Published online Mar 22, 2022. doi: 10.4291/wjgp.v13.i2.41
Peer-review started: March 22, 2022
First decision: October 16, 2021
Revised: October 26, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: March 22, 2022
Electron microscopy has long been used in research in the fields of life sciences and materials sciences. Transmission and scanning electron microscopy and energy-dispersive X-ray spectroscopy (EDX) analyses have also been performed in the field of gastroenterology. Electron microscopy and EDX enable (1) Observation of ultrastructural differences in esophageal epithelial cells in patients with gastroesophageal reflux and eosinophilic esophagitis; (2) Detection of lanthanum deposition in the stomach and duodenum; (3) Ultrastructural and elemental analyses of enteroliths and bezoars; (4) Detection and characterization of microorganisms in the gastrointestinal tract; (5) Diagnosis of gastrointestinal tumors with neuroendocrine differentiation; and (6) Analysis of gold nanop
Core Tip: This review provides an overview of transmission electron microscopy, scanning electron microscopy, and energy-dispersive X-ray spectrometry analyses used in the field of gastroenterology. Previously reported articles have been reviewed, with a focus on electron microscopy applications. The history and present trends in electron microscopy applications in patients and research associated with digestive system diseases are also summarized.
- Citation: Iwamuro M, Urata H, Tanaka T, Okada H. Application of electron microscopy in gastroenterology. World J Gastrointest Pathophysiol 2022; 13(2): 41-49
- URL: https://www.wjgnet.com/2150-5330/full/v13/i2/41.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v13.i2.41
In light microscopy, visible light is used to obtain magnified views of the object. As the resolution is related to the wavelength of light used to image a specimen, the resolution of an optical microscope is theoretically limited to approximately 200 nm. Thus, nanostructures cannot be observed using light microscopy. In contrast, electron beams are used in electron microscopy. As the wavelength of an electron beam is shorter than that of visible light, electron microscopy has extremely high resolution and provides sharp, finely detailed images of the surface or interior of biological and nonbiological specimens. In addition, energy-dispersive X-ray spectroscopy (EDX), which is a chemical microanalysis technique used in conjunction with electron microscopy, enables the analysis of elements or chemical characterization of a sample. Since the development of the first prototype in 1931, electron microscopes have been widely used in various fields, such as physics, chemistry, engineering, biology, and medicine[1]. Based on its versatility, electron microscopy analysis has been used in several studies covering various aspects of clinical samples obtained from patients with gastrointestinal diseases. This paper briefly discusses the fundamentals of electron microscopy and reviews the literature concerning the application of electron microscopy in gastroenterology science.
Analytical methods in electron microscopy can broadly be categorized into three types: Transmission electron microscopy, scanning electron microscopy (SEM), and EDX. The different types of electron microscopes used in these methods are related and often applied concurrently in the field of biology.
A transmission electron microscope irradiates a specimen with an electron beam. The object must be cut into very thin cross-sections because it is visualized through the spatial distribution of the transmitted electron beam. Although the use of transmission electron microscopy is limited to engineering science at the outset, it has been extensively used in the field of biology since the 1950s largely due to improvement of the microtome for ultrathin slice preparation using a diamond knife and the development of staining techniques based on heavy metals, such as osmium.
A scanning electron microscope produces an image using electrons reflected or generated from the surface of the specimen. The specimen is placed in a high vacuum state, and the surface is scanned with an electron beam focused by an electric or magnetic field. SEM produces a characteristic three-dimensional appearance that is useful for understanding the surface ultrastructure of a sample.
EDX is an X-ray system used to identify the elemental composition of a material. It has a semiconductor detector to detect the fluorescent X-rays generated when the primary X-ray beam illuminates the sample. The fluorescent X-rays emitted from the material have a spectrum of wavelengths characteristic of the types of atoms present in the specimen. EDX enables both qualitative and semiquantitative analyses of the elements based on the energy and number of generated electron-hole pairs. EDX is more suited for analyses of inorganic materials than organic materials.
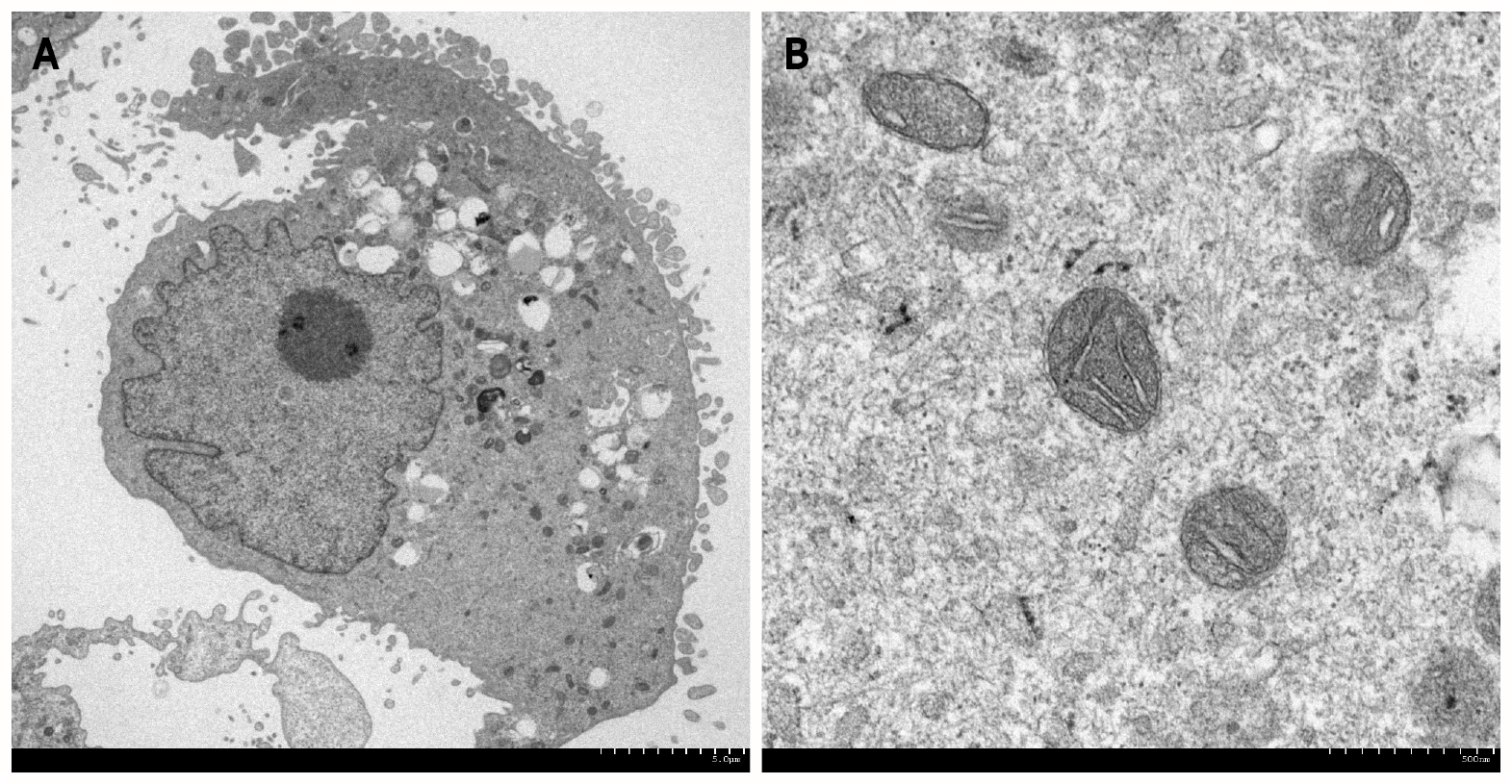
In the field of gastroenterology, transmission and SEM and EDX analyses have been used to visualize cells (Figure 1) and pathogens, including parasites, bacteria, viruses, biofilms, and elements deposited in the gastrointestinal mucosa. Nonbiological materials, such as stents, powders, and bezoars, have also been analyzed at subnanometer resolution. In the following sections, we review examples of electron microscopy analyses in association with the pathophysiology of gastrointestinal disorders.
The most typical example of electron microscopy analysis in gastroenterology is evaluation of the intracellular spaces of esophageal epithelial cells. Notably, some of the articles on this topic have been published in high-impact journals. Intercellular spaces in the esophageal epithelium are known to be dilated in patients with nonerosive reflux disease and in patients with esophagitis. Following several animal studies, endoscopic esophageal biopsy specimens taken from patients with (n = 11) and without (n = 13) recurrent heartburn were investigated in 1996 using transmission electron microscopy[2]. A dilated intercellular space diameter was observed in 8 of the 11 patients with heartburn, while none of the asymptomatic individuals exhibited this feature. Dilated intercellular space was also present in the normal-appearing, nonerosive mucosa of patients with symptomatic reflux disease. Other authors have provided further evidence that detached interepithelial cell junctions, which are observed as dilated intercellular spaces assessed by electron microscopy[3-5], correspond to early esophageal damage induced by acid reflux[6-8]. Dilatation of intercellular spaces in the esophageal epithelium is not observed in patients with functional heartburn, suggesting that this microscopic feature is specific to acid reflux[9]. Proton pump inhibitor therapy resulted in complete recovery of dilated intercellular spaces in > 90% of cases with nonerosive reflux disease and erosive esophagitis, indicating that the electron microscopy features are reversible[10,11].
Dilated intracellular spaces arise along the distal and proximal esophagus of patients with nonerosive reflux disease, suggesting that they may be an underlying mechanism accounting for the enhanced perception of proximal acid reflux[12]. Duodenal gastroesophageal reflux has also been reported to cause dilatation of intercellular spaces in the esophageal epithelium[3,13]. Similarly, in patients with laryngopharyngeal reflux and sore throat, this feature appears at the squamous basal and suprabasal levels in oropharyngeal biopsy specimens[14,15]. An investigation of patients with bronchial asthma[11,16] and children with reflux-related cough[17] revealed that the intracellular spaces in the esophageal epithelium are significantly dilated compared with those in control patients, suggesting a pathophysiological correlation between gastroesophageal reflux and the development of these respiratory tract symptoms.
Although the width of the intracellular spaces can be measured using light microscopy[18], the sensitivity of light microscopy was 79.3%, and the specificity was 75.0%[19]. Owing to the inferior specificity of light microscopy analysis, electron microscopy seems to be more suitable for measuring intercellular spaces in the esophageal epithelium. Chu et al[20] reported the possible utility of in vivo confocal laser endomicroscopy to examine microalterations of the esophagus in patients with nonerosive reflux disease[20].
Eosinophilic esophagitis is a chronic, allergic inflammatory condition of the esophagus. Dilated intracellular spaces are evident in the esophageal epithelium of patients with eosinophilic esophagitis, which are significantly reduced after treatment[21]. Transmission electron microscopy revealed a significant decrease in the number of desmosomes[22] and an increased autophagic vesicle content[23] in active eosinophilic esophagitis compared with observations in normal individuals and inactive eosinophilic esophagitis patients. Thus, electron microscopy may be useful for investigating the pathophysiology of eosinophilic esophagitis.
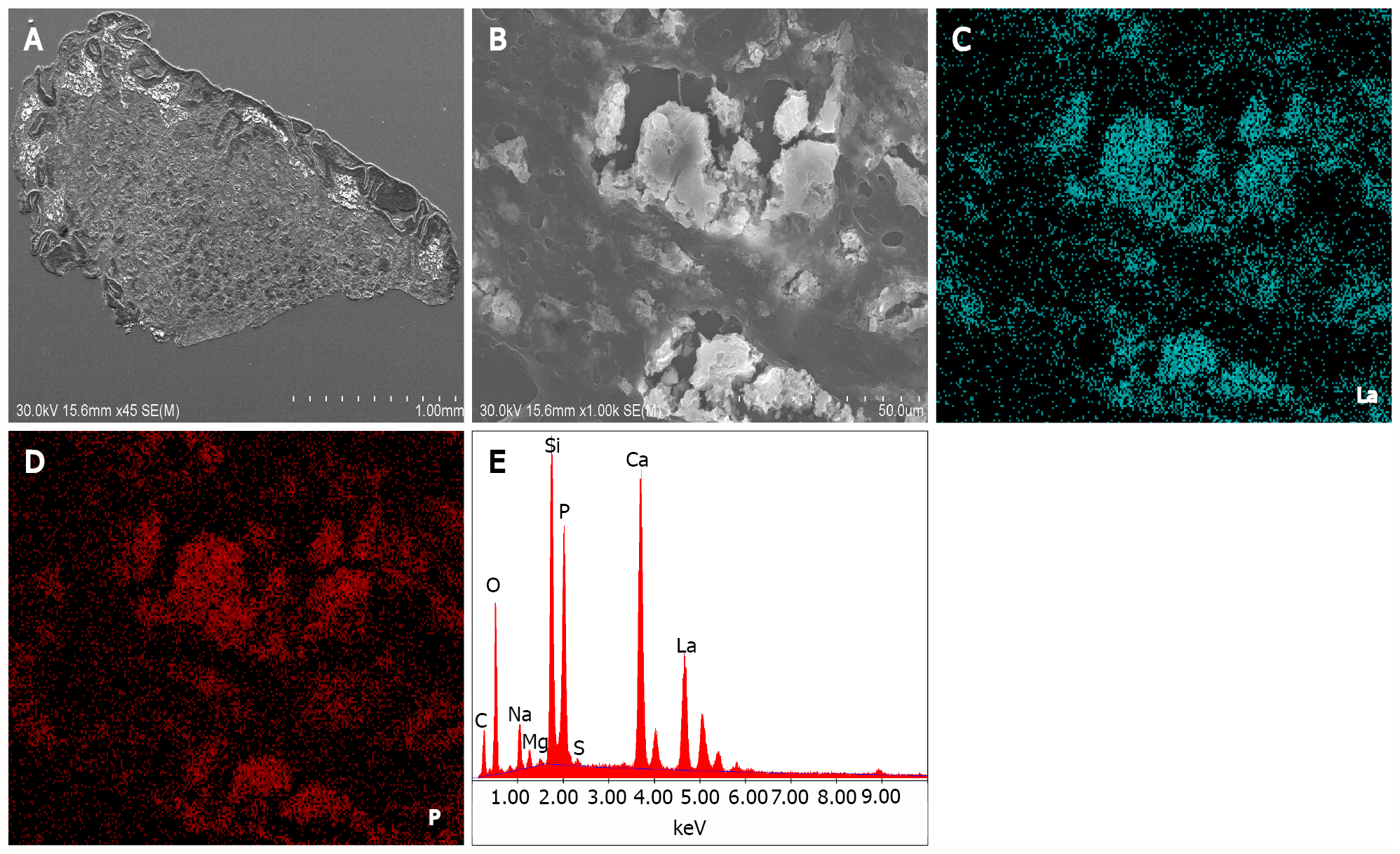
Lanthanum carbonate is a phosphate binder taken orally and is commonly used in patients with chronic kidney disease. Although its tolerability and safety profile have been reported in hemodialysis patients, lanthanum deposition in the gastric and duodenal mucosa of these patients, in the form of lanthanum phosphate, has been reported in the literature[24-28]. On light microscopy examination of hematoxylin and eosin-stained specimens, deposited lanthanum is visible as a fine, amorphous, eosinophilic material. SEM revealed bright areas in the deposited lanthanum (Figure 2A). Images at higher magnification showed deposition as the accumulation of minute particles (Figure 2B). EDX analysis provided evidence directly related to the presence of lanthanum and phosphate (Figure 2C). Elemental mapping by EDX revealed that lanthanum (Figure 2D) and phosphate (Figure 2E) showed an identical location to that of the bright areas on SEM. Although lanthanum deposition in the gastrointestinal tract can be clinically diagnosed with conventional light microscopy observation of the fine, amorphous, eosinophilic material and medication information from the patient’s current or past use of lanthanum carbonate, SEM has advantages in the detection of deposited lanthanum, as it is easily identified as a bright area.
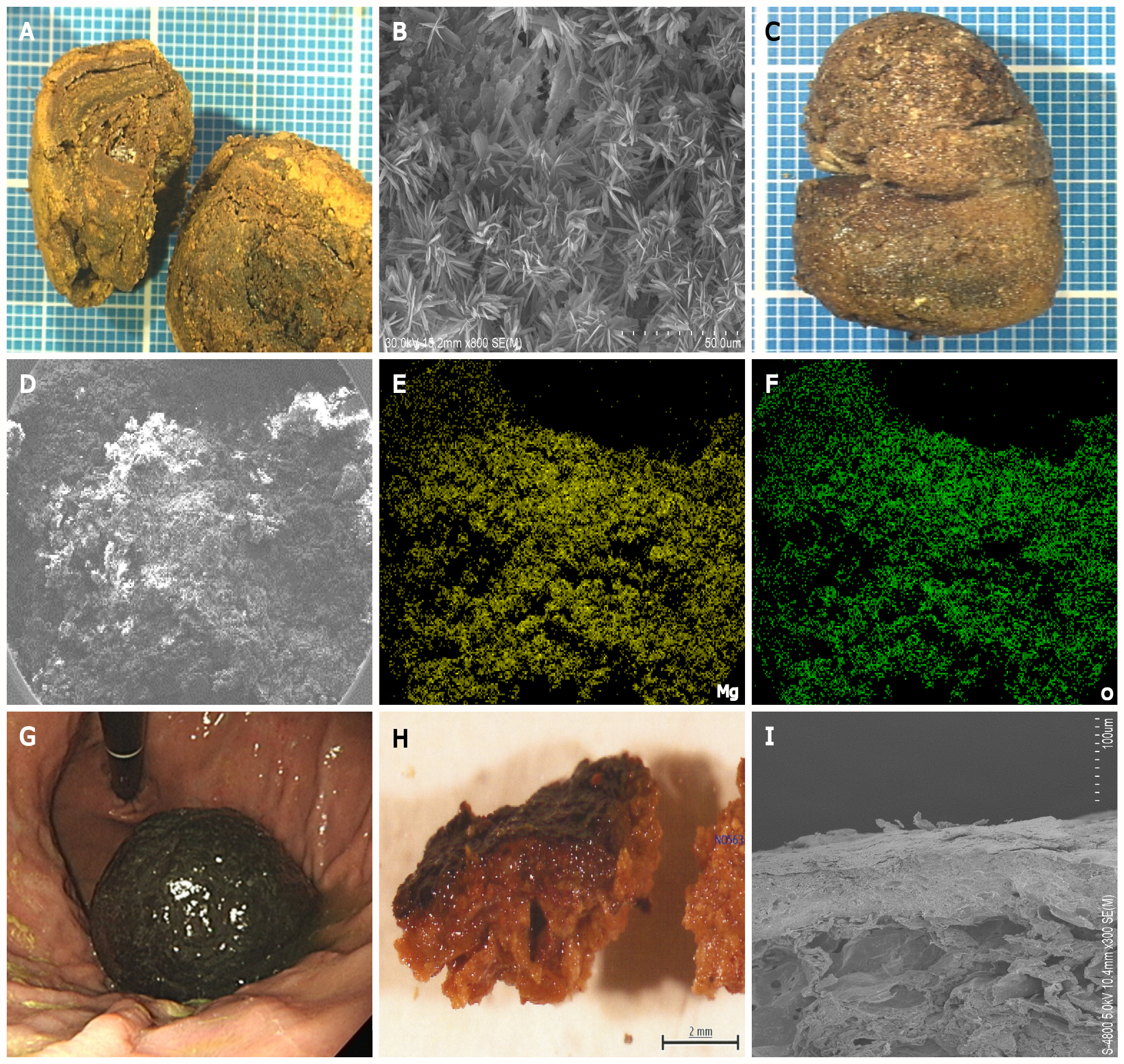
Enteroliths are calculi that occur in the intestines and include two types: “True” and “false” enteroliths. True enteroliths, for example, cholic acid and calcium stones, are generated from the sediments of substances found in enteric contents. False enteroliths, such as bezoars, gallstones, and foreign objects, are formed from indigestible substances stuck in the alimentary tract. Infrared spectroscopy is generally used to identify the chemical substances constituting enteroliths removed from patients. Electron microscopy and EDX have the advantages of imaging the microstructure and analyzing elements, allowing clarification of the nature of enteroliths.
Figure 3 shows examples of enteroliths and bezoars that we previously investigated. One patient had an enterolith in the stomach composed of bilirubin calcium, calcium carbonate, and fatty acid calcium[29] (Figure 3A and B). Another patient had a rare pharmacobezoar in the stomach, which was composed of magnesium oxide (Figure 3C–F)[30]. We also investigated the ultrastructure of the persimmon phytobezoar in the stomach (Figure 3G–I)[31]. Thus, electron microscopy and EDX analyses offer insights into the microstructure and elemental composition of enteroliths.
Electron microscopy has been widely used in microbiology to elucidate the number, distribution, and adherence of microorganisms in clinical samples. One of the typical applications of electron microscopy for pathogens in gastroenterology is the detection of Helicobacter species, such as Helicobacter pylori[32-36] and Helicobacter heilmannii[37]. These bacteria have a spiral form, which is a distinct difference from other bacteria. Another example is Tropheryma whipplei[38-41], which causes the rare systemic infectious disorder Whipple's disease. Electron microscopy revealed that Tropheryma whipplei shows a characteristic trilamellar plasma membrane. Other rare pathogens identified by electron microscopy include anisakiasis[42], amoebiasis[43], intestinal spirochetosis[44], Sutterella wadsworthensis[45], Giardia intestinalis[46], and Brachyspira aalborgi[47].
A biofilm is a thick layer formed by microorganisms attached to the surface of a solid material or liquid. SEM has been used to visualize the shape and localization of biofilms and the steps of the biofilm formation process. For instance, several authors have investigated the efficiency of the cleaning, disinfection, and sterilization processes of biofilm-contaminated endoscopes[48,49].
Neuroendocrine and mixed neuroendocrine neoplasms can arise in most of the epithelial organs of the body and are not rare in the gastrointestinal tract. Transmission electron microscopy revealed that neuroendocrine tumor cells in the gastrointestinal tract contained numerous dense-core secretory granules of variable sizes and shapes in the cytoplasm. Because these neurosecretory granules are characteristic of neuroendocrine tumors, electron microscopy analysis has been used to support its diagnosis. For instance, neuroendocrine differentiation was assessed using electron microscopy images in cases of malignant peripheral nerve sheath tumors of the esophagus[50], gangliocytic paraganglioma in the duodenum[51], mixed acinar-endocrine carcinoma arising in the ampulla of Vater[52], combined adenocarcinoma and neuroendocrine tumors in the stomach[53], neuroendocrine carcinoma in the stomach[54], mixed acinar-endocrine neoplasm in the stomach[55], and large cell neuroendocrine carcinoma in the esophagogastric junction[56].
Based on the properties of absorption and scattering of electromagnetic radiation, gold nanoparticles are emerging as promising agents and are of particular interest for applications in photothermal therapy, in addition to efficient drug carriers and diagnostic agents. For instance, endoscopic fluorescence-guided near-infrared photothermal therapy using gold nanoparticles is in development for the treatment of gastrointestinal tumors[57]. The size, morphology, and composition of synthesized gold nanoparticles and their location within tissue can be assessed using transmission electron microscopy and EDX analysis[58].
Electron microscopy enables (1) Observation of ultrastructural differences in esophageal epithelial cells in patients with gastroesophageal reflux and eosinophilic esophagitis; (2) Detection of lanthanum deposition in the stomach and duodenum; (3) Ultrastructural and elemental analyses of enteroliths and bezoars; (4) Detection and characterization of microorganisms in the gastrointestinal tract; (5) Diagnosis of gastrointestinal tumors with neuroendocrine differentiation; and (6) Analysis of gold nanoparticles potentially used in endoscopic photodynamic therapy. Therefore, electron microscopy has had a profound impact on our knowledge and understanding of various digestive tract diseases. We hope that this article will help gastroenterologists widely utilize electron microscopy analysis for clinical diagnosis and basic research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wu J S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Savage JC, Picard K, González-Ibáñez F, Tremblay MÈ. A Brief History of Microglial Ultrastructure: Distinctive Features, Phenotypes, and Functions Discovered Over the Past 60 Years by Electron Microscopy. Front Immunol. 2018;9:803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 2. | Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, Miglioli M, Di Febo G. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Long JD, Orlando RC. Nonerosive reflux disease. Minerva Gastroenterol Dietol. 2007;53:127-141. [PubMed] [Cited in This Article: ] |
| 5. | Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep. 2009;11:190-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Farré R, Fornari F, Blondeau K, Vieth M, De Vos R, Bisschops R, Mertens V, Pauwels A, Tack J, Sifrim D. Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut. 2010;59:164-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Mancini V, Ribolsi M, Gentile M, de'Angelis G, Bizzarri B, Lindley KJ, Cucchiara S, Cicala M, Borrelli O. Oesophageal mucosal intercellular space diameter and reflux pattern in childhood erosive and non-erosive reflux disease. Dig Liver Dis. 2012;44:981-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Li YW, Sifrim D, Xie C, Chen M, Xiao YL. Relationship Between Salivary Pepsin Concentration and Esophageal Mucosal Integrity in Patients With Gastroesophageal Reflux Disease. J Neurogastroenterol Motil. 2017;23:517-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Vela MF, Craft BM, Sharma N, Freeman J, Hazen-Martin D. Refractory heartburn: comparison of intercellular space diameter in documented GERD vs. functional heartburn. Am J Gastroenterol. 2011;106:844-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Calabrese C, Bortolotti M, Fabbri A, Areni A, Cenacchi G, Scialpi C, Miglioli M, Di Febo G. Reversibility of GERD ultrastructural alterations and relief of symptoms after omeprazole treatment. Am J Gastroenterol. 2005;100:537-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Calabrese C, Fabbri A, Areni A, Scialpi C, Zahlane D, Di Febo G. Asthma and gastroesophageal reflux disease: effect of long-term pantoprazole therapy. World J Gastroenterol. 2005;11:7657-7660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Caviglia R, Ribolsi M, Gentile M, Rabitti C, Emerenziani S, Guarino MP, Petitti T, Cicala M. Dilated intercellular spaces and acid reflux at the distal and proximal oesophagus in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;25:629-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Xue Y, Zhou LY, Lin SR. Dilated intercellular spaces in gastroesophageal reflux disease patients and the changes of intercellular spaces after omeprazole treatment. Chin Med J (Engl). 2008;121:1297-1301. [PubMed] [Cited in This Article: ] |
| 14. | Amin SM, Abdel Maged KH, Naser AY, Aly BH. Laryngopharyngeal reflux with sore throat: an ultrastructural study of oropharyngeal epithelium. Ann Otol Rhinol Laryngol. 2009;118:362-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Park S, Chun HJ, Keum B, Uhm CS, Baek SK, Jung KY, Lee SJ. An electron microscopic study--correlation of gastroesophageal reflux disease and laryngopharyngeal reflux. Laryngoscope. 2010;120:1303-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Park S, Lee EJ, Chun HJ, Keum B, Seo YS, Kim YS, Jeen YT, Lee HS, Um SH, Kim CD, Ryu HS, In KH, Uhm CS, Lee SJ. Electron microscopic study of intercellular space: correlation analysis of bronchial asthma and gastroesophageal reflux disease. J Gastroenterol Hepatol. 2011;26:104-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Borrelli O, Mancini V, Thapar N, Ribolsi M, Emerenziani S, de'Angelis G, Bizzarri B, Lindley KJ, Cicala M. Dilated intercellular space diameter as marker of reflux-related mucosal injury in children with chronic cough and gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2014;39:733-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Villanacci V, Grigolato PG, Cestari R, Missale G, Cengia G, Klersy C, Rindi G. Dilated intercellular spaces as markers of reflux disease: histology, semiquantitative score and morphometry upon light microscopy. Digestion. 2001;64:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Ribolsi M, Perrone G, Caviglia R, Gentile M, Emerenziani S, Luca Guarino MP, Petitti T, Cicala M. Intercellular space diameters of the oesophageal epithelium in NERD patients: head to head comparison between light and electron microscopy analysis. Dig Liver Dis. 2009;41:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Chu CL, Zhen YB, Lv GP, Li CQ, Li Z, Qi QQ, Gu XM, Yu T, Zhang TG, Zhou CJ, Rui-Ji, Li YQ. Microalterations of esophagus in patients with non-erosive reflux disease: in-vivo diagnosis by confocal laser endomicroscopy and its relationship with gastroesophageal reflux. Am J Gastroenterol. 2012;107:864-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ravelli A, Villanacci V, Cadei M, Fuoti M, Gennati G, Salemme M. Dilated intercellular spaces in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2014;59:589-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Capocelli KE, Fernando SD, Menard-Katcher C, Furuta GT, Masterson JC, Wartchow EP. Ultrastructural features of eosinophilic oesophagitis: impact of treatment on desmosomes. J Clin Pathol. 2015;68:51-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Whelan KA, Merves JF, Giroux V, Tanaka K, Guo A, Chandramouleeswaran PM, Benitez AJ, Dods K, Que J, Masterson JC, Fernando SD, Godwin BC, Klein-Szanto AJ, Chikwava K, Ruchelli ED, Hamilton KE, Muir AB, Wang ML, Furuta GT, Falk GW, Spergel JM, Nakagawa H. Autophagy mediates epithelial cytoprotection in eosinophilic oesophagitis. Gut. 2017;66:1197-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Yasunaga C, Haratake J, Ohtani A. Specific Accumulation of Lanthanum Carbonate in the Gastric Mucosal Histiocytes in a Dialysis Patient. Ther Apher Dial. 2015;19:622-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Valika AK, Jain D, Jaffe PE, Moeckel G, Brewster UC. A Nodular Foreign Body Reaction in a Dialysis Patient Receiving Long-term Treatment With Lanthanum Carbonate. Am J Kidney Dis. 2016;67:128-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Iwamuro M, Urata H, Tanaka T, Ando A, Nada T, Kimura K, Yamauchi K, Kusumoto C, Otsuka F, Okada H. Lanthanum Deposition in the Stomach: Usefulness of Scanning Electron Microscopy for Its Detection. Acta Med Okayama. 2017;71:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 27. | Iwamuro M, Urata H, Tanaka T, Okada H. Review of the diagnosis of gastrointestinal lanthanum deposition. World J Gastroenterol. 2020;26:1439-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Ohno A, Miyoshi J, Tanabe H, Kusuhara M, Toki M, Chiba T, Shimoyamada H, Shibahara J, Hisamatsu T. Gastropathy associated with lanthanum phosphate deposition that was endoscopically tracked for 3 years. A case report. BMC Gastroenterol. 2020;20:292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Iwamuro M, Urata H, Hirata S, Ueki T, Hanabata T, Takeda S, Teraoka A, Okada H. A Bezoar Composed of Bilirubin Calcium, Calcium Carbonate, and Fatty Acid Calcium. Case Rep Gastrointest Med. 2019;2019:5742672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Iwamuro M, Saito S, Yoshioka M, Urata H, Ueda K, Yamamoto K, Okada H. A Magnesium Oxide Bezoar. Intern Med. 2018;57:3087-3091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Iwamuro M, Urata H, Furutani M, Kawai Y, Shiraha H, Takaki A, Okada H, Yamamoto K. Ultrastructural analysis of a gastric persimmon phytobezoar. Clin Res Hepatol Gastroenterol. 2014;38:e85-e87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Young KA, Allaker RP, Hardie JM. Morphological analysis of Helicobacter pylori from gastric biopsies and dental plaque by scanning electron microscopy. Oral Microbiol Immunol. 2001;16:178-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Chun HJ, Park DK, Park CH, Park JH, Jeen YT, Um SH, Lee SW, Choi JH, Kim CD, Ryu HS, Hyun JH, Chae YS, Uhm CS. Electron microscopic evaluation of adhesion of Helicobacter pylori to the gastric epithelial cells in chronic gastritis. Korean J Intern Med. 2002;17:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Jhala NC, Siegal GP, Klemm K, Atkinson BF, Jhala DN. Infiltration of Helicobacter pylori in the gastric mucosa. Am J Clin Pathol. 2003;119:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Caruso RA, Fedele F, Di Bella C, Mazzon E, Rigoli L. Foveolar cells phagocytose apoptotic neutrophils in chronic active Helicobacter pylori gastritis. Virchows Arch. 2012;461:489-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Okiyama Y, Matsuzawa K, Hidaka E, Sano K, Akamatsu T, Ota H. Helicobacter heilmannii infection: clinical, endoscopic and histopathological features in Japanese patients. Pathol Int. 2005;55:398-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Lange U, Teichmann J. Whipple arthritis: diagnosis by molecular analysis of synovial fluid--current status of diagnosis and therapy. Rheumatology (Oxford). 2003;42:473-480. [PubMed] [Cited in This Article: ] |
| 39. | Mehta A, Patkar N, Duhan S, Srinivas, Nema S. Atypical Whipple's disease. Indian J Gastroenterol. 2005;24:31. [PubMed] [Cited in This Article: ] |
| 40. | Whittle DO, Williams NP, Nicholson AM, King-Robinson K, Kirsch R, Riddell R, Mazzulli T, Lee MG. Whipple's Disease in an Afro-Caribbean National. West Indian Med J. 2014;63:101-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Vindigni SM, Taylor J, Quilter LA, Hyun TS, Liu C, Rosinski SL, Rakita RM, Fredricks DN, Damman CJ. Tropheryma whipplei infection (Whipple's disease) in a patient after liver transplantation. Transpl Infect Dis. 2016;18:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Ugenti I, Lattarulo S, Ferrarese F, De Ceglie A, Manta R, Brandonisio O. Acute gastric anisakiasis: an Italian experience. Minerva Chir. 2007;62:51-60. [PubMed] [Cited in This Article: ] |
| 43. | Calderaro A, Villanacci V, Bommezzadri S, Gorrini C, Piccolo G, Aquilano MC, Incaprera M, Viviani G, Dettori G, Chezzi C. Colonic amoebiasis and spirochetosis: morphological, ultrastructural and microbiological evaluation. J Gastroenterol Hepatol. 2007;22:64-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Calderaro A, Bommezzadri S, Gorrini C, Piccolo G, Peruzzi S, Villanacci V, Zambelli C, Dettori G, Chezzi C. Infective colitis associated with human intestinal spirochetosis. J Gastroenterol Hepatol. 2007;22:1772-1779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Mukhopadhya I, Hansen R, Nicholl CE, Alhaidan YA, Thomson JM, Berry SH, Pattinson C, Stead DA, Russell RK, El-Omar EM, Hold GL. A comprehensive evaluation of colonic mucosal isolates of Sutterella wadsworthensis from inflammatory bowel disease. PLoS One. 2011;6:e27076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Chen YJ, Li CJ. [Hypothermic effect of intracerebroventricular injections of taurine on endotoxin-induced fever in rabbits]. Zhongguo Yao Li Xue Bao. 1989;10:7-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Heine RG, Ward PB, Mikosza AS, Bennett-Wood V, Robins-Browne RM, Hampson DJ. Brachyspira aalborgi infection in four Australian children. J Gastroenterol Hepatol. 2001;16:872-875. [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Alfa MJ, Ribeiro MM, da Costa Luciano C, Franca R, Olson N, DeGagne P, Singh H. A novel polytetrafluoroethylene-channel model, which simulates low levels of culturable bacteria in buildup biofilm after repeated endoscope reprocessing. Gastrointest Endosc. 2017;86:442-451.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Bhatt S, Mehta P, Chen C, Schneider CL, White LN, Chen HL, Kong MG. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest Endosc. 2019;89:105-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Manger T, Pross M, Haeckel C, Lippert H. Malignant peripheral nerve sheath tumor of the esophagus. Dig Surg. 2000;17:627-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Altavilla G, Chiarelli S, Fassina A. Duodenal periampullary gangliocytic paraganglioma: report of two cases with immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2001;25:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Moncur JT, Lacy BE, Longnecker DS. Mixed acinar-endocrine carcinoma arising in the ampulla of Vater. Hum Pathol. 2002;33:449-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Adhikari D, Conte C, Eskreis D, Urmacher C, Ellen K. Combined adenocarcinoma and carcinoid tumor in atrophic gastritis. Ann Clin Lab Sci. 2002;32:422-427. [PubMed] [Cited in This Article: ] |
| 54. | Jianu CS, Lange OJ, Viset T, Qvigstad G, Martinsen TC, Fougner R, Kleveland PM, Fossmark R, Hauso Ø, Waldum HL. Gastric neuroendocrine carcinoma after long-term use of proton pump inhibitor. Scand J Gastroenterol. 2012;47:64-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Uraizee I, Hart J, Waxman I, Weber CR, Setia N. Unusual gastric subepithelial tumor: primary mixed acinar-endocrine neoplasm in an anemic woman. Gastrointest Endosc. 2018;87:1355-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Ichimata S, Aoyagi D, Takehana T, Uehara T, Shiozawa S. A case of large cell neuroendocrine carcinoma exhibiting rhabdoid features in the esophagogastric junction. Pathol Int. 2019;69:481-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Singh M, Nabavi E, Zhou Y, Gallina ME, Zhao H, Ruenraroengsak P, Porter AE, Ma D, Cass AEG, Hanna GB, Elson DS. Laparoscopic fluorescence image-guided photothermal therapy enhances cancer diagnosis and treatment. Nanotheranostics. 2019;3:89-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Li C, Wang Y, Zhang H, Li M, Zhu Z, Xue Y. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized gold nanoparticles using Cardiospermum halicacabum on AGS gastric carcinoma cells. Int J Nanomedicine. 2019;14:951-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |