see Table 1 in the recent study of Serafini et al[5]).
| Ref. | Sample characteristics | Study type | Location of WMHs | Main findings | Limitations of the study | Conclusion |
| Serafini et al[5] | 148 patients (77 men, 71 women) with BD-I having a mean age of 47.9 yr | Research article | Centrum semiovale (24.4%) and corona radiata (20.2%) regions, cortical and subcortical deep frontal (17.6%), parietal (15.1%), and temporal (8.4%) areas | A total of 73 subjects (49.3%) reported PWMHs and 59 (39.9%) had DWMHs. Overall, 41 (27.7%) subjects had both PWMHs and DWMHs. Patients with BD-I and lower insight for mania had significantly more PWMHs (54.6% vs 22.2%; P < 0.05), significantly higher scores on the HDRS17 (27.05 ± 6.54 vs 23.67 ± 8.64; t146 = -1.98; P < 0.05), and more frequent BHS score ≥ 9 (66.2% vs 38.9%; P < 0.05) when compared to those with higher insight for mania | All participants were inpatients (a potential confounder). The present study did not include a formal measure of insight. The effects of psychoactive medications on insight ratings and image processing were not analyzed | Patients with PWMHs were more likely to have impaired insight than those without. Different insight levels reflected different MRI findings |
| Serafini et al[19] | 85 adult outpatients (16 men and 69 women) with CH and having a mean age of 50.1 | Research article | Not specified | Above 40% of patients had PWMHs and almost 98% had DWMHs. Patients with PWMHs differed from those without periventricular lesions on depression severity (t 77.76 = 2.30; P < 0.05). Patients with PWMHs had lower CES-D scores (13.79 ± 7.51 vs 18.19 ± 9.68) than patients without PWMHs. Patients with more severe DWMHs were older (53.89 ± 13.26 vs 47.40 ± 11.91) and reported lower scores on the drive dimension (9.97 ± 2.86 vs 11.14 ± 2.52) than patients with mild lesions or without any deep lesion | Different mechanisms may be considered in the emergence of WMHs and it is possible that WMHs may represent only the ‘tip of the iceberg’ in terms of structural white matter lesions | Patients with PWMHs were 1.06 times more likely to have lower CES-D scores (P < 0.05) than patients without PWMHs. Patients with more severe DWMHs were 1.04 times more likely to be older (P < 0.05) than patients with mild or without any DWMHs |
| Serafini et al[16] | 247 patients (118 men, 129 women) with major affective disorders, specifically 143 BD-I, 42 BD-II, and 62 with MDD | Research article | Centrum semiovale (24.4%) and corona radiata (20.2%) regions, cortical and subcortical deep frontal (17.6%), parietal (15.1%), and temporal (8.4%) areas | 48% of patients had PWMHs (more than 15% had PWMHs of 2 or higher on the Fazekas modified scale), and 39% had DWMHs (more than 7% had DWMHs of 2 or higher on the Fazekas modified scale). Patients in the high dysthymic, cyclothymic, irritability, and anxiety group were more likely to have higher BHS ≥ 9 = 77% vs 52%; P > 0.001), more DWMHs (46% vs 29%; χ2n =3 = 9.90; P < 0.05), higher MINI suicidal risk (54% vs 42%; P < 0.05), and more recent suicide attempts (24% vs 14%; P < 0.05), than patients in the hyperthymia group | The small sample size did not allow to generalize findings. The association between the lethality or number of suicide attempts and the presence, severity, or number of hyperintensities was not assessed. The study lacks of accounting for the cognitive effects of medications | Differences among temperament groups as measured by the TEMPS-A are supported by differences at the MRI indicating that different temperament profiles are associated with differences in subcortical brain structures |
| Serafini et al[20] | A 76-year-old woman with BD hospitalized for a mixed state | Case report with a 2-yr follow-up | Not specified | Patient had severe WMHs, she took lithium and haloperidol during the hospitalization. She was euthymic at discharge as well as after two-years of follow-up. Her nutrition had a high concentration of Vitamin-D | A second MRI was not performed | Although WM lesions were persistent, the patient improved in both mood and quality of life. Lithium and Vitamin-D may have exerted possible protective effects |
| Serafini et al[18] | 54 patients (30 men and 24 women) with LOBD (≥ 60 yr old) having a mean age of 68 yr. 76% had a diagnosis of BD-I, and 24% had a diagnosis of BD-II | Letter to the Editor including research data | Centrum semiovale (22%), corona radiata (15%), paratrigonal regions (6%), cortical, subcortical deep frontal (46%), parietal (24%) areas | Confluence of DWMH lesions were found in 17% of the patients (modified Fazekas scale ≥ 2) whereas in 28% PWMH confluent lesions (modified Fazekas scale ≥ 2) were reported. No significant association resulted between diagnosis and PWMHs or DWMHs. BD-II with DWMHs had a poorer quality of life than BD-I subjects | The link between clinical features of bipolar disorders and deep brain lesions on MRI remains quite unknown | MRI findings of DWMHs could be a useful biological predictor of severity in patients with BD-II |
| Pompili et al[17] | 47 LOBD patients (55.3% men and 44.7% women) | Review article including research data | Frontal (26.1) and centrum semiovale areas (26.1%), corona radiata (17.4%), parietal (17.4%), and paratrigonal regions (8.7%) | 55.3% of these patients had periventricular WMHs, 46.4% had WMHs of mild severity, 50% WMHs of moderate severity, and only 3.6 WMHs of high severity. 34% of LOBD patients had both deep and periventricular WMHs. A significant relationship between older age with LOBD and WMHs was reported | Vascular-related mechanisms cannot be the only factors implicated in the pathophysiology of the WMHs in LOBD subjects. The study did not assess how cerebro-vascular risk factors are related to the type /intensity of medications, and the progression of WMHs | MRI findings of WMHs could be a useful biological predictor of severity in patients with LOBD |
| Pompili et al[15] | 99 patients having a mean age of 46.5 yr. 40.4% were diagnosed as BD-I, 21.2% as BD-II, and 38.4% as unipolar MDD | Research article | Corona radiate (n = 10), centrum semiovale (n = 6), and frontal subcortical white matter (n = 18) | It has been suggested that 27.3% of patients showed evidence of PWMHs and 36.4% of DWMHs whereas 14.1% of patients had hyperintensities in both locations. The presence of PWMHs was the only variable significantly associated with attempted suicide even after controlling for age. Subjects with PWMHs were 8 times more likely to have attempted suicide than individuals without PWMHs [OR = 8.08 (95%CI: 2.67-24.51)] | The small sample size may affect the generalization of results. PWMHs were able to explain only a small part of the variability of suicide attempt risk, indicating that one single variable is not sufficient to predict suicidality | Patients with affective disorders and PWMHs are more likely to have a history of suicide attempts even after controlling for potential confounding variables such as cardiovascular risk factors and age |
| Pompili et al[12] | 65 subjects, 29 (44.6%) with a history of at least one suicide attempt, and 36 (65.4%) without. Subjects had a mean age of 44.61 yr | Research article | Not specified | After logistic regression analyses, the prevalence of WMHs was significantly higher in subjects with past suicide attempts (P = 0.01) and other clinical indicators of elevated suicide risk | The association between WMHs and suicidality holds true for both unipolar and bipolar depressed patients | WMHs in patients with major affective disorders might be useful biological markers of suicidality |
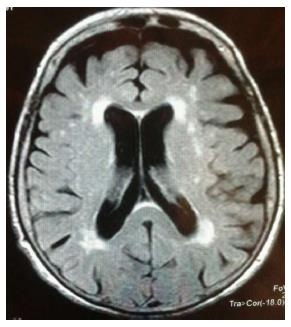
WMHs are hyperintense signals on T2-weighted magnetic resonance images (MRI) identifying ependymal loss and altered brain myelination. According to their localization, WMHs may be classified in periventricular white matter hyperintensities (PWMHs) having a debated origin and deep white matter hyperintensities (DWMHs) with a predominant vascular aetiology[6].WMHs are known to be commonly associated with older age and several risk factors such as arterial hypertension and diabetes mellitus (Figure 1).
