Published online Aug 28, 2014. doi: 10.4329/wjr.v6.i8.583
Revised: July 9, 2014
Accepted: July 17, 2014
Published online: August 28, 2014
In acute promyelocytic leukemia, differentiation therapy based on all-trans-retinoic acid can be complicated by the development of a differentiation syndrome (DS). DS is a life-threatening complication, characterized by respiratory distress, unexplained fever, weight gain, interstitial lung infiltrates, pleural or pericardial effusions, hypotension and acute renal failure. The diagnosis of DS is made on clinical grounds and has proven to be difficult, because none of the symptoms is pathognomonic for the syndrome without any definitive diagnostic criteria. As DS can have subtle signs and symptoms at presentation but progress rapidly, end-stage DS clinical picture resembles the acute respiratory distress syndrome with extremely poor prognosis; so it is of absolute importance to be conscious of these complications and initiate therapy as soon as it was suspected. The radiologic appearance resembles the typical features of cardiogenic pulmonary edema. Diagnosis of DS remains a great skill for radiologists and haematologist but it is of an utmost importance the cooperation in suspect DS, detect the early signs of DS, examine the patients’ behaviour and rapidly detect the complications.
Core tip: Aim of this review is to illustrate the spectrum of chest imaging findings which lead to suspect a diagnosis of differentiation syndrome, which arise in patients suffering of Acute Promyelocytic Leukemia after treatment with all-trans-retinoic acid or other differentiating drugs, in order to facilitate the differential diagnosis with other life-threatening pulmonary complications occurring in this subset of highly immunocompromised patients.
- Citation: Cardinale L, Asteggiano F, Moretti F, Torre F, Ulisciani S, Fava C, Rege-Cambrin G. Pathophysiology, clinical features and radiological findings of differentiation syndrome/all-trans-retinoic acid syndrome. World J Radiol 2014; 6(8): 583-588
- URL: https://www.wjgnet.com/1949-8470/full/v6/i8/583.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i8.583
The treatment of acute promyelocytic leukemia with agents capable of inducing the differentiation of leukemic cells can be complicated by a peculiar syndrome, named differentiation syndrome (DS); this was previously classified as retinoic acid syndrome, as all-trans-retinoic acid (ATRA) was the first agent to be involved in this complication. DS is a syndrome of cardiac and respiratory distress which represents a life-threatening complication, and is associated with severe morbidity and mortality; so early diagnosis and immediate therapeutic intervention are essential in reducing the risk of death[1].
In this work we illustrate the imaging findings on chest X-ray, and in standard or high-resolution computed tomography (CT) useful to suggest this uncommon diagnosis. The aim of this work is to underline the importance of an early diagnosis of DS, to show how radiologists can confirm the diagnosis of DS, to stimulate the cooperation and communication between radiologists and clinicians.
Acute promyelocytic leukemia (APL), identified as acute myeloid leukaemia (AML) M3 by the French-American-English classification, is an acute myeloid disorder due to a maturative block of myeloid precursor at the promyelocytic stage, leading to peculiar clinical manifestations, and characterized by a reciprocal balanced translocation between chromosome 15 and 17 t(15, 17)[2]. APL represents about 10% of all AML in United States and Europe and is more frequent in adults; median age at diagnosis is 40[3,4].
The finding of a translocation t(15, 17), with a consequent transcriptional fusion between promyelocytic leukaemia (PML) gene and retinoic acid receptor-α (RARα) gene, responsible of the maturative and differentiative stop, offered the basis to develop a specific target therapy with retinoic acid. PML-RARα rearrangement is detectable in approximately 95% of APL cases[2,3].
APL is usually a primitive disorder, but there are also cases of APL arising following a previous exposure to chemotherapy or radiotherapy[5,6].
On the clinical side APL represents a hematologic emergency because of its typical presentation with pancytopenia and a life-threatening disseminated intravascular coagulation, that enforces a quick start of treatment, even before a cytogenetic and molecular diagnosis. Diagnosis of APL is usually based on peripheral blood and bone marrow morphology, supported by typical laboratory and clinical picture. Confirmation then comes from the molecular finding of PML-RARα rearrangement. Treatment of APL is based on the association of ATRA with conventional chemotherapy (usually anthracyclines), or with arsenic trioxide (ATO). Since the introduction of ATRA in the treatment, the complete remission rate raised up to 90% and the 5-year disease free survival to 74%[7-9].
Induction treatment with ATRA and ATO, either as a single agent or in combination with cytotoxic drugs, can induce the DS[10] in 2% to 31% of APL patients[10,11], while the association with chemotherapy compared with ATRA as single-agent lead to a reduction risk of DS (9% vs 18%-25%)[12,13]. Mortality due to DS has declined from 30% to 2%-10% because of the early recognition and clinical intervention[10]. Notably, DS is rarely seen during consolidation and maintenance phases[12,14,15].
The pathophysiology of DS is not completely understood. Both ATRA and ATO exert their action by degradation of the PML-RARα fusion product. As a consequence, malignant leukemic cells undergo differentiation and final maturation[16,17].
Exposition to ATRA and ATO cause continued generation of inflammatory cytokines and adhesion molecules, with the consequent extravasation into the tissues from the blood. Increased in cytokine and adhesion molecules level also occurs in liver, heart and spleen[11,18].
DS is typically diagnosed during the first induction treatment, more often 10 to 12 d after therapy start, with a range of 2 to 46 d[10,11,18-20]. Main symptoms at presentation are fever, respiratory failure and fluid retention with weight increase, occurring in around 80% of the cases[10,18]. Additional common findings are lung infiltrates (50%), pericardial and pleural effusions (30%) and acute renal failure (10% of the cases)[18]. DS has to be distinguished from other clinical conditions which may occur in the setting of acute leukaemia, including pulmonary infection, leukostasis, and heart failure. Diagnosis of DS is made when three or more of the symptoms and signs listed in Table 1 are present. Clinical management and outcome are greatly influenced by early pharmacological treatment. Dexamethasone must be used at a dosage of 10 mg twice daily iv as soon as DS is suspected. Steroid treatment should continue until DS resolves, and then the dosage can be gradually tapered in the following weeks[21,22]. Hemodynamic and ventilatory support is also indicated in severe cases, which may require admission at the intensive care unit. In Table 2, modified from[1], the measures to be taken at suspicion of DS are reported. Discontinuation of treatment with ATRA (or ATO) is mandatory in severe DS cases[4]. Corticosteroid are commonly used as DS prophylaxis, although there is no evidence that such treatment may ameliorate the morbidity and mortality of DS. However, in patients with leukocytosis at diagnosis (white blood cells > 5 × 109/L), preemptive steroids administration has been shown useful in reducing incidence and severity of DS. No definite risk factors for developing DS were found, but a close monitoring of patients presenting with hyperleukocytosis is recommended[22,23].
| Differentiation syndrome: Sign and symptoms | |
| Elevated white blood cell count | Weight gain > 5 kg |
| Dyspnea | Bone pain |
| Respiratory distress | Headache |
| Fever | Hypotension |
| Pulmonary edema | Congestive heart failure |
| Pulmonary infiltrates | Acute renal failure |
| Pleural and pericardial effusion | Hepatotoxicity |
| Measures at suspicion of DS |
| Chest X-ray, renal function (creatinine and urea), hepatic function (amino transferases and bilirubin), blood cell counts, coagulation test, oxygen saturation |
| Weight monitoring |
| Ventilatory support/O2 supplementation |
| Blood pressure maintenance measures |
| Fluid restriction (renal failure) |
| Steroid administration at first suspicion: dexamethasone 10 mg twice daily until clinical resolution, then tapered dose for a few days |
| Suspend ATRA or ATO in severe cases, which can be restarted after clinical improvement. If DS recurs after restart, ATRA must be definitively discontinued during induction |
A better knowledge and early identification of DS, with rapid initiation of treatment, allowed to obtain a decrease in mortality associated with APL in the recent years.
As stated before, there are no clinical signs or laboratory tests to diagnose DS, nor is there a radiological finding pathognomonic for DS.
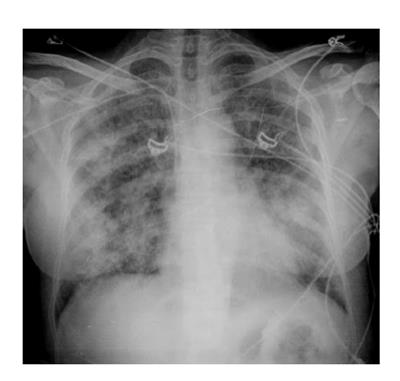
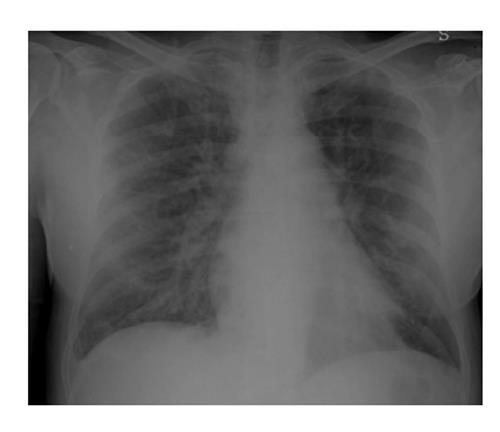
Radiologic features may be explained by the proposed hypotheses of pathophysiology of the DS[24]. Most of the patients with DS showed cardiomegaly, widening of the vascular pedicle width, increased pulmonary blood volume, peribronchial cuff, ground-glass opacity, septal lines, and pleural effusion: these findings are similar to those of congestive heart failure with pulmonary edema, but they could also probably be produced by leukemic lung infiltration and endothelial leakage[24].
If the disease progresses, acute respiratory distress syndrome develops. Diffuse alveolar damage and massive intra-alveolar hemorrhage were found in a necropsy patient study by Frankel et al[25]. Endothelial cell damage, including intra-alveolar oedema, intra-alveolar hemorrhage, and fibrinous exudate, were found by Tallman et al[26] and by Nicolls et al[27]. Others histological analysis of lungs reported extensive interstitial and alveolar lung infiltration by maturing myeloid cells, endothelial cells damage, oedema, hemorrhage, and fibrinous exudates that correspond in poorly defined centrolobular nodules and ground-glass opacity with or without interlobular septal thickening.
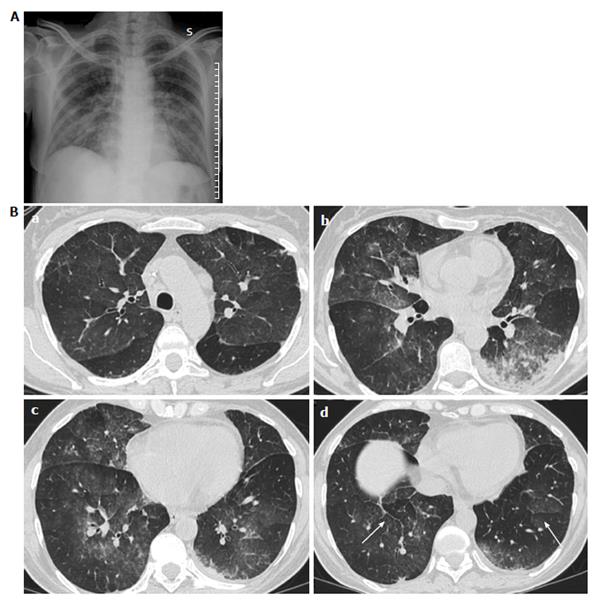
To our knowledge only a few cases are reported describing the CT aspect in DS: Davis et al[28] reported CT findings in three patients with DS. CT findings were peripheral nodules reticular and ground-glass opacity and pleural effusions. They also reported the case of a patient with DS who developed pneumothorax[28].
In mild DS, lesions are prevalent in the lower lobes, while in severe DS, lesions are ubiquitary, with no difference within peripheral or central regions[6,9].
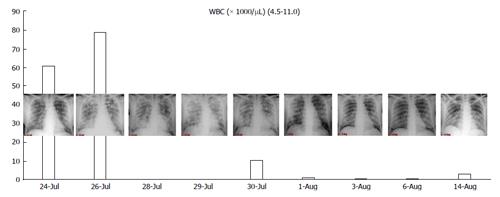
In Figure 1, we present the correlation with radiological findings and white blood cells (WBC). The pathogenesis of DS suggest that this syndrome is due to a massive lung interstitial invasion by leukemic cells. The finding of a negative peak of WBC nearly contemporary to the massive lung oedema seems to confirm this theory.
Patients with PML and DS have an highly compromised immune system; therefore, it is not a rare event to find other concurrent pathologic conditions as pneumonia or fungine infections which can confound the radiological picture of DS/ATRA syndrome.
CT findings of ATRA syndrome are nonspecific (Figure 2).
All findings have differential diagnoses: leukemic infiltrates, drug toxicity, pulmonary edema, hemorrhage, can all have similar appearances.
Although the imaging features are not characteristic, in combination with the clinical picture, they may contribute to the early identification of DS and consequently to its fast resolution (Figures 3 and 4).
Diagnosis of DS requires a great skill for radiologists and hematologists, and cooperation and treatment is of utmost importance when DS is confirmed.
Chest X-ray still remains the first imaging step, but often, in mild cases, it is not sufficient.
In summary chest X-ray features include increased cardiothoracic ratio and vascular pedicle width, interstitial edema with peribronchial cuffing and Kerley lines.
The chest CT is useful to evaluate the lung parenchyma and also to discover other signs of severity, as pericardial and pleural effusions, and sometimes to find others concurrent lung infections.
Take home message: (1) Suspect DS in patient with acute APL under treatment with ATRA and/or arsenic trioxide; (2) Detect the early signs of DS to confirm the clinical diagnosis; (3) Examine the patient’s behaviour; and (4) Rapidly detect and treat the complications.
P- Reviewer: Agarwal R, Sureka B S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Rego EM, De Santis GC. Differentiation syndrome in promyelocytic leukemia: clinical presentation, pathogenesis and treatment. Mediterr J Hematol Infect Dis. 2011;3:e2011048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167-3215. [PubMed] [Cited in This Article: ] |
| 3. | Rogers JE, Yang D. Differentiation syndrome in patients with acute promyelocytic leukemia. J Oncol Pharm Pract. 2012;18:109-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875-1891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 594] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 5. | Pulsoni A, Pagano L, Lo Coco F, Avvisati G, Mele L, Di Bona E, Invernizzi R, Leoni F, Marmont F, Mele A. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood. 2002;100:1972-1976. [PubMed] [Cited in This Article: ] |
| 6. | Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, Parry A, Walz C, Wiemels JL, Segal MR. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352:1529-1538. [PubMed] [Cited in This Article: ] |
| 7. | Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505-2515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 871] [Cited by in F6Publishing: 886] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 8. | Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Willman CL. Hypergranular promyelocytic leukemia: correlation between morphology and chromosomal translocations including t(15; 17) and t(11; 17). Leukemia. 2000;14:1197-1200. [PubMed] [Cited in This Article: ] |
| 9. | de la Serna J, Montesinos P, Vellenga E, Rayón C, Parody R, León A, Esteve J, Bergua JM, Milone G, Debén G. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395-3402. [PubMed] [Cited in This Article: ] |
| 10. | Patatanian E, Thompson DF. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008;33:331-338. [PubMed] [Cited in This Article: ] |
| 11. | Luesink M, Pennings JL, Wissink WM, Linssen PC, Muus P, Pfundt R, de Witte TJ, van der Reijden BA, Jansen JH. Chemokine induction by all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia: triggering the differentiation syndrome. Blood. 2009;114:5512-5521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | De Botton S, Dombret H, Sanz M, Miguel JS, Caillot D, Zittoun R, Gardembas M, Stamatoulas A, Condé E, Guerci A. Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1998;92:2712-2718. [PubMed] [Cited in This Article: ] |
| 13. | de Botton S, Chevret S, Coiteux V, Dombret H, Sanz M, San Miguel J, Caillot D, Vekhoff A, Gardembas M, Stamatoulas A. Early onset of chemotherapy can reduce the incidence of ATRA syndrome in newly diagnosed acute promyelocytic leukemia (APL) with low white blood cell counts: results from APL 93 trial. Leukemia. 2003;17:339-342. [PubMed] [Cited in This Article: ] |
| 14. | Rust DM, Soignet SL. Risk/benefit profile of arsenic trioxide. Oncologist. 2001;6 Suppl 2:29-32. [PubMed] [Cited in This Article: ] |
| 15. | Gupta V, Yi QL, Brandwein J, Lipton JH, Messner HA, Schuh AC, Wells RA, Minden MD. Role of all-trans-retinoic acid (ATRA) in the consolidation therapy of acute promyelocytic leukaemia (APL). Leuk Res. 2005;29:113-114. [PubMed] [Cited in This Article: ] |
| 16. | Roche Laboratories, Inc. Vesanoid (tretinoin) package insert. 2004; Available from: http: // www.accessdata.fda.gov/drugsatfda_docs/label/2004/20438s004lbl.pdf. [Cited in This Article: ] |
| 17. | Nasr R, Lallemand-Breitenbach V, Zhu J, Guillemin MC, de Thé H. Therapy-induced PML/RARA proteolysis and acute promyelocytic leukemia cure. Clin Cancer Res. 2009;15:6321-6326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Larson RS, Tallman MS. Retinoic acid syndrome: manifestations, pathogenesis, and treatment. Best Pract Res Clin Haematol. 2003;16:453-461. [PubMed] [Cited in This Article: ] |
| 19. | Luesink M, Jansen JH. Advances in understanding the pulmonary infiltration in acute promyelocytic leukaemia. Br J Haematol. 2010;151:209-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Montesinos P, Bergua JM, Vellenga E, Rayón C, Parody R, de la Serna J, León A, Esteve J, Milone G, Debén G. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113:775-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Acute Myeloid Leukemia NCCN Clinical Practice Guidelines in Oncology (Version 2.2014). . [Cited in This Article: ] |
| 22. | Wiley JS, Firkin FC. Reduction of pulmonary toxicity by prednisolone prophylaxis during all-trans retinoic acid treatment of acute promyelocytic leukemia. Australian Leukaemia Study Group. Leukemia. 1995;9:774-778. [PubMed] [Cited in This Article: ] |
| 23. | Sanz MA, Martín G, González M, León A, Rayón C, Rivas C, Colomer D, Amutio E, Capote FJ, Milone GA. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103:1237-1243. [PubMed] [Cited in This Article: ] |
| 24. | Jung JI, Choi JE, Hahn ST, Min CK, Kim CC, Park SH. Radiologic features of all-trans-retinoic acid syndrome. AJR Am J Roentgenol. 2002;178:475-480. [PubMed] [Cited in This Article: ] |
| 25. | Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292-296. [PubMed] [Cited in This Article: ] |
| 26. | Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Rowe JM, François C, Larson RS. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95:90-95. [PubMed] [Cited in This Article: ] |
| 27. | Nicolls MR, Terada LS, Tuder RM, Prindiville SA, Schwarz MI. Diffuse alveolar hemorrhage with underlying pulmonary capillaritis in the retinoic acid syndrome. Am J Respir Crit Care Med. 1998;158:1302-1305. [PubMed] [Cited in This Article: ] |
| 28. | Davis BA, Cervi P, Amin Z, Moshi G, Shaw P, Porter J. Retinoic acid syndrome: pulmonary computed tomography (CT) findings. Leuk Lymphoma. 1996;23:113-117. [PubMed] [Cited in This Article: ] |