WMHs are frequently associated with demyelinating disorders, in particular multiple sclerosis, an illness involving the presence of different causative mechanisms[7,8]. As suggested[7,8], distinct patterns of demyelination have been documented over time and subcortical WMHs have been repeatedly recorded in patients with multiple sclerosis. MRI may be commonly used to monitor disease progress in the white matter of patients with multiple sclerosis[9]. However, MRI techniques may be considered as not sufficiently sensitive to detect purely cortical MS lesions[10] and their sensitivity can be improved using higher field strength or voxel-based morphometry.
Several studies suggested that WMHs are consistently associated with major affective disorders and suicidal behaviour in children as well as in young adults[11,12]. Overall, the association between WMHs and major affective disorders has been confirmed by several lines of evidence, not only in patients with major depressive disorder (MDD) but also in those with bipolar disorder (BD)[13], respectively. This manuscript aimed to selectively review our research studies that have been published to date about the association between white matter hyperintensities assessed by MRI, major affective disorders, and suicidal behaviour.
A critical review of the eight studies that have been published by our research group about white matter abnormalities, major affective disorders, and suicidal behaviour has been conducted.
This is, in summary, an educational commentary reviewing the evidence derived by our research studies concerning the presence and significance of white matter abnormalities in subjects with major affective disorders.
Eight research articles have been performed by our research group about the association between WMHs, major affective disorders, and suicidal behaviour. The presence of WMHs was assessed by a neuroradiologist blind to all clinical information, using the modified Fazekas four-point rating scale describing MRI hyperintensities on an ascending scale of intensity and frequency[14]. A second neuroradiologist, blind to all clinical information and previous ratings, usually reviewed all MRI films.
Pompili et al[12] initially suggested that WMHs in patients with major affective disorders might be useful biological markers of suicidality. The authors investigated a sample of 65 subjects of which 44.6% had a history of at least one suicide attempt. After logistic regression analyses they reported that the prevalence of WMHs was significantly higher in subjects with past suicide attempts and elevated suicide risk.
Subsequently, Pompili et al[15] analyzed 99 patients of which 40.4% were diagnosed as BD-I, 21.2% were diagnosed as BD-II and 38.4% as unipolar MDD. They found that 27.3% of patients showed evidence of PWMHs, 36.4% presented DWMHs whereas 14.1% had hyperintensities in both periventricular and deep locations. Interestingly, the presence of PWMHs was the only variable significantly associated with attempted suicide even after controlling for age. Subjects with PWMHs were 8 times more likely to have attempted suicide when compared to individuals without PWMHs. Therefore, patients with major affective disorders and PWMHs are more likely to have a history of suicide attempts even after controlling for potential confounding variables such as cardiovascular risk factors and age.
In 2011, Serafini et al[16] reported that differences among temperament groups as measured by the Temperament Evaluation of Memphis, Pisa, Paris and San Diego-autoquestionnaire (TEMPS-A) are supported by differences at the MRI indicating that different temperament profiles are associated with differences in the subcortical brain structures of 247 patients with major affective disorders (specifically 143 with BD type I, 42 with BD type II, and 62 with MDD). TEMPS-A is a self-report questionnaire designed to measure temperamental variations in psychiatric patients and healthy volunteers and has been used by the authors on the basis of the diagnostic criteria for affective temperaments (cyclothymic, dysthymic, irritable, hyperthymic, and anxious). They found that 48% of patients had PWMHs (specifically, more than 15% had PWMHs of 2 or higher on the Fazekas modified scale), and 39% had DWMHs (in particular, more than 7% had DWMHs of 2 or higher on the Fazekas modified scale). Patients in the high- dysthymic, cyclothymic, irritability, and anxiety group were more likely to have higher Beck Hopelessness Scale (BHS), more DWMHs, higher Mini International Neuropsychiatric Interview (MINI) suicidal risk, and more recent suicide attempts when compared with patients in the hyperthymia group. BHS is a 20-item psychometric instrument designed to measure negative attitudes about the future whereas MINI is a short structured interview developed to explore psychiatric disorders according to DSM-III-R; importantly, one section of this instrument is developed to assess suicidal risk with questions about past and current suicidality.
Pompili et al[17] conducted an overview of the literature (including research data) in which reported that 55.3% of patients had periventricular WMHs (46.4% of them of mild severity, 50% of moderate severity, and only 3.6 of high severity, respectively). They also found that 34% of late-onset bipolar disorder (LOBD) patients had both deep and periventricular WMHs and a significant relationship has been suggested between older age with LOBD and WMHs. The authors concluded that MRI findings of WMHs could be a useful biological predictor of severity in patients with LOBD.
Subsequently, Serafini et al[18] reported that in a sample of 54 patients (30 men and 24 women) with LOBD (≥ 60 years old) 76% had a diagnosis of BD type I, 24% a diagnosis of BD type II whereas confluence of deep lesions (modified Fazekas scale ≥ 2) was found in 17% of the patients and in 28% periventricular confluent lesions (modified Fazekas scale ≥ 2). The authors suggested that no significant association between diagnosis and PWMHs/DWMHs emerged but, interestingly, subjects with BD type II and DWMHs had a poorer quality of life compared to paients with BD type I.
Finally, Serafini et al[5] suggested that in a sample of 148 patients with BD type I (having a mean age of 47.9 years) a total of 49.3% reported PWMHs and 39.9% had DWMHs. Overall, 27.7% of subjects had both PWMHs and DWMHs. They reported that patients with BD type I and lower insight for mania had significantly more PWMHs (54.6% vs 22.2%; P < 0.05), significantly higher scores on the HDRS17 (27.05 ± 6.54 vs 23.67 ± 8.64; t146 = -1.98; P < 0.05), and more frequent BHS scores ≥ 9 (66.2% vs 38.9%; P < 0.05) when compared to patients with BD type I and higher insight for mania. Importantly, different insight levels reflected different MRI findings. The authors concluded that patients with PWMHs were more likely to have impaired insight than those without.
Serafini et al[19] found that in a sample of 85 adult outpatients with a chronic headache above 40% of patients had PWMHs and almost 98% DWMHs, respectively. Patients with PWMHs significantly differed from those without periventricular lesions on depression severity. Patients with PWMHs had lower Center for Epidemiologic Studies Depression Scale (CES-D) scores (13.79 ± 7.51 vs 18.19 ± 9.68) when compared with patients without PWMHs. Also, patients with more severe DWMHs were older (53.89 ± 13.26 vs 47.40 ± 11.91) and reported lower scores on the drive dimension (9.97 ± 2.86 vs 11.14 ± 2.52) than patients with mild or without any deep lesion.
Overall, patients with PWMHs were 1.06 times more likely to have lower CES-D scores compared to patients without PWMHs. Patients with more severe DWMHs were 1.04 times more likely to be older than patients with mild or without DWMHs.
Based on a case report study of a 76-year-old woman with BD hospitalized for a mixed state and having severe WMHs treated with lithium and haloperidol during the hospitalization, Serafini et al[20] found that she was euthymic at discharge as well as after two-years of follow-up. The authors suggested that, although WM lesions were persistent, the patient improved in both mood and quality of life. Lithium and Vitamin-D (highly present in her nutrition) may have exerted possible neuroprotective effects.
Patients with WMHs, particularly those with abnormalities in the white matter of prefrontal cortex, amygdala-hippocampus complex, thalamus and basal ganglia whose integrity implicates adequate mood regulation may be at higher risk for developing mood disorders because of possible alterations of neuroanatomic pathways[21]. Also, volumetric studies clearly indicated the possible involvement of the frontal cortex, temporal lobes, basal ganglia and cerebellum in BD and, recently, subgenual cingulate cortex in adolescents with BD type I[22].
Understanding the nature and significance of white matter abnormalities in major affective disorders is critical as these lesions may represent neurobiological markers able to indicate the risk for subsequent development of more aggressive illness subtypes and the need of more targeted interventions[23].
WMH location may be critical in the expression of certain affective dysfunctions (e.g., cognitive/emotional impairments). Periventricular lesions seem to be more common in BD type I compared to BD-type II, and healthy controls[5,12,18]. This may indicate that these neuroimaging findings are sensitive and even subtype selective diagnostic tools in bipolar patients whereas DWMHs are predictors of a less favourable outcome being associated with a poorer response to treatment, and recurrent illness episodes[24].
A relevant association between increased rates of WMHs and a history of suicide attempts has also been suggested in both unipolar and bipolar patients[12]. This finding has been replicated in a sample of 99 consecutively admitted inpatients with major affective disorders where neuroimaging measures were found to be markers of risk for suicidal attempts. Specifically, attempters and nonattempters differed only for the presence of PWMHs, with the former who were more likely to have PWMHs[15]. Moreover, a significant association between older age and WMHs[17], and between DWMHs and poor prognosis was reported in a sample of patients with late-onset bipolar II disorder[18] demonstrating that WMHs could be useful biological predictors of illness severity.
Differences in MRI profiles were also found to be associated with differences among temperament groups measured by the TEMPS-A[16]. Specifically, patients with higher scores for dysthymic and lower scores for hyperthymic temperament were more likely to have higher BHS scores, more DWMHs, higher MINI suicidal risk, and more recent suicide attempts than patients with higher scores for hyperthymic and lower scores for dysthymic temperament. The presence of a dysthymic temperament profile together with DWMHs may presumably play a critical role in the emergence of hopelessness as assessed by BHS. These differential characteristics may be used for grouping subjects with mood disorders potentially helping in optimizing reliable treatment strategies.
It has also been found that in contrast to hyperthymic temperament the short allele of the serotonin transporter gene was significantly related to depressive, cyclothymic, irritable and anxious temperaments[25] and with violent suicidal behavior in nonclinical populations. A study on elderly depressed patients showed that individuals with the short allele of the serotonin transporter gene had more microstructural white matter abnormalities in the frontolimbic and other brain regions[26] compared to those without. These findings indicate that the short allele of the serotonin transporter gene, DWMHs, affective temperaments containing more or less depressive component, and suicidal behaviour (in chronological order) are strongly related to each other and the presence of the first three in the same subject could serve as a powerful marker for predicting future suicidal behaviour.
However, also negative associations have been reported[5,19,20]. In particular, patients with chronic migraine and PWMHs reported fewer depressive symptoms as assessed by the Center for Epidemiologic Studies Depression Scale (CES-D) than those with chronic migraine without PWMHs[19]. In contrast with the generally poor outcome related to the presence of WMHs in patients with affective disorders, the possible protective effect of lithium and Vitamin-D in ameliorating mood and psychosocial functioning in a patient with BD has also been suggested[20].
Additionally, our last study found that subjects with PWMHs were more likely to have impaired insight compared to those without, but any association between PWMHs and suicidal behaviour as assessed using BHS has been found[5].
These latter findings[5] seem to contradict our previous results regarding the association between WMHs and poor outcome including suicidality in both unipolar and bipolar depressed individuals. However, in these studies we recruited a sample of subjects with chronic migraine and a sample of patients with BD type I respectively, whereas the previous results were reported in mixed samples (including both bipolar and unipolar depressed patients)[12,15] or other specific subgroups[5,16-20]. It’s possible to speculate that the association between PWMHs and suicidal behaviour is significant only in some specific subgroups of patients with major affective disorders in which WMHs may serve as a marker of disease. WMHs could not be a risk factor for suicide among inpatients with other predominant conditions (e.g., chronic migraine) or in those with certain specific illness subtypes.
Another consideration needs to be critically addressed. WMHs were in most cases able to explain only a small part of the variability related to suicide attempt risk, indicating that one single variable is not sufficient to predict a complex behaviour such as suicide[15]. Table 1 summarized the most relevant findings of our studies.
MRI studies investigating the presence of WMHs should be considered in the light of the following limitations. First, the small sample size of the studies did not allow for generalization of the present findings. In addition, samples are often mixed including inpatients admitted to a psychiatric hospital for more severe affective symptoms that were compared to outpatients usually exhibiting less severe/more stable illness episodes. Also, in most cases the absence of a direct comparison between patients with major depression and other subjects with different mood disorders limited the clinical relevance of the findings.
Not all studies evaluated the presence, severity, or number of hyperintense lesions and most of them used visual scales such as the Fazekas modified rating scale that is a less objective evaluation method than many volumetric methods available.
Moreover, most studies recruited patients treated with psychoactive medications or having a history of substance abuse, but not all analyzed the effects of these variables on insight ratings and image processing. The lack of accounting for the cognitive effects of medications may be considered an important limitation. In order to comprehensively examine the effect of different medications on the neurobiology of clinical symptoms, studies should investigate patients with early affective symptoms or first illness episodes. Also, not all studies include an healthy comparison group and this may limit conclusive statements about the specificity of results.
Other methodological issues concern the procedure. MRI studies were of quite low spatial resolution especially if performed using a 1.5 T scanner. Studies using 3 T scanner and/or higher resolution techniques would likely yield a much higher number and extent of WMHs than studies using a 1.5 T scanner. An analysis quantifying total white matter lesion volume would strengthen the findings of most studies. In addition, diffusion tensor imaging techniques may be more sensitive for detecting white matter abnormalities associated with mood disorders. Finally, although some studies reported that WMHs were predominant in some brain regions, not all analyzed WMHs using regional analyses.
In summary, although it is possible that WMHs may represent only the ‘tip of the iceberg’ and an extreme consequence of underlying microstructural processes that affect brain connectivity, we believe that they may represent relevant biological markers of poor outcome in patients with major affective diseases. The presence of WMHs may be used for grouping subjects who will manifest more severe illness impairments from those who will present a better outcome, potentially helping clinicians in optimizing the best treatment strategy. WMHs may be considered as a proxy for identifying subgroups of patients with more severe illness subtypes requiring more targeted interventions. For example, the early identification of individuals at risk for highly lethal suicide attempts through the assessment of WMHs may help to closely monitor this clinical subgroup of subjects with poor outcome.
Based on our studies, it has been demonstrated that WMHs are a useful biological marker of poor outcome including suicidality in the specific subpopulations of patients which were investigated. However, as studies using MRI techniques are biased by several limitations, further prospective studies are needed in order to provide a better understanding of the biological processes involved in WMH progression as well as to elucidate the nature of the association between WMHs and major affective disorders.
Xénia Gonda is recipient of the Janos Bolyai Research Fellowship of the Hungarian Academy of Sciences.
P- Reviewers: Brasic JR, Yang YK S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Cho DY, Lee WY, Chen CC. Limbic leukotomy for intractable major affective disorders: a 7-year follow-up study using nine comprehensive psychiatric test evaluations. J Clin Neurosci. 2008;15:138-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Rihmer Z. Suicide risk in mood disorders. Curr Opin Psychiatry. 2007;20:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Rihmer Z, Angst J. Epidemiology of bipolar disorder. Handbook of bipolar disorder. New York: Taylor and Francis 2005; 21-35. [Cited in This Article: ] |
| 4. | Vasudev A, Thomas A. ‘Bipolar disorder’ in the elderly: what’s in a name? Maturitas. 2010;66:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Serafini G, Pompili M, Innamorati M, Girardi N, Strusi L, Amore M, Sher L, Gonda X, Rihmer Z, Girardi P. The impact of periventricular white matter lesions in patients with bipolar disorder type I. CNS Spectr. 1-12 Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 6. | Thomas AJ, Perry R, Barber R, Kalaria RN, O’Brien JT. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann N Y Acad Sci. 2002;977:333-339. [PubMed] [Cited in This Article: ] |
| 7. | Morris G, Maes M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013;11:205. [PubMed] [Cited in This Article: ] |
| 8. | Kanekar S, Devgun P. A pattern approach to focal white matter hyperintensities on magnetic resonance imaging. Radiol Clin North Am. 2014;52:241-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Neema M, Stankiewicz J, Arora A, Guss ZD, Bakshi R. MRI in multiple sclerosis: what’s inside the toolbox? Neurotherapeutics. 2007;4:602-617. [PubMed] [Cited in This Article: ] |
| 10. | Bö L, Geurts JJ, Mörk SJ, van der Valk P. Grey matter pathology in multiple sclerosis. Acta Neurol Scand Suppl. 2006;183:48-50. [PubMed] [Cited in This Article: ] |
| 11. | Ehrlich S, Breeze JL, Hesdorffer DC, Noam GG, Hong X, Alban RL, Davis SE, Renshaw PF. White matter hyperintensities and their association with suicidality in depressed young adults. J Affect Disord. 2005;86:281-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Pompili M, Ehrlich S, De Pisa E, Mann JJ, Innamorati M, Cittadini A, Montagna B, Iliceto P, Romano A, Amore M. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur Arch Psychiatry Clin Neurosci. 2007;257:494-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 424] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187-189. [Cited in This Article: ] |
| 15. | Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, Serafini G, Rigucci S, Romano A, Tamburello A. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1501-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Serafini G, Pompili M, Innamorati M, Fusar-Poli P, Akiskal HS, Rihmer Z, Lester D, Romano A, de Oliveira IR, Strusi L. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J Affect Disord. 129:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 17. | Pompili M, Serafini G, Innamorati M, Serra G, Forte A, Lester D, Ducci G, Girardi P, Tatarelli R. White matter hyperintensities, suicide risk and late-onset affective disorders: an overview of the current literature. Clin Ter. 2010;161:555-563. [PubMed] [Cited in This Article: ] |
| 18. | Serafini G, Pompili M, Innamorati M, De Rossi P, Ferracuti S, Girardi P, Tatarelli R. Deep white matter hyperintensities as possible predictor of poor prognosis in a sample of patients with late-onset bipolar II disorder. Bipolar Disord. 2010;12:755-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Serafini G, Pompili M, Innamorati M, Negro A, Fiorillo M, Lamis DA, Erbuto D, Marsibilio F, Romano A, Amore M. White matter hyperintensities and self-reported depression in a sample of patients with chronic headache. J Headache Pain. 2012;13:661-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Serafini G, Pompili M, Angelone M, Lester D, Girardi P, Tatarelli R. Clinical and functional outcome in a subject with bipolar disorder and severe white matter hyperintensities. Eur J Psychiatry. 25:41-45. [Cited in This Article: ] |
| 21. | Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry. 2010;11:165-180. [PubMed] [Cited in This Article: ] |
| 22. | Singh MK, Chang KD, Chen MC, Kelley RG, Garrett A, Mitsunaga MM, Bararpour L, Howe M, Reiss AL, Gotlib IH. Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar Disord. 2012;14:585-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Borgwardt S, Fusar-Poli P. White matter pathology--an endophenotype for bipolar disorder? BMC Psychiatry. 2012;12:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Moore PB, Shepherd DJ, Eccleston D, Macmillan IC, Goswami U, McAllister VL, Ferrier IN. Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry. 2001;178:172-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Gonda X, Fountoulakis KN, Rihmer Z, Lazary J, Laszik A, Akiskal KK, Akiskal HS, Bagdy G. Towards a genetically validated new affective temperament scale: a delineation of the temperament phenotype of 5-HTTLPR using the TEMPS-A. J Affect Disord. 2009;112:19-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Glatt CE, Latoussakis V, Kelly RE, Kanellopoulos D, Klimstra S, Lim KO, Young RC. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. 2009;119:132-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |